Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

20.3 Electrocyclic Reactions 1039
2
to
3
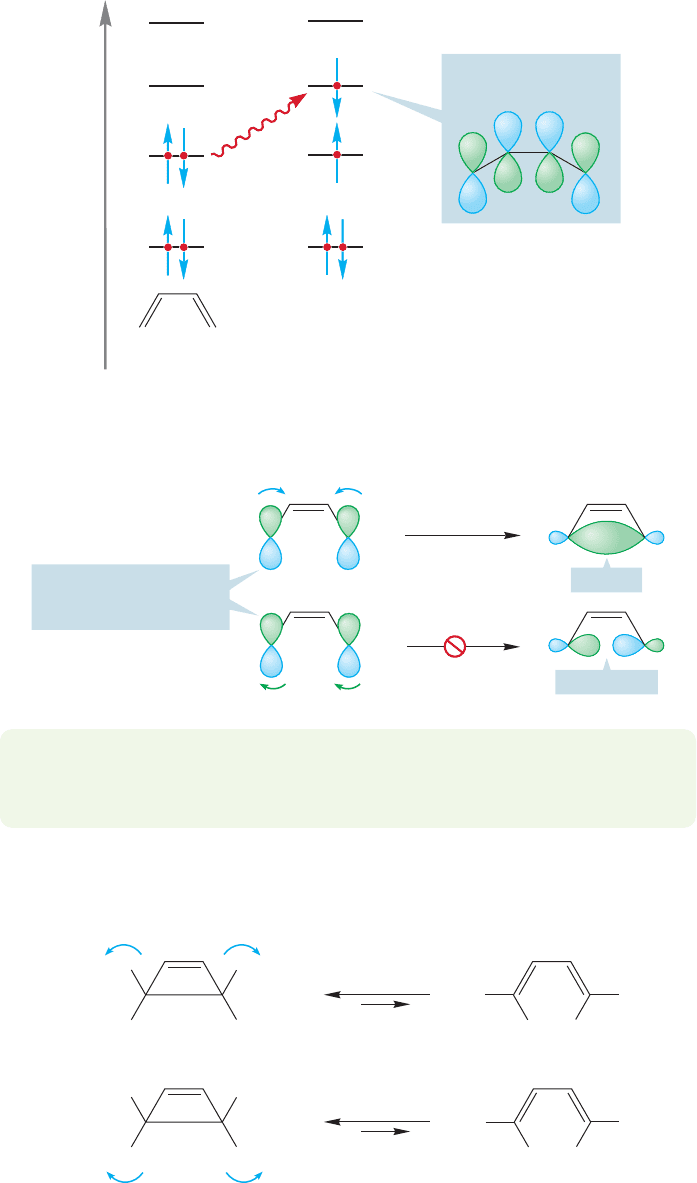
, creating a new, “photochemical HOMO” (Fig. 20.13). No longer does
conrotation in the HOMO, now
3
, create a bond between the end carbons.£
££
PROBLEM 20.2 Figure 20.14 shows only one disrotatory and one conrotatory
mode. Verify that the other possible disrotation produces a bond between the two
carbons, and that the other possible conrotation produces an antibond.
Energy
HOMO = Φ
2
hν
Φ
4
Φ
3
Φ
2
Φ
1
HOMO = Φ
3
New “photochemical
HOMO” is Φ
3
FIGURE 20.13 The HOMO involved
in the photochemical reaction of
butadiene is .£
3
Bonding
disrotation
conrotation
End lobes of ⌽
3
of butadiene, the
“photochemical HOMO”
Antibonding
FIGURE 20.14 The photochemical
HOMO ( ) demands disrotation.
In this molecular orbital, conrotation
produces an antibond.
£
3
E = COOCH
3
COOCH
3
CH
3
OOC
H
cis
cis
H
EE
cis,cis (Z,Z)
H
disrotation
H
COOCH
3
HH
E
H
trans,trans (E,E)
H
disrotation
E
CH
3
OOC
hν
hν
FIGURE 20.15 Disrotation
interconverts the cis disubstituted
cyclobutene and the cis,cis and
trans,trans dienes.
Two disrotatory modes interconvert the cis 3,4-disubstituted cyclobutene and the
cis,cis and trans,trans isomers of the butadiene (Fig. 20.15).
For an orbital of this symmetry, conrotation creates an antibond. In the photochem-
ical reaction, it is disrotation that creates the bonding interaction (Fig. 20.14).
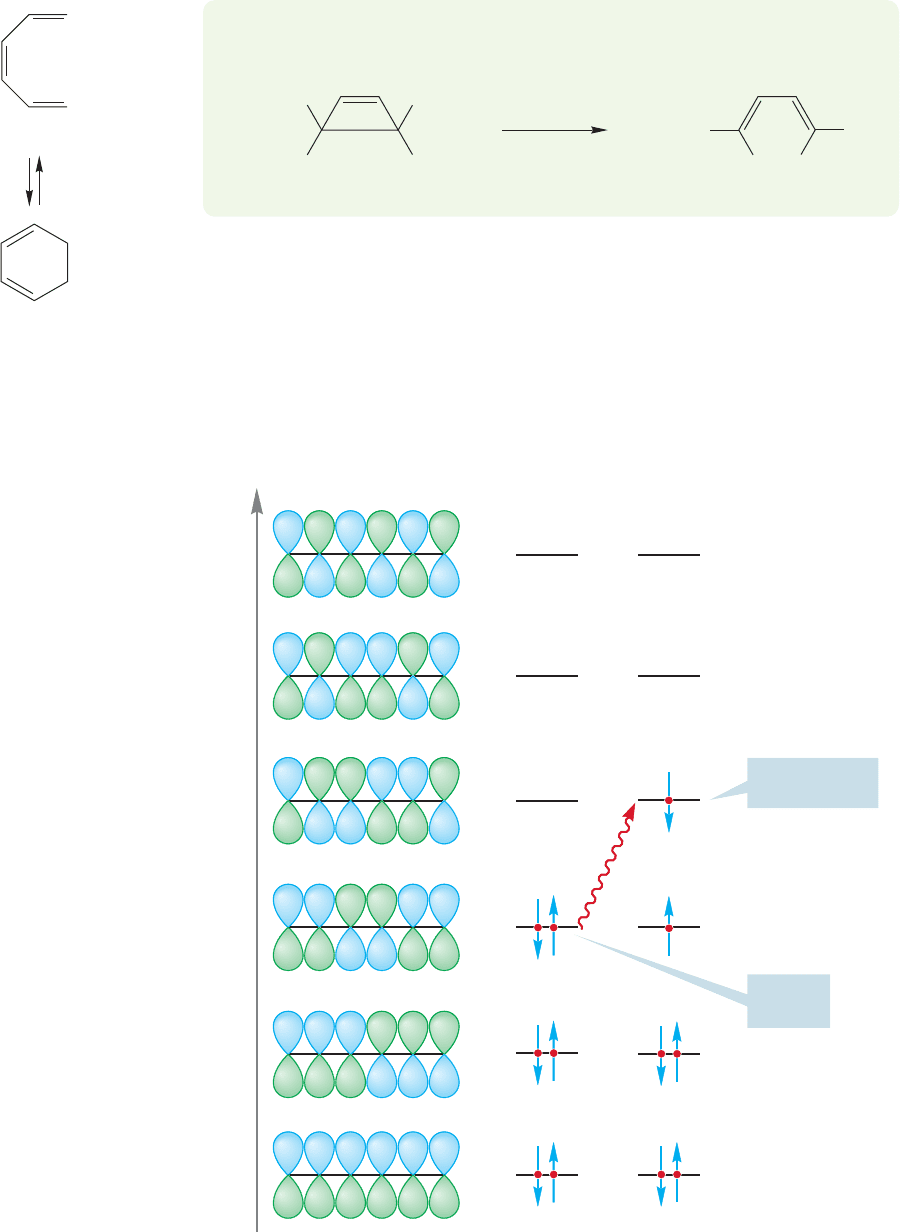
Let’s now use this technique for analyzing electrocyclic reactions to make
some predictions about the 1,3,5-hexatriene–1,3-cyclohexadiene system (Fig.
20.16).
Because it is always easier to look first at the open polyene system, we will con-
sider the π molecular orbitals for hexatriene (Fig. 20.17).The HOMO for the ther-
mal reaction is , and that for the photochemical reaction, .£
4
£
3
1040 CHAPTER 20 Reactions Controlled by Orbital Symmetry
PROBLEM 20.3 Show that disrotation in the photochemical reaction of trans
3,4-disubstituted cyclobutene leads to the cis,trans isomer of the butadiene.
COOCH
3
COOCH
3
CH
3
OOC
CH
3
OOC
H
H
H
cis,trans (Z,E )
H
disrotation
hν
cis-1,3,5-Hexatriene
1,3-Cyclohexadiene
FIGURE 20.16 Another electrocyclic
reaction interconverts cis-1,3,5-
hexatriene and 1,3-cyclohexadiene.
Φ
6
Φ
5
Φ
4
Energy
Φ
3
Φ
2
Φ
1
hν
Thermal
HOMO
Photochemical
HOMO
FIGURE 20.17 The π molecular orbitals for 1,3,5-hexatriene.The thermal HOMO is
and the photochemical HOMO is .
£
4
£
3
20.3 Electrocyclic Reactions 1041
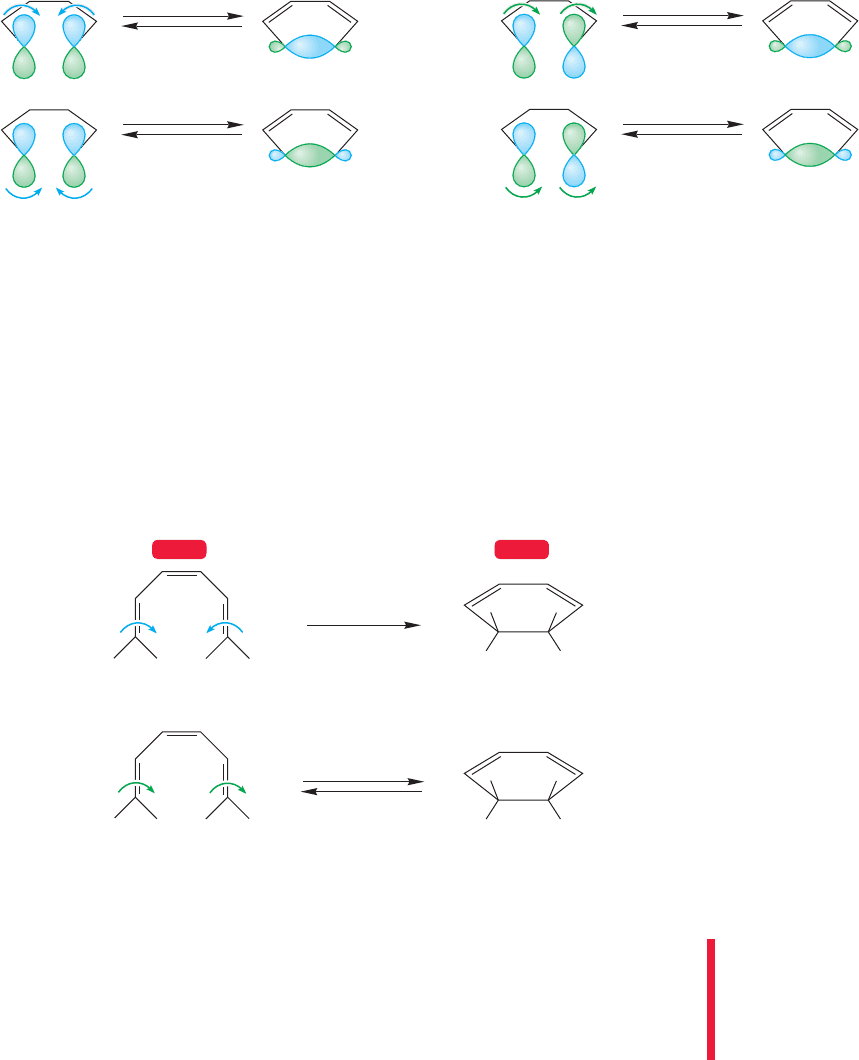
As the open triene closes to the cyclohexadiene (Fig. 20.18),the only way to cre-
ate a bonding interaction between the end carbons is to close in a disrotatory fash-
ion from , and in a conrotatory fashion from . So, we predict that the thermal
interconversion will occur in a disrotatory way, and that the photochemical reaction
must involve conrotation.
£
4
£
3
To test these predictions we need some labeled molecules. The trans,cis,trans-
2,4,6-octatriene shown in Figure 20.19 will do nicely. A disrotatory thermal closure
to cis-5,6-dimethylcyclohexa-1,3-diene and a conrotatory photochemical closure to
the trans isomer are both known.
disrotation
Δ
End lobes of Φ
3
(the HOMO)
disrotation
Thermal reactions
Δ
conrotation
End lobes of
Φ
4
of hexatriene
(photochemical HOMO)
conrotation
Photochemical reactions
hν
hν
FIGURE 20.18 For the hexatriene–cyclohexadiene system, in order to produce a bonding interaction, the thermal
reactions must take place in a disrotatory fashion and the photochemical reactions in a conrotatory way.
Δ
hν
HH
disrotation
conrotation
H H
H H
CH
3
CH
3
CH
3
trans,cis,trans
trans,cis,trans
cis
>95% trans
H
3
C
HCH
3
CH
3
H
H
3
C
H
3
C
WEB 3D WEB 3D
FIGURE 20.19 The experimental
results bear out the predictions.
The thermal reaction is disrotatory
and the photochemical reaction is
conrotatory.
Figure 20.19 shows the apparent formation of a single enantiomer of trans-5,6-
dimethylcyclohexa-1,3-diene from an achiral precursor, which cannot be possible.
We have not taken the trouble to draw both enantiomers of the racemic mixture of
the trans-5,6-dimethylcyclohexa-1,3-diene formed in the reaction. This omission
leads directly to Problem 20.4.
CONVENTION ALERT
1042 CHAPTER 20 Reactions Controlled by Orbital Symmetry
The first two rows of Table 20.1 summarize the results so far. They also point
out that the cyclobutene–1,3-butadiene interconversion is a four-electron process,
whereas the hexatriene–1,3-cyclohexadiene reaction involves six electrons. In
the cyclobutene–butadiene reaction, the four electrons in the σ and π orbitals of the
cyclobutene come to occupy the
1
and
2
orbitals of 1,3-butadiene. Similarly, the
six electrons in the σ,
1
, and
2
of 1,3-cyclohexadiene become the six electrons
in
1
,
2
, and
3
of the 1,3,5-hexatriene molecule.£££
££
££
TABLE 20.1 Rotatory Motions in Electrocyclic Reactions
Number of
Reaction Electrons Thermal Photochemical
Cyclobutene–butadiene 4 (4n) Conrotation Disrotation
Hexatriene–cyclohexadiene 6 (4n 2) Disrotation Conrotation
All 4n electrocyclic reactions 4n Conrotation Disrotation
All 4n 2 electrocyclic reactions 4n 2 Disrotation Conrotation
Δ
trans
“Dot” means
“H up”
CH
3
CH
3
CH
3
CH
3
(a)
PROBLEM 20.4 Figure 20.19 shows only one conrotatory and one disrotatory
mode. Show that the other disrotation and conrotation give the same relative
stereochemical results.
PROBLEM 20.5 Why are cyclohexadienes formed only from the trans,cis,trans
isomer shown in Figure 20.19? Why does the trans,trans,trans triene not give a
similar reaction?
PROBLEM 20.6 Work out the predicted products from the cis,cis,trans and
cis,cis,cis isomers of 2,4,6-octatriene for both photochemical and thermal
reactions.
It turns out that all 4n systems behave like the four-electron case (thermal
reactions conrotatory and photochemical reactions disrotatory) and all 4n 2
systems behave like the six-electron case (thermal reactions disrotatory and pho-
tochemical reactions conrotatory). This generalization is shown in the second
half of Table 20.1, which gives the rules for all electrocyclic reactions. Now, if
you can count the number of electrons correctly you can easily predict the stereo-
chemistry of any electrocyclic reaction without even working out the molecu-
lar orbitals.
PROBLEM 20.7 Provide mechanisms for the following reactions:
(continued)
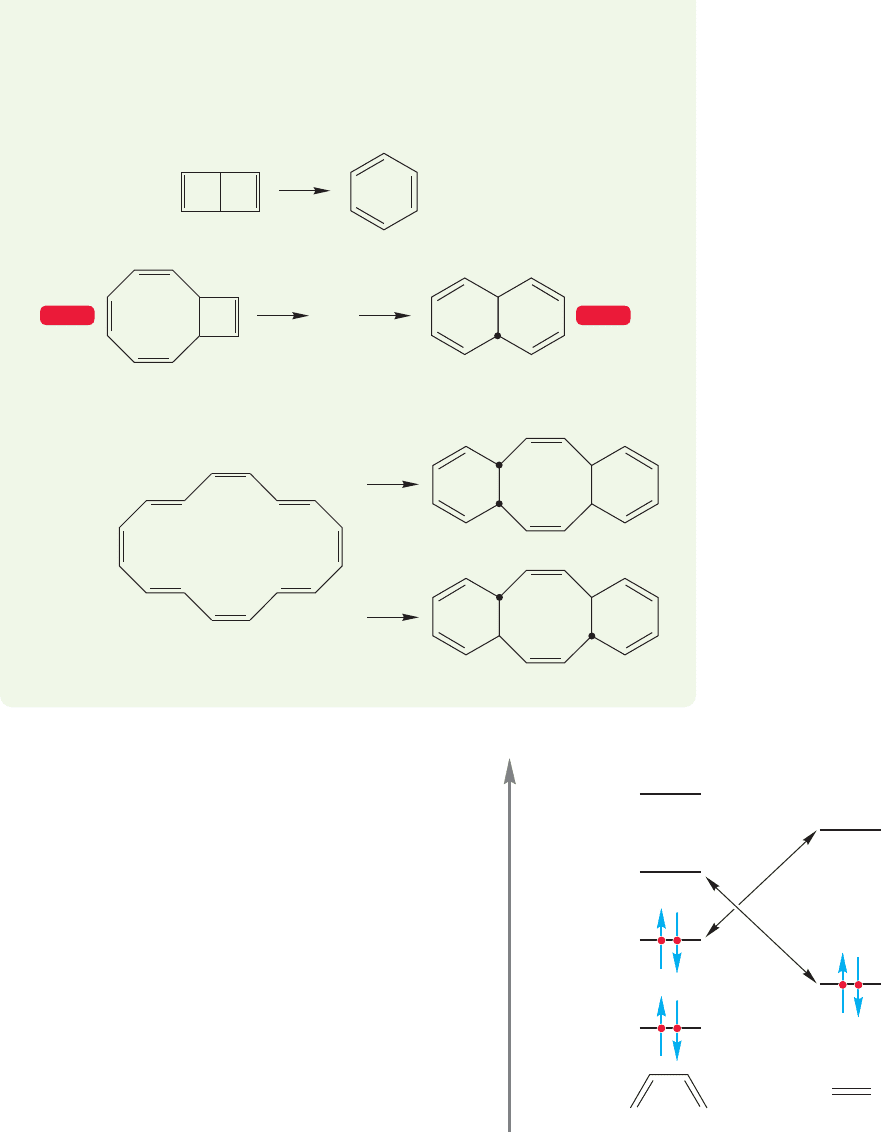
20.4 Cycloaddition Reactions 1043
(d)
(c)
Δ
trans
cis,cis
trans,trans
X
hν
Δ
Δ
Δ
WEB 3D WEB 3D
(b) Dewar benzene (p. 573) lies perched some 60 kcal/mol higher in energy than
its isomer benzene. It would seem that a simple stretching of the central bond in
Dewar benzene would inevitably and easily give benzene. Yet Dewar benzene
rearranges to benzene only quite slowly. Why?
20.4 Cycloaddition Reactions
Cycloaddition reactions are also orbital symmetry-
controlled, pericyclic reactions. We have seen one
example already, the Diels–Alder reaction, and we will
use it as our prototype. We found the Diels–Alder
cycloaddition to be a thermal process that takes place
in a concerted (one-step) fashion, passing over a cyclic
transition state. Several stereochemical labeling exper-
iments were described in Chapter 12 (p. 549), all of
which showed that the reaction involved neither
diradical nor polar intermediates. This stereospecifici-
ty is important because orbital symmetry considerations
apply only to concerted reactions. Of course, all reac-
tions can be subdivided into series of single-step, sin-
gle-barrier processes, and each of these steps could be
analyzed separately by orbital symmetry. However, it makes no sense to speak of the
overall orbital symmetry control of a multistep process.
When a diene and dienophile approach each other in the Diels–Alder reaction,
the important orbital interactions are between the HOMOs and LUMOs. As
Figure 20.20 shows, there are two possible HOMO–LUMO interactions,
HOMO
(diene)
–LUMO
(dienophile)
and HOMO
(dienophile)
–LUMO
(diene)
.The stronger of
Φ
4
π∗
π
LUMO
(dienophile)
HOMO
(dienophile)
LUMO
(diene)
Diene Dienophile
HOMO
(diene)
Φ
3
Φ
2
Φ
1
Energy
FIGURE 20.20 The two possible
HOMO–LUMO interactions in the
prototypal Diels–Alder reaction
between butadiene and ethylene.
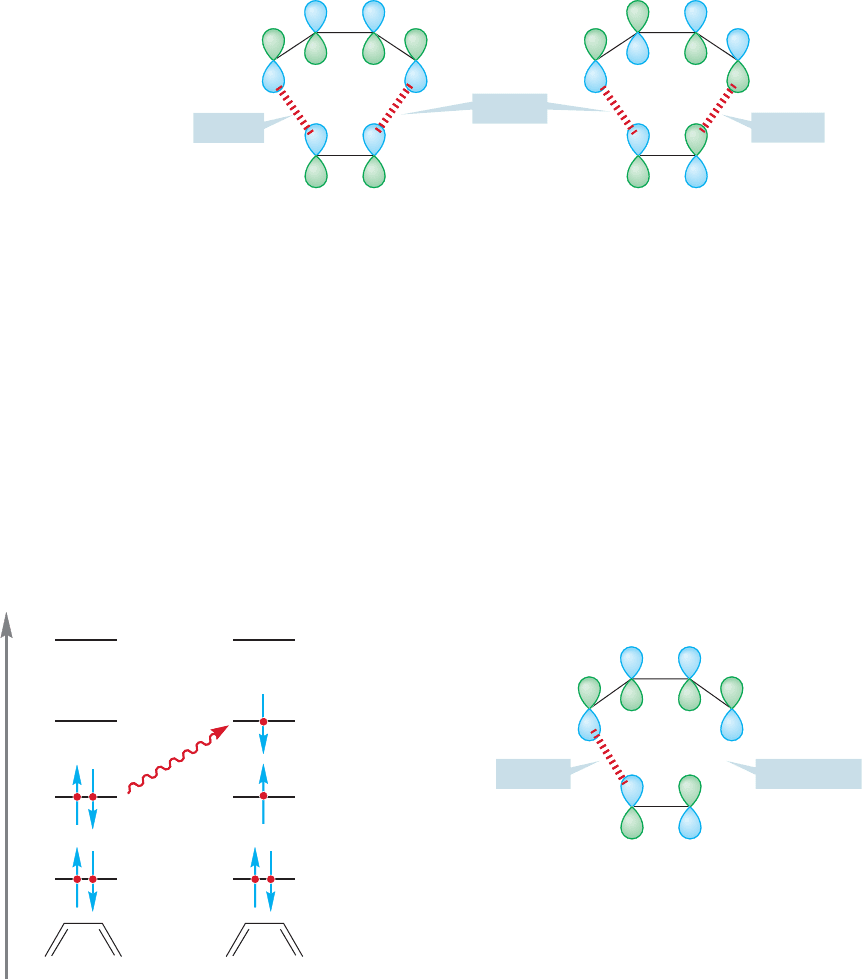
1044 CHAPTER 20 Reactions Controlled by Orbital Symmetry
these two will control the reaction. Remember: The strength of orbital overlap, and
the magnitude of the resulting stabilization, depends on the relative energies of the
two orbitals that are mixing.The closer the two are in energy, the stronger the inter-
action. In the simplest Diels–Alder reaction between ethylene and 1,3-butadiene,
the two HOMO–LUMO interactions are of equal magnitude and so we must look at
the orbital symmetry of both HOMO
(diene)
–LUMO
(dienophile)
and HOMO
(dienophile)
–
LUMO
(diene)
.Figure 20.21 shows the symmetry relationships for this reaction. Don’t
forget that in the Diels–Alder and related reactions the two participants approach
each other in parallel planes (p. 548).
π (HOMO) π* (LUMO)
Φ
2
(HOMO)Φ
3
(LUMO)
HOMO
(dienophile)
–LUMO
(diene)
HOMO
(diene)
–LUMO
(dienophile)
BondingBonding
Bonding
FIGURE 20.21 The orbital overlaps
in the two HOMO–LUMO
interactions of the Diels–Alder
reaction. Either of the HOMO–
LUMO interactions can lead to
product.
Both interactions involve bonding overlaps at the points of formation of the two
new σ bonds.Accordingly, it’s no surprise to find that the thermal Diels–Alder reac-
tion occurs. One says that the reaction is “allowed by orbital symmetry.”
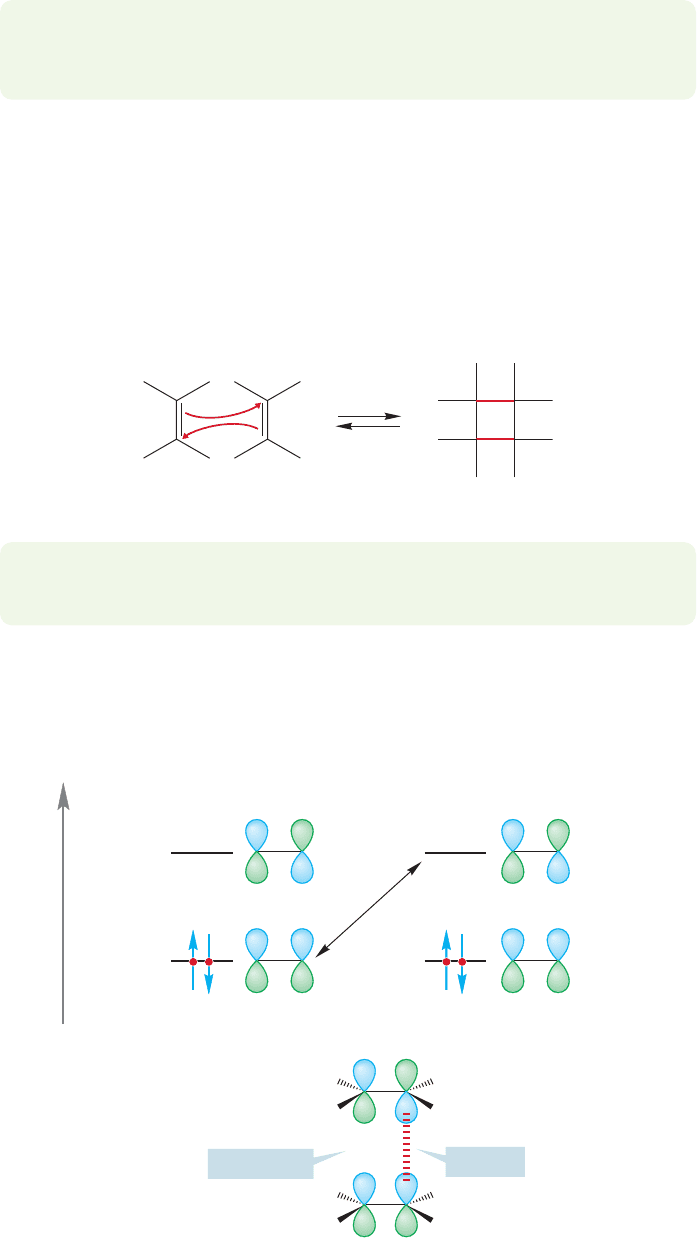
What about the photochemical Diels–Alder reaction? The observation that this
reaction is most uncommon leads us to the immediate suspicion that there is some-
thing wrong with it. Usually, the absorption of a photon will promote an electron
from the HOMO to the LUMO. In this case, the lower energy HOMO–LUMO
gap is that in the diene partner. Absorption of light creates a new photochemical
HOMO for the diene,
3
, and now the HOMO–LUMO interaction with the
dienophile partner involves one antibonding overlap. Both new bonds cannot be
formed at the same time (Fig. 20.22). So this photochemical Diels–Alder reaction
is said to be “forbidden by orbital symmetry.”
£
Φ
4
Φ
3
Φ
2
Φ
1
Photochemical
HOMO
Δ
Energy
hν
hν
π* (LUMO)
Photochemical
HOMO =
Φ
3
Bonding
Antibonding!
FIGURE 20.22 Absorption of a photon results in the formation of a new HOMO,
3
.The HOMO
(diene)
–LUMO
(dienophile)
interaction involves one antibond.The photo-Diels–Alder reaction is forbidden by orbital symmetry.
£
20.4 Cycloaddition Reactions 1045
PROBLEM 20.8 What would happen if the light were used to promote an electron
from the HOMO of the alkene (π) to the LUMO of the alkene (π*)? Would the
Diels–Alder reaction be allowed or forbidden? Show your analysis.
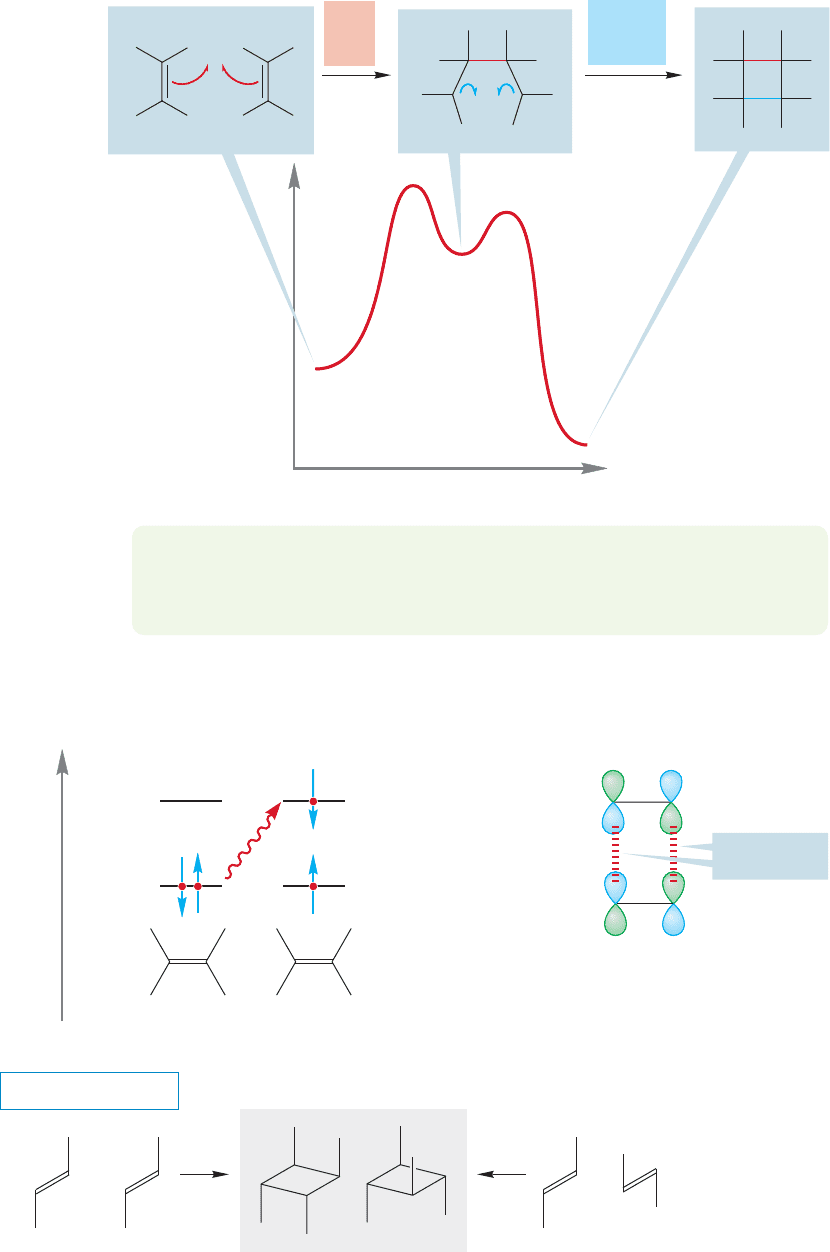
Let’s now analyze another potential cycloaddition, the joining together of a
pair of alkenes to give a cyclobutane (Fig. 20.23). This process can be called
a2 2 reaction because it involves a two-electron π bond combining with
another two-electron π bond (alkene alkene). Using this same terminology, a
Diels–Alder reaction (diene dienophile) is a 4 2 reaction. But be careful; using
this terminology makes most sense in describing the electrons involved in the reac-
tion, whereas another common use of 2 2, 4 2, etc., is to describe the number
of atoms involved in a cyclization.
Two alkenes
A cyclobutane
FIGURE 20.23 The hypothetical
combination of two alkenes to give
a cyclobutane.
PROBLEM 20.9 Will the 2 2 reaction between a pair of ethylenes to produce a
cyclobutane be exothermic or endothermic? Explain.
The HOMO and LUMO in an alkene are easy to identify, and it is quickly obvi-
ous that here, too, the apparently easy bond formation shown by the arrow formal-
ism used in Figure 20.23 is thwarted by an antibonding interaction as the two
molecules come together (Fig. 20.24).
π* (LUMO)
π
π
*
π
(HOMO)
(HOMO)
(LUMO)
Bonding
Antibonding
Energy
FIGURE 20.24 An analysis of the
HOMO and LUMO for the reaction
of two alkenes to give a cyclobutane
shows that it is forbidden by orbital
symmetry. There is an antibonding
interaction.
Therefore we might guess that the thermal dimerization of ethylenes would be
a rare process and we would be exactly correct. Moreover, in most of the reactions
1046 CHAPTER 20 Reactions Controlled by Orbital Symmetry
PROBLEM 20.10 Predict the stereochemistry of the product formed in the 2 2
cycloaddition of a pair of cis disubstituted ethylenes, (see
Fig. 20.25).
What about the photochemical dimerization of alkenes? Promotion of a single
electron creates a new photochemical HOMO (Fig. 20.26a), and now the symme-
tries are perfect for a HOMO–LUMO interaction involving two bonding
X
O
CH
P
CH
O
X
Energy
Reaction progress
form
one
bond
form
the other
bond
. .
R
R R
R R R
R
R
R R
R R
R
R
R R
R
R
R R R R
R R
FIGURE 20.25 The known
dimerizations of alkenes can
be shown to involve diradical
intermediates.This reaction
is not concerted.
π*
(LUMO)
π
π
*
π
*
(photochemical
HOMO)
Photochemical
HOMO
(HOMO)
(LUMO)
Energy
hν
Both bonding
interactions
+++
hν
hν
A REAL EXAMPLE
(a)
(b)
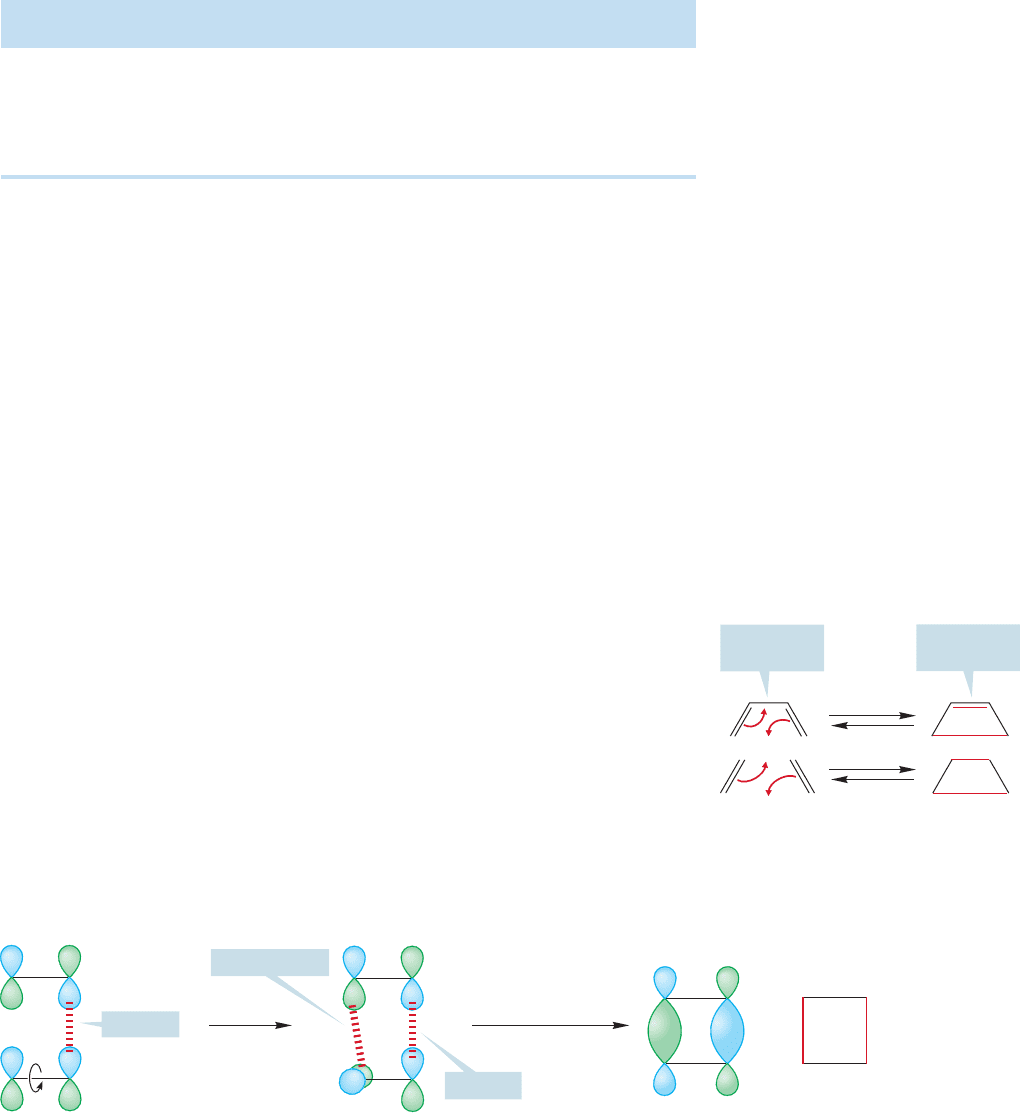
FIGURE 20.26 The photochemical dimerization of two butenes.
of this kind that do occur, it is clear that the two new bonds are formed sequentially
and not in a concerted manner (Fig. 20.25).
20.4 Cycloaddition Reactions 1047
overlaps. In contrast to the thermal reaction, the photochemical dimerization of two
ethylenes is allowed.For example, the photochemical dimerization of trans-2-butene
gives the two cyclobutanes shown in Figure 20.26b.
Again, it’s time for generalization. The first two rows of Table 20.2 show what
we have so far.
TABLE 20.2 Rules for Cycloaddition Reactions
Number of
Reaction Electrons Thermal Photochemical
Alkene alkene cyclobutane 4 (4n)No Yes
Alkene diene cyclohexene 6 (4n 2) Yes No
(Diels–Alder)
All 4n reactions 4n No Yes
All 4n 2 reactions 4n 2Yes No
Z
U
Z
U
Table 20.2 summarizes all 4n and 4n 2 reactions. Other 4n processes will fol-
low the rules for the 2 2 dimerization of a pair of alkenes, and 4n 2 processes
will resemble the 4 2 cycloaddition we know as the Diels–Alder reaction. Perhaps
you can see the relationship to aromaticity (4n 2) that plays a role in this analysis.
The transition state for these cycloaddition reactions is cyclic and will be allowed only
in the cases where the number of electrons makes the transition state aromatic,
4n 2 electrons for thermal processes and 4n for photochemical reactions.
Now compare Table 20.1 and Table 20.2. This comparison should be a bit dis-
quieting to you. Notice how much more information is available for electrocyclic
reactions than for cycloadditions.For the electrocyclic reactions,we have a clear stereo-
chemical prediction of how things must happen. For example, the 4n processes
undergo conrotatory thermal ring-closing reactions and disrotatory photochemical
ring-closing reactions. On the other hand, for cycloadditions, all we get is a crude
“thermal no,” “photochemical yes” message; a much less detailed picture.
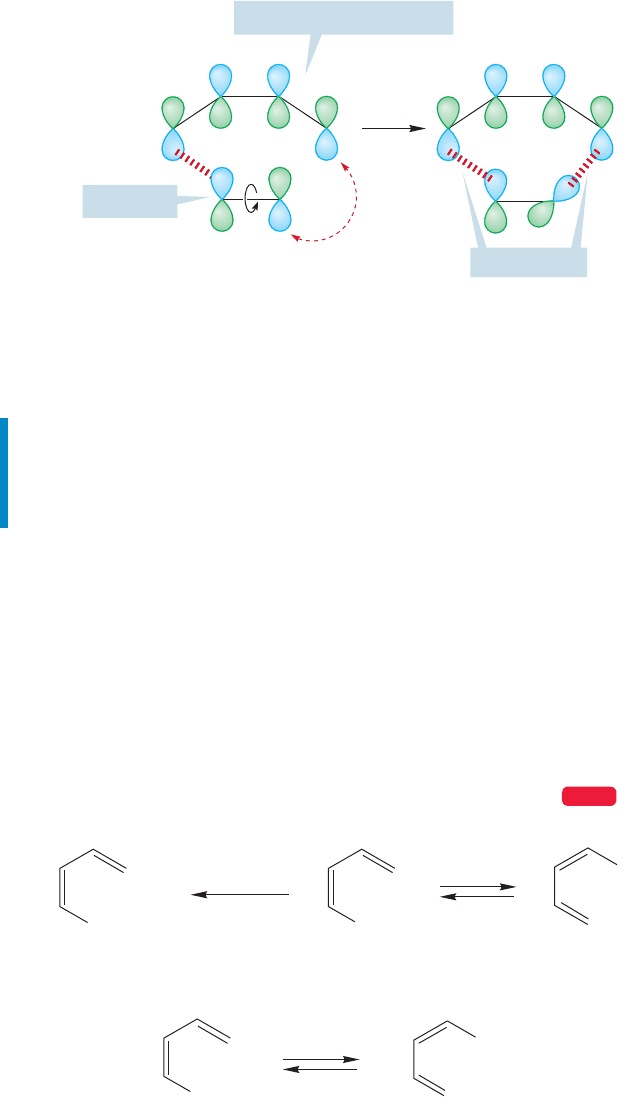
Electrocyclic reactions are not really different from cycloadditions.Figure 20.27 com-
pares the equilibration of 1,3-butadiene and cyclobutene with the 2 2 dimerization
of a pair of ethylenes.The only difference is the extra σ bond in butadiene,and this bond
is surely not one of the important ones in the reaction—it seems to be just going along
for the ride. Why should its presence or absence change the level of detail available to
us through an orbital symmetry analysis? It shouldn’t, and in fact, it doesn’t.
Look again at Figure 20.24, from which we derived the information that the ther-
mal 2 2 dimerization of a pair of ethylenes was forbidden. It’s only forbidden if
we insist on smushing the two ethylenes together head to head exactly as shown in
the figure. If we are more flexible, and allow one ethylene to rotate about its
carbon–carbon σ bond as the reaction takes place, the offending antibonding over-
lap vanishes. What orbital symmetry really says is that for the concerted thermal
dimerization of two alkenes to take place, there must be this rotation (Fig. 20.28).
π*
π
(HOMO)
(LUMO)
Bonding
“2 + 2” Product
a cyclobutane
Bonding
rotate
=
continue to rotate
Also bonding!
90⬚
FIGURE 20.28 The thermal 2 2 dimerization is allowed if one end of one ethylene can rotate as the reaction occurs.
σ Bond
(single bond)
σ Bond of
double bond
FIGURE 20.27 The equilibration
of cyclobutene and 1,3-butadiene
and the 2 2 dimerization of a pair
of ethylenes are very closely related
reactions.
1048 CHAPTER 20 Reactions Controlled by Orbital Symmetry
Don’t forget; these rules do not include any consideration of thermodynamics.
The processes allowed with a rotation may well not be very favorable thermodynam-
ically, even though they are permitted electronically.
Summary
Cycloaddition reactions involve the coming together of two (or, rarely,even more)
molecules to make a ring.The easiest analysis involves examining the interactions
of the HOMOs and LUMOs of the two fragments forming the ring compound.
20.5 Sigmatropic Shift Reactions
When one heats (Z)-1,3-pentadiene nothing obvious happens, as the starting ma-
terial is recovered, apparently unchanged. Finally, at very high temperatures, radical
reactions begin as carbon–hydrogen bonds are broken. However, a deuterium-label-
ing experiment reveals that much is happening long before the energies necessary
for the formation of radicals have been reached (Fig. 20.30).
Both bonding
rotate
π* (LUMO)
Φ
3
(photochemical HOMO)
FIGURE 20.29 The photochemical
4 2 reaction (the Diels–Alder
reaction) is also allowed if a rotation
occurs during the cycloaddition or if
the orientation is favorable.
Δ Δ
Δ
Δ
H
.
.
CH
2
CH
2
D
CH
3
CD
3
(high
temperature)
Radicals
Labeling experiment
“No reaction”
(Z)-1,3-Pentadiene
(Z)-5,5,5 -Trideuterio-
1,3-pentadiene
(Z)-1,1,5 -Trideuterio-
1,3-pentadiene
(Z)-1,3 -Pentadiene
CD
2
CH
3
(a)
(b)
WEB 3D
FIGURE 20.30 A labeling
experiment reveals the degenerate
thermal rearrangement of
(Z)-1,3-pentadiene.
In practice,this rotation is a very difficult thing to achieve. It is this difficulty that
accounts for the absence of the thermal 2 2 reaction,not the absolute “No”of Table
20.2. Similarly, the photochemical 4 2,Diels–Alder reaction also becomes allowed
if we permit one of the partners to undergo a rotation during the reaction or orient
the two reagents at an angle so that the bonding overlap is possible (Fig. 20.29).
