Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

6.3 Alkyl Halides as Sources of Organometallic Reagents: A Synthesis of Hydrocarbons 229
RX
Li
(R
Li)
n
+
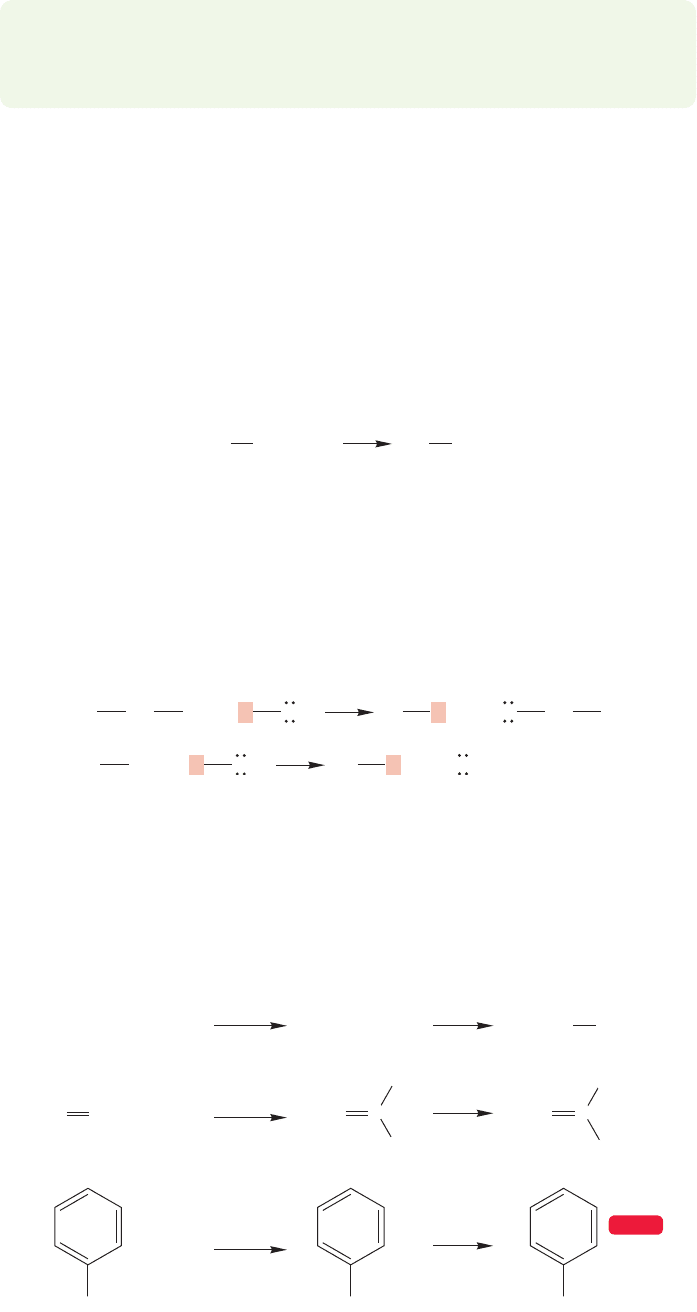
FIGURE 6.8 Organolithium reagents
are not monomeric, but oligomeric.
The exact value of n depends on the
solvent and the structure of R.
PROBLEM 6.3 Draw an interaction diagram to show the stabilization when two
methyl radicals combine. How great is that stabilization? For an example of an
interaction diagram see Figure 1.39, p. 33.
Coupling of R and MgX generates RMgX, and further radical reactions can
equilibrate this species with the dialkylmagnesium. Most halides (except fluorides)
can be made into Grignard reagents.
Organolithium reagents are even more complex. Here the ether solvent is not
essential, but the nature of the organolithium reagent does depend on the solvent.
Organolithium reagents are known not to be monomeric; they form aggregates the
size of which depends on the nature of the solvent and the structure of the R group.
As in RMgX, the representation of organolithium reagents as RLi, even with the
addition of the polar resonance form shown in Figure 6.5, is a convenient simplifi-
cation (Fig. 6.8).
..
RX
Mg
++
R
H
HO
X
Mg
OH
H
R Li
++
R
H
LiOH
OH
H
FIGURE 6.9 The powerfully basic
organometallic reagents are easily
protonated by water to give
hydrocarbons and metal hydroxides.
One of the end products of the reactions in Figure 6.9 is a hydrocarbon. This
sequence constitutes a synthesis of hydrocarbons from most halides. This reaction
can often be put to good use in the construction of isotopically labeled reagents,
because D
2
O can be used in place of H
2
O to produce a specifically deuteriolabeled
hydrocarbon (Fig. 6.10).
WEB 3D
CH
3
CH
2
D
Mg
ether
D
2
O
+
CH
3
CH
2
Br
CH
3
CH
2
MgBr
Li
D
2
O
+
H
2
C
CHBr
C
H
Li
H
2
CH
2
C
C
H
D
Li
D
2
O
+
Cl Li D
FIGURE 6.10 Treatment of an
organometallic reagent with D
2
O
gives deuteriolabeled molecules.
Both of these organometallic reagents are extraordinarily strong bases, and they
must be carefully protected from moisture and oxygen. Chemists form them under
a strictly dry and inert atmosphere. Although these reagents do not contain free car-
banions, they act as if they did.They are sources of R
.The very polar carbon–metal
bond attacks all manner of Lewis and Brønsted acids. Water is more than strong
enough to protonate a Grignard or organolithium reagent (Fig. 6.9).
:
D
D
230 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
PROBLEM 6.4 Given inorganic reagents of your choice (including D
2
O), devise
syntheses of the following molecules from the indicated starting materials:
WEB 3D WEB 3D
2
3
CH
3
OH
Methanol
(methyl alcohol)
CH
3
CH
2
OH
Ethanol
(ethyl alcohol)
CH
3
CH
2
CH
2
OH
1-Propanol
(propyl alcohol)
OH
CH
OH
2-Propanol
(isopropyl alcohol)
CH
3
CH
2
CH
2
CH
2
OH
1-Butanol
(butyl alcohol)
CH
2
OH
CH
2-Methyl-1-propanol
(isobutyl alcohol)
CH
3
CH
2
CHOH
CH
3
H
3
C
CH
3
C OH
CH
3
2-Methyl-2-propanol
(tert-butyl alcohol)
CH
3
(CH
2
)
3
CH
2
OH
1-Pentanol
(amyl alcohol)
H
3
C
H
3
C
H
3
C
H
3
C
1
Cl
2-Chloro-1-butanol
Cl
3-Chloro-4-fluoro-
2-pentanol
OH
F
2-Butanol
(sec-butyl alcohol)
OH
Cyclopentanol
(cyclopentyl alcohol)
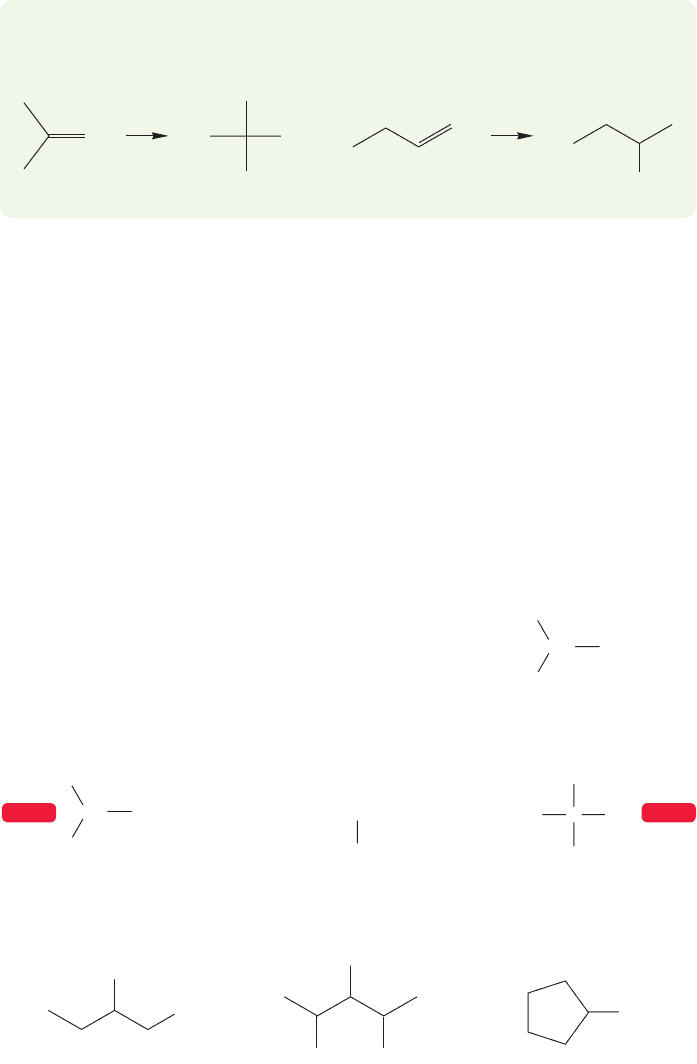
FIGURE 6.11 Some systematic and commonly used names for alcohols.
6.4 Alcohols
6.4a Nomenclature There are two ways to name alcohols: the IUPAC system-
atic way, and the way chemists often do it, by using the splendidly vulgar common
names. As usual, these common names are used only for the smaller members of the
class, and the system takes over as complexity increases.
Alcohols are named systematically by dropping the final “e” of the parent hydro-
carbon and adding the suffix “ol.” The functional group, OH, is given the lowest
number possible,taking precedence over other groups in the molecule including the
related thiol group, SH.The longest carbon chain is identified and the substituents
numbered appropriately (Fig. 6.11).
The smaller alcohols, however, are given common names with the word alcohol
written after the appropriate group name. Figure 6.11 gives some systematic and
common names for the smaller alcohols. When we get past approximately five car-
bons, the systematic naming protocol takes over completely, and even the delightful
6.4 Alcohols 231
name amyl for the five-carbon alcohols is disappearing.
2
However, some common
names seem to be quite hardy. For example, the correct IUPAC name for hydroxy-
benzene is benzenol, but the common name phenol is still accepted in the IUPAC
system and probably will survive for a long time (Fig. 6.12).
2
Amyl derives from the Latin word for starch, amylum. The first amyl alcohol was isolated from the fermen-
tation of potatoes.
WEB 3D
WEB 3D
Allyl alcohol
Phenol Amyl alcohol Ethylene
g
l
y
col
Benzyl alcohol
OH
OH
trans-Crotyl alcohol
OH
OH
OH
Glycerol
(g
l
y
cerin
)
OH OH
OH
OH
H
H
H
3
C
CH
2
OH
FIGURE 6.12 Some widely used
common names for alcohols.
PROBLEM 6.5 Provide systematic names for the following alcohols:
OH
OH
OH
OH
OH
HO
OCH
3
OH
OH
Br
Cl
OH
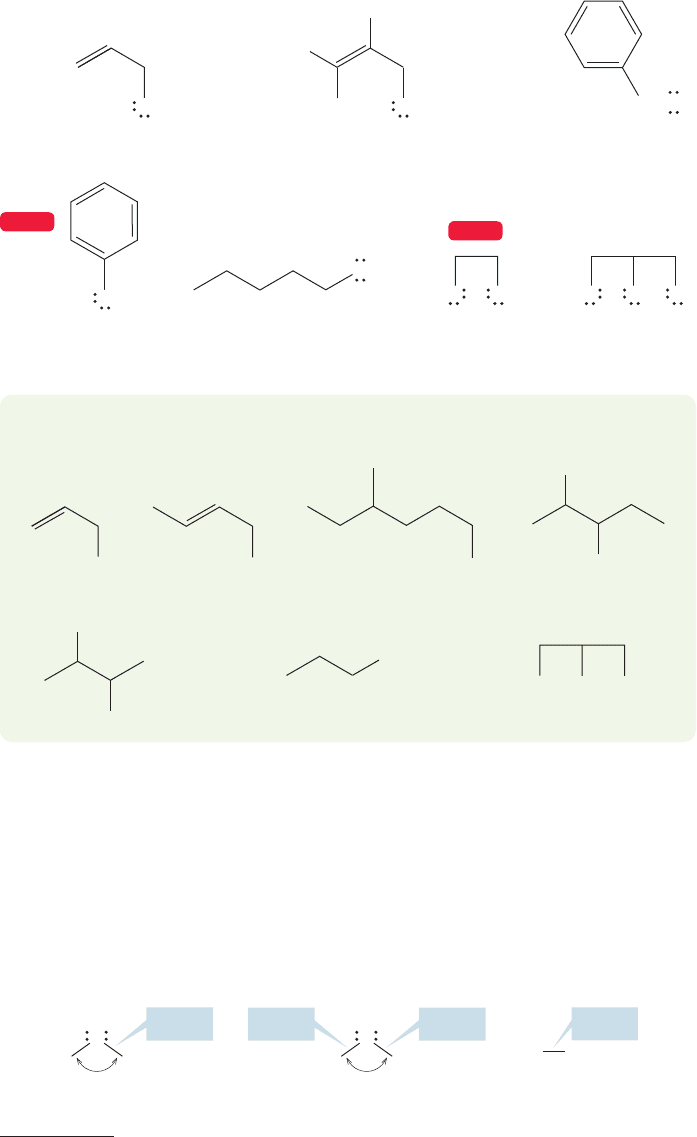
6.4b Structure of Alcohols Alcohols are derivatives of water, and it will be no
surprise to see that they rather closely resemble water structurally. The bond angle
is expanded a bit from 104.5° in to approximately 109° in simple
alcohols .
The oxygen–hydrogen bond length is very little changed from that of water, but
the carbon–oxygen bond is a bit shorter and stronger than the carbon–carbon bond
in ethane (Fig. 6.13).
R
O
O
O
H
H
O
O
O
H
104.5⬚ 108.9⬚
H
3
C
H
3
C
O
H
O
HH
CH
3
0.96 A
⬚
1.43 A
⬚
0.96 A
⬚
1.54 A
⬚
FIGURE 6.13 Bond lengths and
angles for water, methyl alcohol,
and ethane.
232 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
PROBLEM 6.6 What is the approximate hybridization of the oxygen atom in
methyl alcohol ( angle 108.9°). How do you know?
H
3
C
O
O
O
H
(C)
C
sp
3
H
H
H
H
C
O
Energy
C
C
O
O
O
C O
(O)
sp
3
105 kcal/mol
92 kcal/mol
σ*
C Oσ
96 kcal/mol
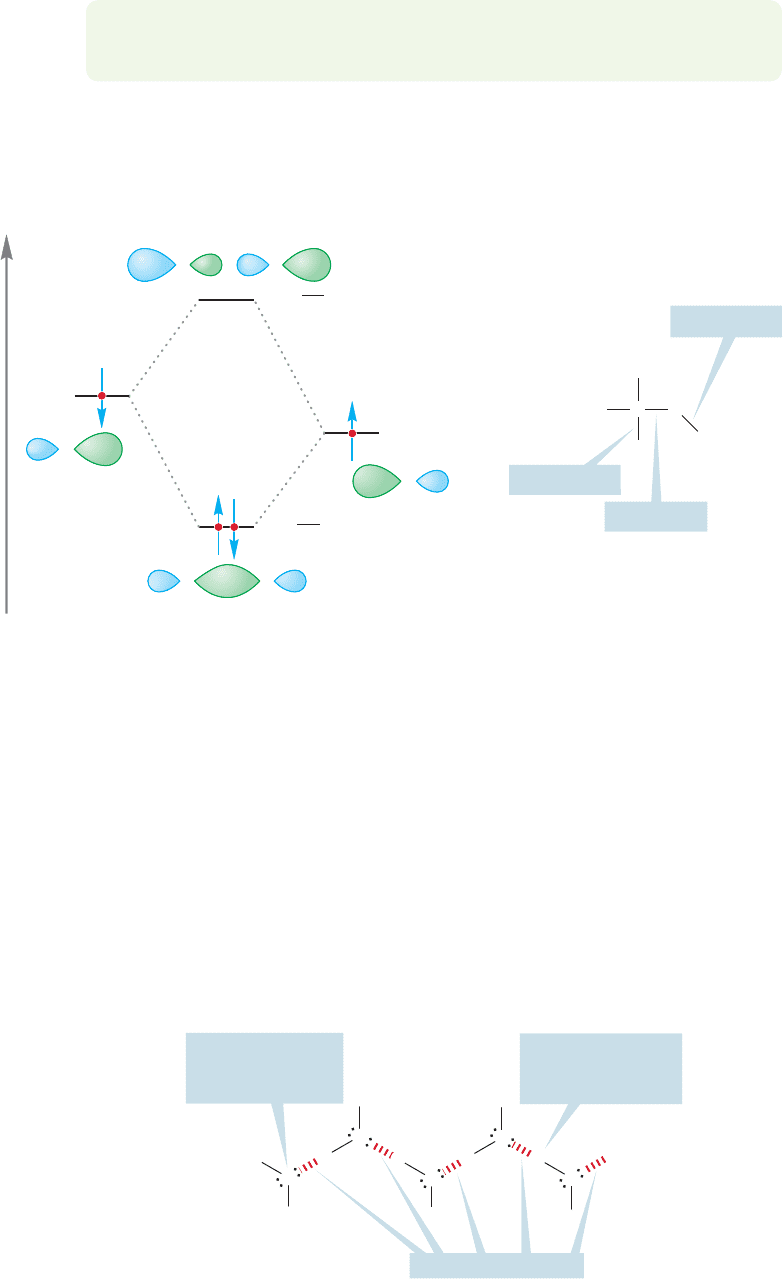
FIGURE 6.14 A graphical description of the formation of the carbon–oxygen σ bond.
R = H (water) or alkyl (alcohols)
Here, an alcohol
acts as Brønsted
acid (proton donor)
H
R
H
R
H
R
R
O
H
O
etc.
H
O
O
R
O
An alcohol acts as
Brønsted base
(proton acceptor)
Hydrogen bonds
FIGURE 6.15 Water and alcohols are
both Brønsted acids and Brønsted
bases.This figure shows hydrogen
bonding between molecules of water
(R H) and alcohols.
The orbital interaction diagram of Figure 6.14 shows the construction of the
carbon–oxygen bond, as well as typical bond energies. Notice that an electron in
an orbital near the very electronegative oxygen is more stable than it would be in an
orbital near carbon.
6.4c Physical Properties of Alcohols The presence of the electronegative
oxygen atom ensures that bonds will be strongly polarized in alcohols and that sub-
stantial dipole moments will exist. In the liquid phase, alcohols are strongly associ-
ated both because of dipole–dipole attractions and because of hydrogen bonding in
which the basic oxygen atoms form partial bonds to the acidic hydroxyl hydrogens.
Such bonds can be quite strong, on the order of 5 kcal/mol, a value not high enough
to make permanent dimeric or polymeric structures but quite sufficient to raise the
boiling points of alcohols far above those of the much less strongly associated
alkanes. Figure 6.15 shows an ordered, hydrogen-bonded structure for an alcohol.
In the absence of hydrogen bonding, water with a molecular weight of only 18, and
the low molecular weight alcohols, would surely be gases. The polarity of alcohols
6.4 Alcohols 233
makes them quite water soluble, and most of the smaller molecules are miscible
(soluble in all proportions) with, or at least highly soluble in, water. Table 6.3 gives
some physical properties of alcohols. For comparisons with the parent alkanes see
Table 2.4 (p. 79).
TABLE 6.3 Some Physical Properties of Alcohols
Compound bp (°C) mp (°C) Dipole Moment (D)
CH
3
OH
Methyl alcohol
65.15 –93.9 1.7
78.5 –117.3 1.69
(CH
3
)
2
CHOH 82.4 –89.5 1.68
Isopropyl alcohol
117.2 –89.5 1.66
Density (g/cm
3
)
0.79
0.79
0.80
0.81
CH
3
CH
2
OH
CH
3
CH
2
CH
2
CH
2
OH
Butyl alcohol
Ethyl alcohol
(CH
3
)
2
CHCH
2
OH –108 1.64108
99.5 –115
sec-Butyl alcohol
(CH
3
)
3
COH 82.3 25.5
181.7 43 1.45
0.80
0.81
0.79
1.06
Isobutyl alcohol
tert-Butyl alcohol
OH
Phenol
CH
3
CH
2
CH(CH
3
)OH
Even though these intramolecular hydrogen bonds are relatively weak ( 5 kcal/mol)
they are critically important. For example, life as we know it is clearly not possible
without water and there would be no liquid water without hydrogen bonds.
Moreover, hydrogen bonds are used to maintain the proper structures in proteins
and nucleic acids, polymeric structures essential to our existence that we will meet
later in this book.
6.4d Acid and Base Properties of Alcohols We have just seen water and
alcohols acting as Brønsted acids and bases. Hydrogen bonding is a perfect exam-
ple of such behavior. In Chapter 7, we are going to look closely at a number of
reactions of alcohols. These processes depend on the ability of alcohols to act as
both acids and bases. Accordingly, we first make sure that we can see these
roles clearly.
A Brønsted acid is any compound that can donate a proton. A Brønsted base
is any compound that can accept a proton. Let’s look first at a very simple process,
the reaction of solid potassium hydroxide (KOH) with gaseous hydrochloric acid
(HCl).This reaction is nothing but a competition of two Brønsted bases (Cl
and
HO
) for a proton, H
. The stronger Brønsted base (HO
) wins the competi-
tion (Fig. 6.16).
'
K
+
K
+
OH
HCl
HOH
Cl
..
++
–
–
FIGURE 6.16 Two Brønsted bases
(blue) competing for a proton.
234 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
Hydrochloric acid (HCl) is called the conjugate acid of Cl
, and Cl
is the
conjugate base of hydrochloric acid. Conjugate bases and acids are related to each
other through the gain and loss of a proton (Fig. 6.17).
H
+
H
Caution! This is a formalized picture and does not
imply the reaction of bases with a bare proton, H
+
.
Outside of the gas phase, bare protons do not exist
B
H B is the
conjugate
acid of B
B is the
conjugate
base of H B
B
–
–
–
FIGURE 6.17 Conjugate acids and
bases.
–
–
ClCl
H
2
O
OH
HO
–
HO
HO
H
2
O
+
H
H
H H
+
++
+
(a)
(b)
FIGURE 6.18 Water is both a
Brønsted acid and base.
PROBLEM 6.7 What are the conjugate acids of the following molecules?
(a) H
2
O (b)
OH (c) NH
3
(d) CH
3
OH (e) (f)
CH
3
PROBLEM 6.8 What are the conjugate bases of the following molecules?
(a) H
2
O (b)
OH (c) NH
3
(d) CH
3
OH (e) CH
4
(f) HOSO
2
OH (g)
OSO
2
OH
H
2
C
P
O
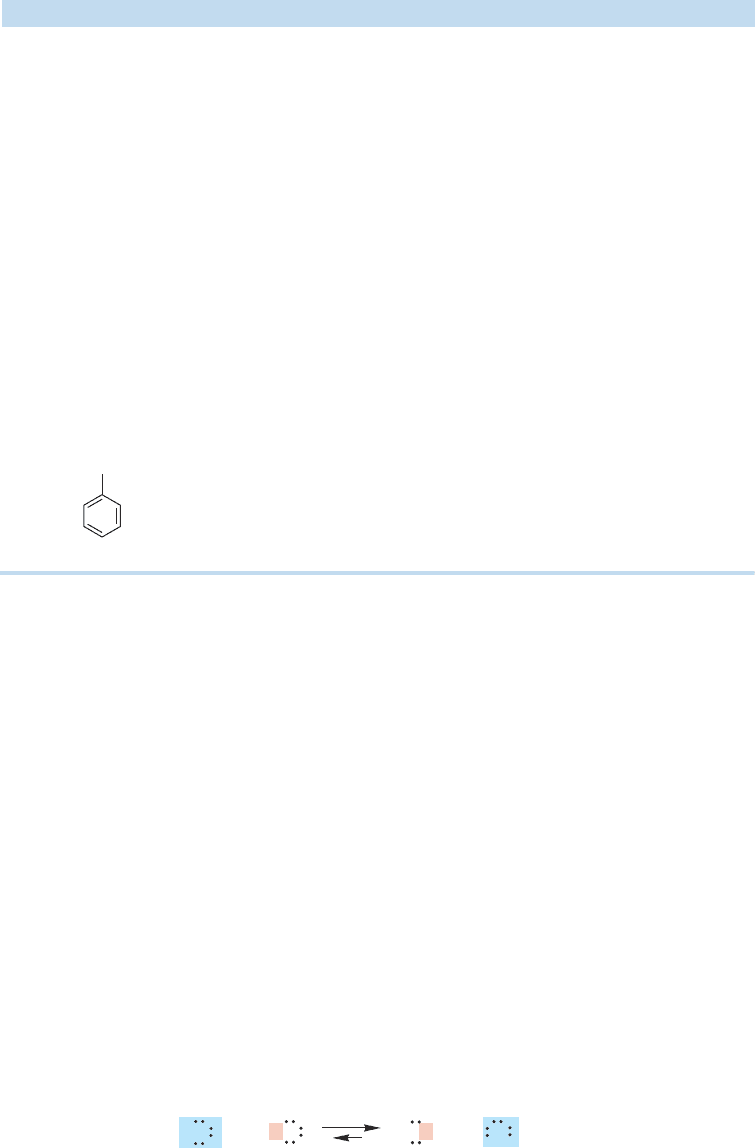
As we saw in Figure 6.15, a molecule may be both a Brønsted acid and a
Brønsted base. Consider water, for example. Water can both donate a proton (act
as a Brønsted acid) and accept a proton (act as a Brønsted base). Figure 6.18 shows
two reactions, (a) the protonation of water by hydrogen chloride, in which water
acts as a base, and (b) the protonation of hydroxide ion by water, in which water is
acting as the acid.
Many reactions begin with a protonation step. Because strong acids are better
able to donate a proton than weaker acids, it will be important for us to know which
molecules are strong acids and which are weak acids.The dissociation of an acid HA
in water can be described by the equation
HA H
2
OH
3
O
A
This equation leads to an expression for the equilibrium constant, K.
Because it is present as solvent, in vast excess, the concentration of water remains
constant in the ionization reaction. This equation is usually rewritten to give the
acidity constant, K
a
,
where the square brackets indicate concentration.
K
a
=
[H
3
O
+
] [A
-
]
[HA]
K =
[H
3
O
+
] [A
-
]
[HA] [H
2
O]
U
Z
6.4 Alcohols 235
By analogy with pH, we can define a quantity pK
a
.
pK
a
log K
a
The stronger the acid, the lower is its pK
a
. Table 6.4 gives pK
a
values for some
representative compounds. This short list spans about 80 powers of 10 (pK
a
is a log
function). In practice, any compound with a pK
a
lower than about 5 is regarded
as a reasonably strong acid; those with pK
a
values below 0 are very strong. As we
discuss new kinds of molecules, more pK
a
values will appear. Should you memorize
this list? We think not, but you will need to have a reasonable idea of the approxi-
mate acidity of different kinds of molecules.
TABLE 6.4 Some pK
a
Values for Assorted Molecules
a
Compound pK
a
Compound pK
a
HI 10 H
4
N
9.2
H
2
SO
4
3CH
3
OH 15.5
HBr 9H
2
O 15.7
HCl 8ROH
16–18
224
H
3
O
1.7 NH
3
38
HNO
3
1.3 50
HF 3.2 (CH
2
)
3
(cyclopropane) 46
RCOOH
4–5 CH
4
50–60
H
2
S 7.0 (CH
3
)
3
CH 50–70
a
When there is a choice, it is the underlined H that is lost. A longer list is provided on the back inside cover.
H
2
C
P
CH
2
HC
q
CHRO
+
H
2
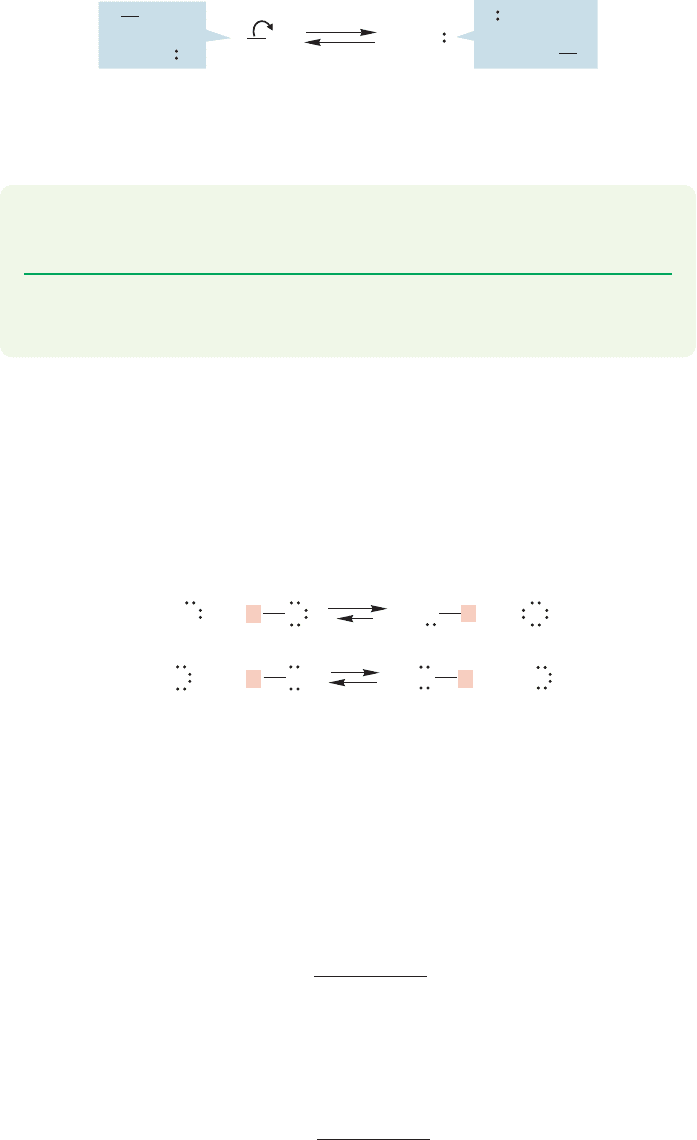
In alcohols and water, Brønsted basicity is centered on the nonbonding elec-
trons of the oxygen atom, which form bonds with a variety of acids. Protonation
converts an alcohol, ROH, into an oxonium ion (RO
H
2
). This conversion will
have profound chemical consequences, which we can preview here. Breaking the
bond to form ions is very difficult, because both positive (R
) and
negative (HO
) ions must be formed.Hydroxide, HO
, is very difficult to form—
one says that hydroxide is a poor leaving group—and alcohols do not ionize easily
(Fig. 6.19). After protonation, however, the “leaving group” is no longer hydroxide,
but water. Formation of the neutral molecule water is a much easier process
(Fig. 6.19).
R
O
OH
An oxonium ion
(a strong acid)
HB
B
+
+
–
+
R
O
H
..
R
O
+
+
R
+
R
OH
2
OH
H
H
A very poor
leaving group
A good
leaving group
Leaving group
(
–
OH) is very poor
Now, leaving group
(OH
2
) is much better
FIGURE 6.19 Protonation of an
alcohol leads to an oxonium ion.
In this process, the very poor leaving
group OH is transformed into the
good leaving group OH
2
.
236 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
TABLE 6.5 Some pK
a
Values
for Simple Oxonium Ions
Compound pK
a
1.74
2.2
1.9
2.3
2.2
2.6
About 3.5RO
+
HR¿
(CH
3
)
3
CO
+
H
2
CH
3
CH
2
CHO
+
HCH
3
CH
3
CH
2
CH
2
CH
2
O
+
H
2
CH
3
CH
2
O
+
H
2
CH
3
O
+
H
2
H
3
O
+
Note that the acidity order in water is CH
3
OH CH
3
CH
2
OH
(CH
3
)
2
CHOH (CH
3
)
3
COH. Apparently, the more alkyl groups on the alcohol,
the weaker an acid it is. For many years, this pK
a
order was explained by assuming
that alkyl groups are intrinsically electron donating. If this were true, the product
alkoxides would be destabilized by alkyl groups and the acidity of the correspon-
ding alcohol would be reduced (Fig. 6.21).
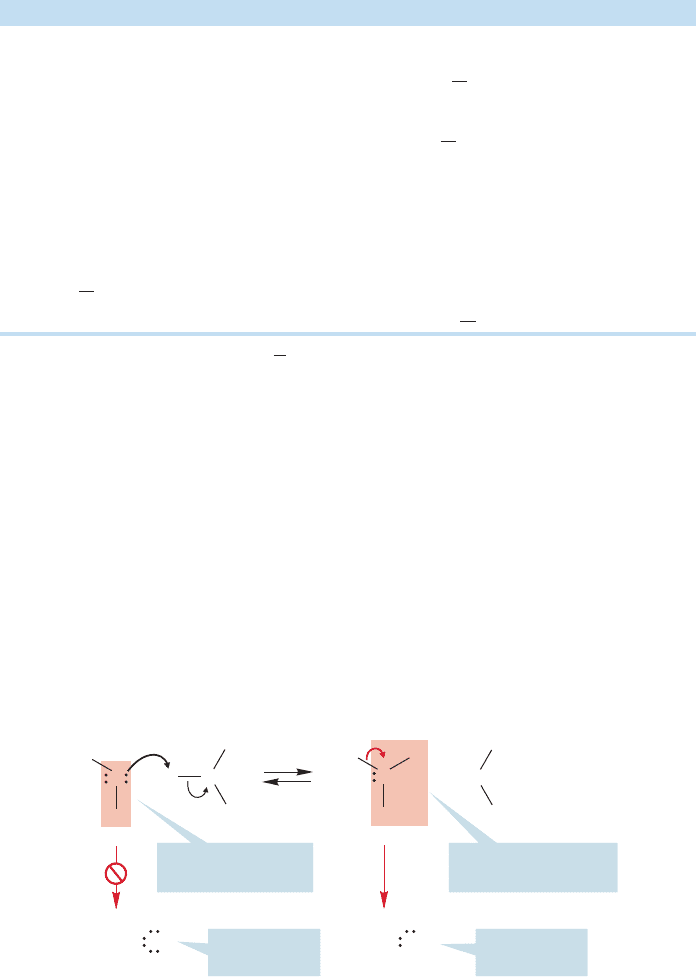
R
B
H
+
+
H
O
R
–
Alkoxide
ion
Brønsted base
B
O
FIGURE 6.20 Loss of a proton to a general
Brønsted base leads to an alkoxide ion. Alkoxides
are named very simply as oxides of the parent
alcohol.Thus, methyl alcohol, CH
3
OH, is
deprotonated to give methoxide, CH
3
O
, ethyl
alcohol, CH
3
CH
2
OH, is deprotonated to give
ethoxide, CH
3
CH
2
O
, and so on.
TABLE 6.6 Some pK
a
Values for Simple Alcohols in Water
Compound pK
a
(loss of underlined H)
Water H
2
O 15.7 Strongest acid
Methyl alcohol CH
3
OH 15.5
Ethyl alcohol CH
3
CH
2
OH 15.9
Isopropyl alcohol (CH
3
)
2
CHOH 16.5
tert-Butyl alcohol (CH
3
)
3
COH 17.0 Weakest acid
CH
3
–
+
Destabilized
by dipole in
CH
3
–––
C bond
δ
–
δ
+
δ
–
δ
+
C
B
H
O
CH
3
C
O
+
H
B
FIGURE 6.21 If alkyl groups were
electron-releasing, formation of an
alkoxide from an alcohol would be
retarded by alkyl groups.
Protonated alcohols are very strong Brønsted acids. Therefore, in order
to protonate an alcohol, it takes a very strong acid indeed. The conjugate
base (B
) of the protonating acid (HB) must be a weaker base than the
alcohol. The base must be an ineffective competitor in the equilibrium of
Figure 6.19.
Oxonium ions are much better proton donors than are the alcohols themselves.
Table 6.5 gives the pK
a
values for a few protonated alcohols and water. Oxonium
ions formed from alcohols have pK
a
values in the 2 range, and those from ethers
( ) are even more acidic.
Brønsted acidity, of course, is centered on the hydroxyl hydrogen. Loss of this
hydrogen to an acceptor, a Brønsted base, leads to the conjugate base of the alcohol,
the alkoxide ion (RO
). The proton is not just lost as H
but removed by a base
(Fig. 6.20).
R
O
O
O
R¿
:
Alcohols are approximately as acidic as water.Table 6.6 gives the pK
a
values for
some simple alcohols in aqueous solution as well as water itself.
H
δ
+
δ
–
CCH
2
OHF
F
F
δ
+
δ
+
δ
–
CCH
2
OF
F
F
δ
–
δ
+
δ
–
CCH
2
O
–
F
F
F
6.4 Alcohols 237
Inductive effects, the result of polarized sigma bonds, are important. For exam-
ple,the pK
a
of 2,2,2-trifluoroethanol (CF
3
CH
2
OH) is 12.5,whereas the pK
a
of ethyl
alcohol is 15.9. The fluorinated alcohol is more than a thousand times as acidic as
ethyl alcohol (remember the pK
a
scale is logarithmic). Fluorinated alcohols are always
stronger acids than their hydrogen-substituted counterparts.
WORKED PROBLEM 6.9 Explain in detail why 2,2,2-trifluoroethanol is a stronger
acid than ethyl alcohol.
ANSWER As fluorine is very electronegative, the fluorines are strongly electron-
withdrawing and will help stabilize the alkoxide ion. They will also stabilize the
transition state leading to the alkoxide because partial negative charge has begun
to develop on the oxygen atom. So the fluorines will have an effect on both the
kinetic and thermodynamic properties of the alcohol.
Yet this traditional, and simple, explanation that treats alkyl groups as intrinsi-
cally electron-donating is not correct. In 1970, Professor John Brauman (b. 1937)
and his co-workers at Stanford University showed that in the gas phase the opposite
acidity order obtained.The intrinsic acidity of the four alcohols of Table 6.6 is exact-
ly opposite to that found in solution.The acidity order measured in solution reflects
a powerful effect of the solvent, not the natural acidities of the alcohols themselves.
Organic ions are almost all unstable species,and the formation of the alkoxide anions
depends critically on how easy it is to stabilize them through interaction with sol-
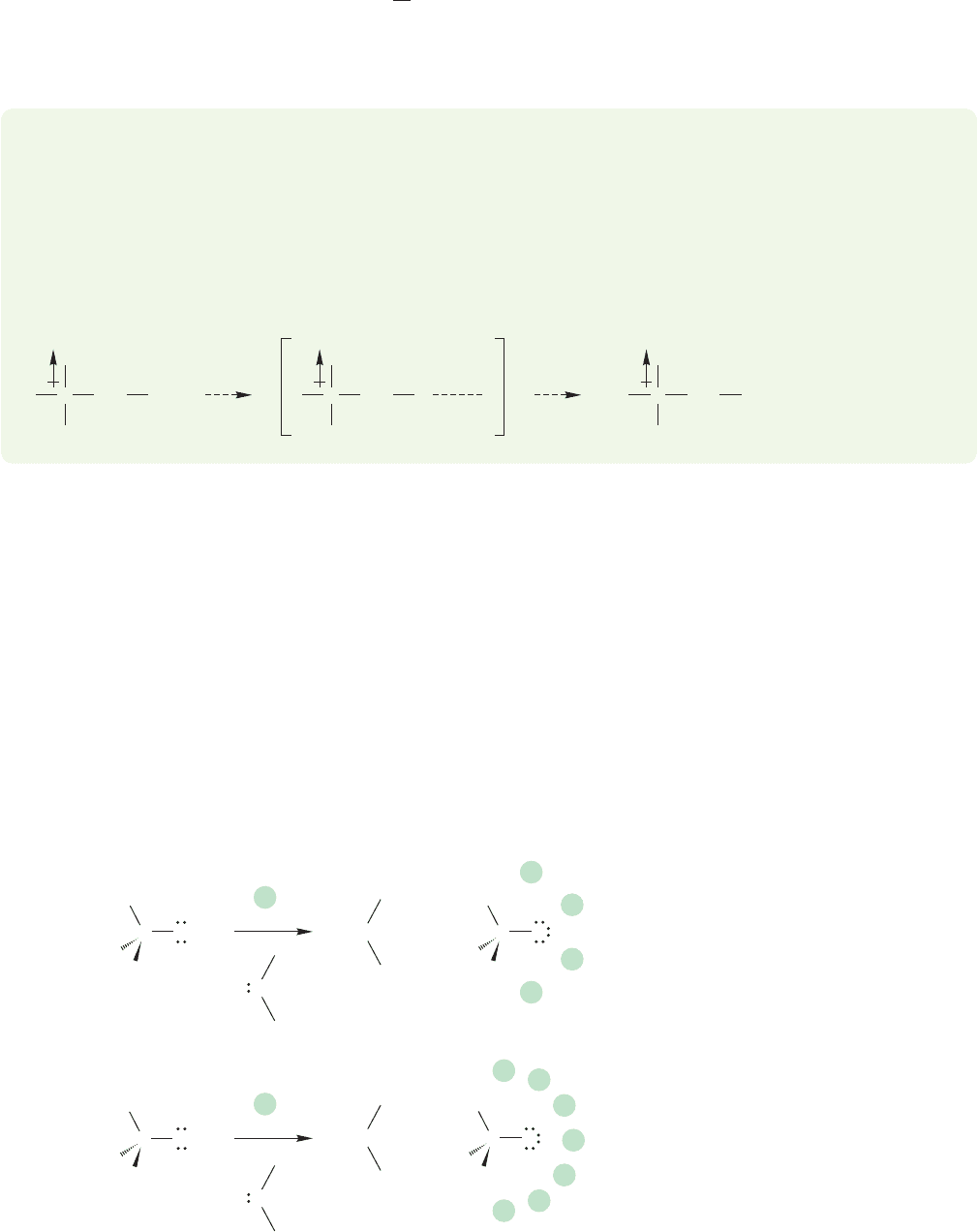
vent molecules, a process called solvation. tert-Butyl alcohol is a weaker acid in
solution than methyl alcohol because the large tert-butyl alkoxide ion is difficult to
solvate. The more alkyl groups, the more difficult it is for the stabilizing solvent
molecules to approach (Fig. 6.22). Of course, in the gas phase where solvation is
impossible, the natural acidity order is observed.
–
+
base
C
+
CH
3
H
3
C
H
3
CH
3
C
C
CH
3
H
3
C
–
S
S
S
S
S
S
S
S
S
S
S
S
S
solvent
base
solvent
C
H
H
H
Difficult to
solvate
Easy to
solvate
C
H
H
H
B
B
+
HB
+
HB
OH
OH
O
O
FIGURE 6.22 The smaller the
alkoxide ion, the easier it is for
solvent molecules to approach and
stabilize it.
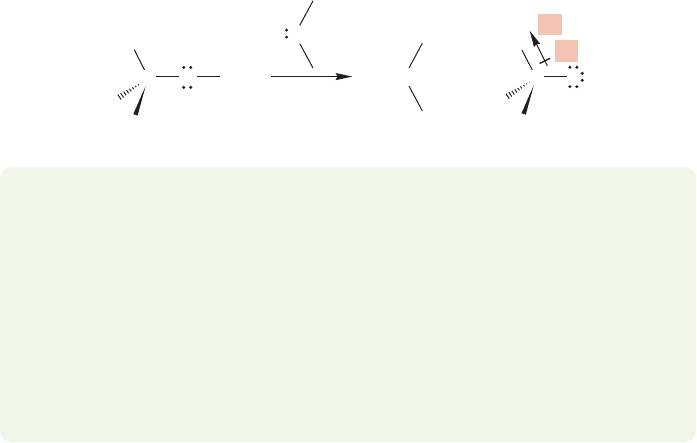
238 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
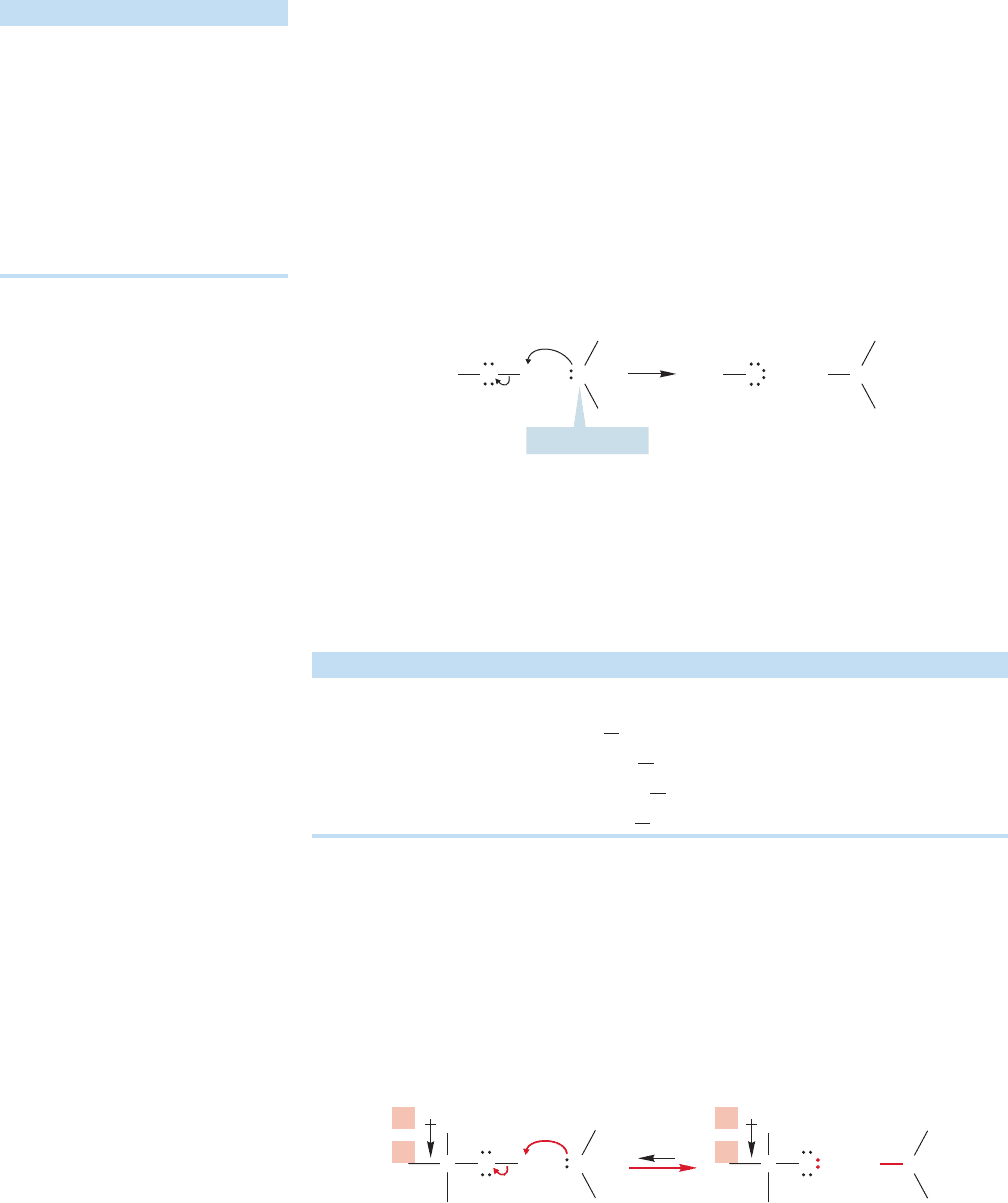
In the gas phase, the alkyl groups are actually operating so as to stabilize the
charged alkoxide ions, presumably by withdrawing electrons, exactly the opposite
from what had been thought (Fig. 6.23).
δ
–
δ
+
–
+
C
H
3
C
C
H
3
C
O
B
+
HB
O H
FIGURE 6.23 Alkyl groups can be
electron-withdrawing.
WORKED PROBLEM 6.10 Can you describe this phenomenon—the stabilization of
a pair of electrons by an adjacent alkyl group—in orbital terms? No orbital con-
struction or complicated argument is necessary. A simple statement is all that is
needed.
ANSWER Alkyl groups have both filled and empty molecular orbitals (see the
problems at the end of Chapter 1 for several examples). A pair of electrons adja-
cent to an alkyl group can be stabilized through overlap with the LUMO of the
alkyl group. (Similarly, an alkyl group stabilizes an adjacent empty orbital through
overlap with the alkyl HOMO.)
What lessons are to be drawn from this discussion? First, solvation is important
and not completely understood. We are certain to find other phenomena best
explained in terms of solvation in the future.Second,the gas phase is the ideal medi-
um for revealing the intrinsic properties of molecules. (Some would go further and
say that calculation is the best way.) However, the practical world of solvated reac-
tions is certainly real, and for us to understand reactivity we can no longer afford to
ignore the solvent.
6.5 Solvents in Organic Chemistry
We have just seen an example of a solvent playing a crucial role in determining
the acidities of alcohols. The greater acidity of methyl alcohol over tert-butyl
alcohol in a polar solvent is a result of the greater ease of solvating the
conjugate base of methyl alcohol, the methoxide ion, over the tert-butoxide ion
(Fig. 6.22). Let’s take a quick look at the properties of solvents and the process
of solvation.
6.5a Polar Solvents We have seen that one kind of stabilization by solvent—
solvation—is a consequence of hydrogen bonding.For hydrogen bonding to be pos-
sible, there clearly must be both a proton donor (usually a hydroxy or amino group,
OH or NH
2
) and a proton acceptor available (a pair of electrons). Solvents that can
donate a proton are called protic solvents. Protic solvents are usually also quite
polar; that is, they have relatively high dielectric constants, ε, a measure of the
ability of a solvent to separate charges. Solvents without available protons—in other
words, solvents that are not proton donors—are aprotic solvents and can be either
polar or nonpolar. It is distinctly possible for a solvent to be quite polar, to have a
high ε, but not be a proton donor. Of course, many molecules are both nonpolar and
