Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

14.5 Carbon–Carbon Bond Formation: Friedel–Crafts Alkylation 639
Summary
Benzene fails to react with most HX reagents under conditions that easily result
in addition to alkenes and dienes. The X
2
reagents, such as bromine and chlo-
rine, are similarly unreactive. Benzene lies in too deep a well, and it is protected
by activation energy barriers that are too high for these reactions to occur.
Sulfonation, nitration, chlorination, and bromination all serve to underscore
the general principles of electrophilic aromatic substitution. In each reaction, an
electrophile powerful enough to react with the weakly nucleophilic benzene ring
must be generated. The aromatic ring and the powerful electrophile together
will have a high enough energy that the reaction can proceed over the energy
barrier.The addition reaction first generates a resonance-stabilized (but not aro-
matic) cyclohexadienyl cation that can be deprotonated to regenerate an aromatic
system. The general mechanism is shown again in Figure 14.33.
+
H
E
BH
E
+
–
B
..
E
+
FIGURE 14.33 Once again, here is
the general mechanism for aromatic
substitution, where E
D
,
SO
2
OH,
NO
2
,
Cl, or
Br.
14.5 Carbon–Carbon Bond Formation:
Friedel–Crafts Alkylation
We are now able to make a few substituted aromatic compounds, and this capability
will shortly prove quite useful. However, much of the interest in synthetic chemistry
involves the construction of carbon–carbon bonds. We have made no progress at all
in finding ways to make bonds from benzene to carbon. Here and in the next section
we do just that by making use of another electrophilic aromatic substitution reaction
quite closely related to the bromination and chlorination reactions we have just seen.
The Friedel–Crafts alkylation reaction is an electrophilic aromatic substitution that
attaches an alkyl group to the aromatic ring.It is named for Charles Friedel (1832–1899)
and James M. Crafts (1839–1917), who discovered it by accident when they tried to
synthesize pentyl chloride from pentyl iodide through reaction with aluminum chlo-
ride in an aromatic solvent. Instead of the hoped-for chloride, substituted aromatic
hydrocarbons appeared. The Friedel–Crafts reaction is closely related to bromination
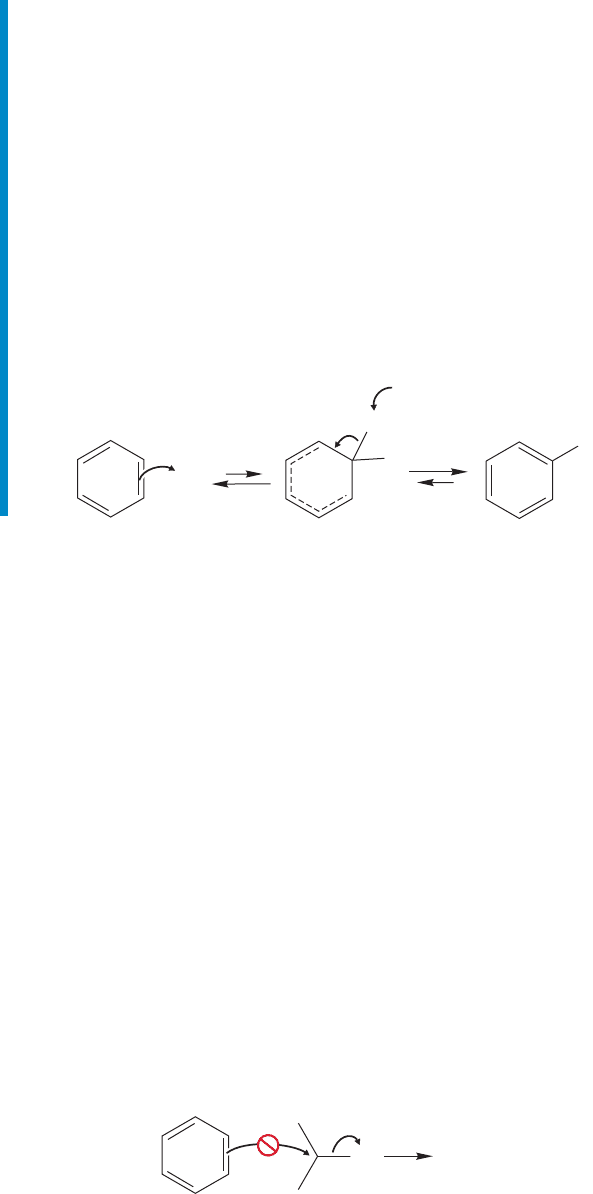
and chlorination of benzene. If we treat benzene with isopropyl bromide in the hope
that a nucleophilic attack of benzene on isopropyl bromide will produce isopropylben-
zene (cumene), we are sure to be disappointed (Fig. 14.34). Benzene is by no means a
strong nucleophile and isopropyl bromide can scarcely be described as a powerful elec-
trophile.The proposed reaction is utterly hopeless, and fails to give product.
Br No reaction
FIGURE 14.34 Benzene fails to react
with isopropyl bromide.
So why even bring the subject up? Notice that this process does look a bit like
another reaction that fails to give product—the attempt to form chlorobenzene or
bromobenzene from chlorine or bromine and benzene. Those halogenations are
transformed into a success by the addition of a catalyst, either FeCl
3
or FeBr
3
, which
converts the halogen, a weak electrophile, into a much stronger Lewis acid
640 CHAPTER 14 Substitution Reactions of Aromatic Compounds
~100% in 0.0005 s
AlBr
3
AlBr
3
AlBr
3
H
H
+
+
–
Br
..
..
..
..
..
..
+
+
Br
Br
..
..
..
Br
..
..
Br
AlBr
3
–
Br
..
..
..
AlBr
3
–
WEB 3D
(+)
+(+)
FIGURE 14.35 The Friedel–Crafts
alkylation of benzene.
(Figs. 14.31 and 14.32). Perhaps a related technique will work here.Indeed it does,
as the addition of a small amount of the catalyst AlBr
3
or AlCl
3
to a mixture of
benzene and isopropyl bromide or chloride results in the formation of isopropyl-
benzene (Fig. 14.35). The mechanism should be easy to write now. A complex is
first formed between isopropyl bromide and AlBr
3
. This complex can be attacked
by benzene to give a resonance-stabilized cyclohexadienyl cation. Deprotonation
of the intermediate cyclohexadienyl cation gives isopropylbenzene.
R
X
AIX
3
R
FIGURE 14.36 Friedel–Crafts
alkylation succeeds for a wide variety
of alkyl halides.
..
AlBr
3
+
–
..
..
Br
C
CH
3
CH
3
H
3
C
AlBr
3
+
–
..
..
Br
C
CH
3
CH
3
H
3
C
H
(CH
3
)
3
C
(+)
(+) +
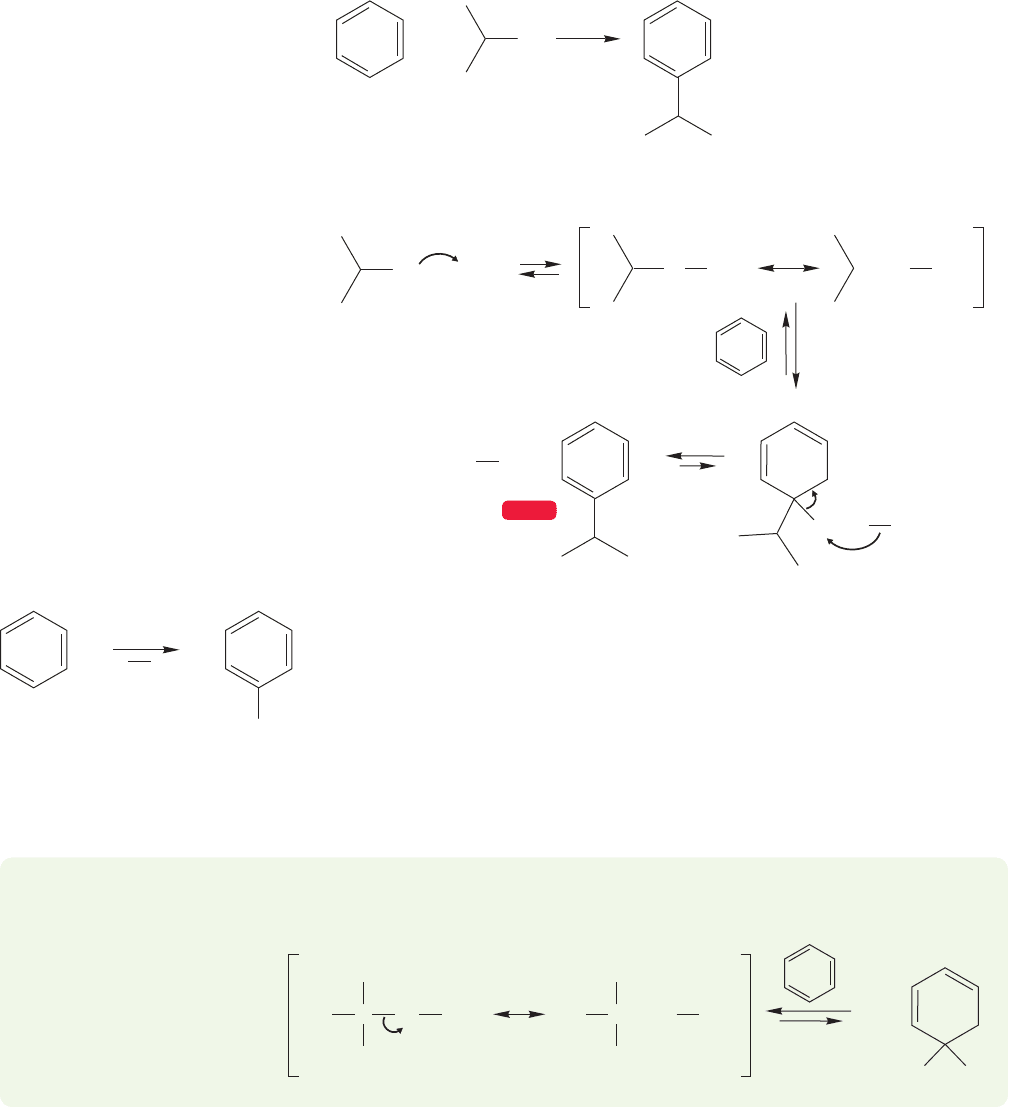
Whether or not the complex in Figure 14.35 can actually form a free carbocation
depends on the alkyl halide used in the reaction. Friedel–Crafts alkylation succeeds
with a variety of alkyl halides,including methyl-, ethyl-, isopropyl-,and tert-butyl chlo-
rides and bromides (Fig. 14.36).
We would not expect the methyl or ethyl cation to be formed in these reactions,
and surely these alkylations take place by attack of benzene on the complexed halide.
tert-Butyl chloride, on the other hand, can produce the relatively stable tert-butyl
cation, not an unlikely intermediate at all. So, the detailed mechanism of the
Friedel–Crafts alkylation depends on the nature of the R group.
PROBLEM 14.8 Why is attack by benzene on complexed tert-butyl bromide to
give a cyclohexadienyl cation an unlikely mechanistic step?
14.5 Carbon–Carbon Bond Formation: Friedel–Crafts Alkylation 641
AlCl
3
5 ⬚C
(55%) (45%)
( Total yield ~ 50%)
2-Phenylpropane
(isopropylbenzene)
1-Phenylpropane
(propylbenzene)
Cl
++
FIGURE 14.37 An attempt to make
propylbenzene by Friedel–Crafts
alkylation gives isopropylbenzene
as the major product.
PROBLEM 14.9 The synthesis of toluene by the aluminum chloride–catalyzed
Friedel–Crafts alkylation of benzene with methyl chloride is badly complicat-
ed by the formation of di-, tri-, and polymethylated benzenes. It appears that
the initial product of the reaction, toluene, is more reactive in the
Friedel–Crafts reaction than is benzene. Analyze the mechanism of elec-
trophilic aromatic substitution to see why toluene is more reactive than ben-
zene. Hint: Look carefully at substitution in the position directly across the
ring from the methyl group (the para position) for toluene, and look for
differences from the reaction with benzene.
PROBLEM 14.10 How would you minimize the overalkylation mentioned in
Problem 14.9 and achieve a reasonable synthesis of toluene from benzene?
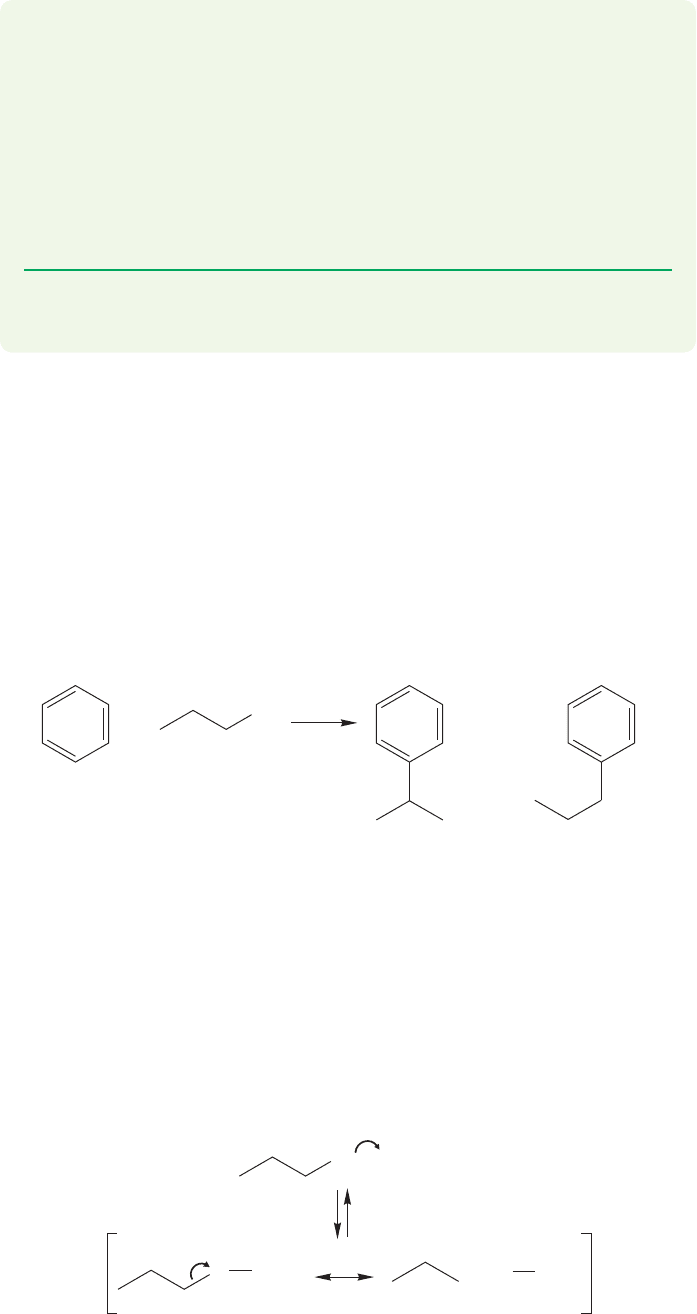
Not only do the mechanistic details of the Friedel–Crafts alkylation depend
on the alkyl group (R), but even the gross outcome of the reaction depends on the
structure of R. Although the alkyl halides we have mentioned so far all succeed in
making the related alkylated benzenes, there are many that do not. Suppose we
set out to make propylbenzene by the Friedel–Crafts alkylation of benzene using
1-chloropropane, benzene, and aluminum chloride. As we run this reaction, all looks
well at first. The products formed are of the correct molecular weight, but a clos-
er look uncovers a problem. Some propylbenzene is formed, but the major prod-
uct is isopropylbenzene (Fig. 14.37).
A careful look at the mechanism of the reaction shows the problem. In the
complex between the 1-chloropropane and aluminum chloride (AlCl
3
), the carbon–
chlorine bond is weakened and the positive charge is shared by the chlorine and the
adjacent carbon. The resonance form shown in Figure 14.38 shows this clearly.
Cl
+
+
AlCl
3
–
..
..
..
..
Cl
..
..
Cl
AlCl
3
AlCl
3
–
..
..
FIGURE 14.38 In the complex
formed between 1-chloropropane and
aluminum chloride, the positive
charge is partially on the primary
carbon adjacent to the chlorine.

642 CHAPTER 14 Substitution Reactions of Aromatic Compounds
–
Cl
..
..
Cl
δ
+
δ
+
δ
+
δ
+
..
..
δ
+
H
3
C
H
H
3
CCH
2
HC
AlCl
3
–
AlCl
3
–
AlCl
4
Cl
..
..
–
AlCl
3
+
H
3
CCH
3
HC
HC
H
3
CCH
3
In this complex no
rearrangement
is possible, but...
on a primary carbon into a partial positive charge on a secondary
carbon—a much more energetically favorable situation
A secondary carbon
ComplexFree cation
A primary carbon
... in this one, hydride migration can convert a partial positive charge
δ
+
FIGURE 14.39 A hydride shift can convert a less stable complex with a partial positive charge on a primary
carbon into a more stable complex in which the partial positive charge is on a secondary carbon. The
substitution reaction follows.
+
–
–
+
H
Br
Br Br
Tertiary carbocation
(more stable)
Secondary carbocation
(less stable)
R
R = H or alkyl
HH
H
R
R
HH
R
Br
HH
R
Br
FIGURE 14.40 Hydride shifts are
commonly encountered in addition
reactions whenever a more stable
carbocation can be generated from a
less stable one.
The complex contains a partial positive charge on a primary carbon, a most
unhappy state of affairs.When this happens in reactions of methyl chloride or ethyl
chloride, there is nothing that can be done about it, but propyl chloride has a
way out. A rearrangement of hydride generates the much more stable complex,
or even a free cation in which a secondary carbon bears the positive charge.
Straightforward alkylation now gives the observed major product, isopropylben-
zene (Fig. 14.39).
These rearrangements are very common, and are encountered whenever a hydride
or alkyl shift will generate a more stable cation, or complex. This phenomenon is
not new. Recall, for example,the rearrangements of hydride and alkyl groups encoun-
tered in reactions involving carbocations (Fig. 14.40; also p. 388).
14.6 Friedel–Crafts Acylation 643
C
R
O
..
..
C
Acid chloride
(acyl chloride)
The acyl group
R
O
..
..
Cl
..
..
..
FIGURE 14.41 The acyl group.
C
R
O
..
..
S
An acid chlorideA carboxylic acid
O
..
..
Cl
..
..
..
..
Cl
..
..
..
CSO
2
+
HCl
R
O
..
..
Cl
..
..
..
OH
..
..
+
WEB 3D
FIGURE 14.42 Acid chlorides can be
made by the reaction of carboxylic
acids with thionyl chloride.
PROBLEM 14.11 tert-Butylbenzene can be obtained from treatment of 1-bromo-
2-methylpropane with aluminum chloride. Write a mechanism for this reaction.
PROBLEM 14.12 tert-Butylbenzene is converted into benzene on treatment with
AlCl
3
and a trace of HCl. Write a mechanism for this reaction.
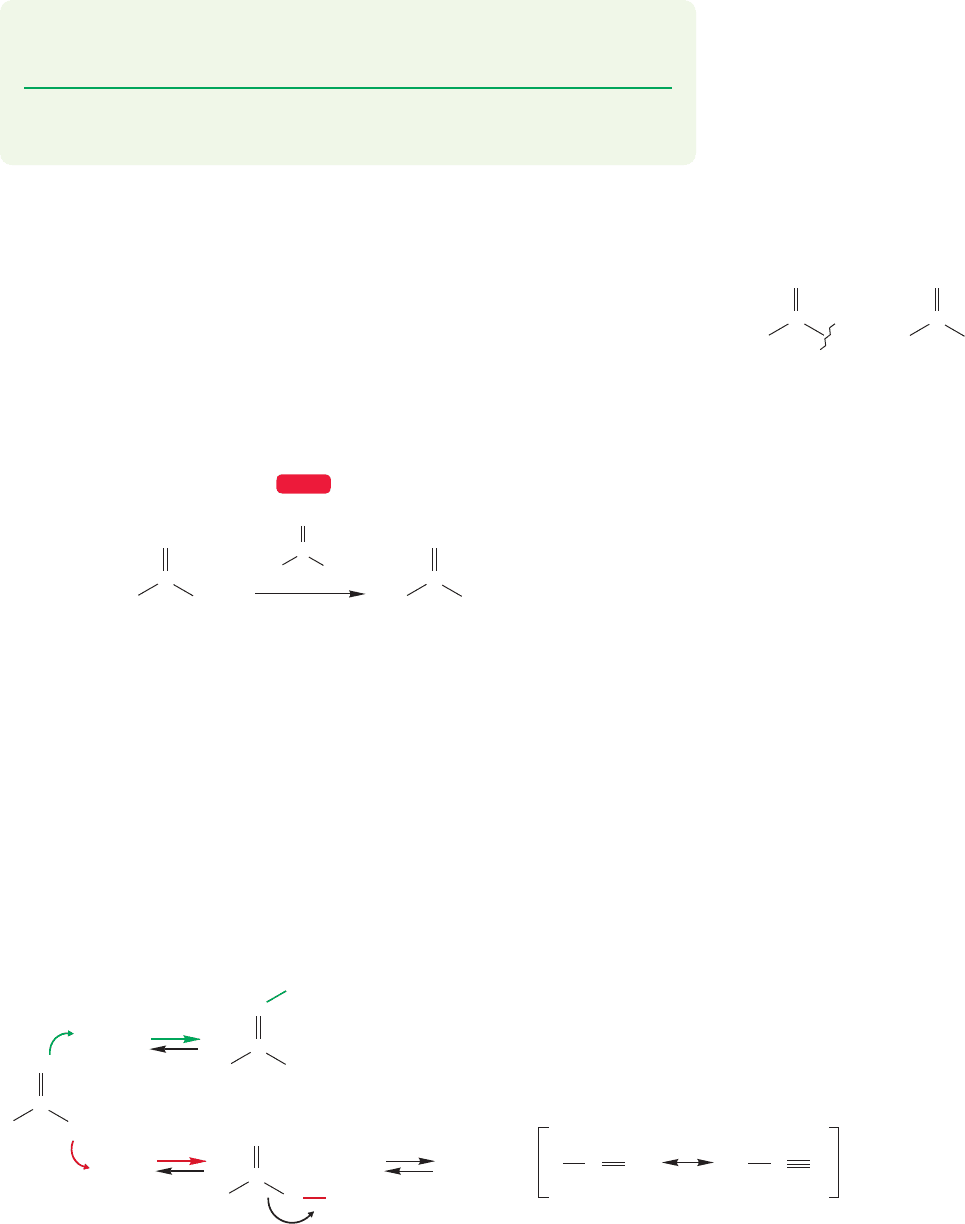
14.6 Friedel–Crafts Acylation
The Friedel–Crafts acylation reaction places an acyl group ( ) onto an
aromatic ring (Fig. 14.41). We will take up the chemistry of all manner of acyl
derivatives in Chapter 18, but here we need to use acyl chlorides (RCOCl), which
are more commonly known as acid chlorides. In practice, acid chlorides are rather
easy to make from the relatively available carboxylic acids, RCOOH, by treatment
with thionyl chloride (SOCl
2
) (Fig. 14.42).
RC
P
O
Acid chlorides react with benzene under the influence of an equivalent (not
a catalytic amount) of AlCl
3
. A molecule of AlCl
3
is needed for each molecule
of product. By now it should be easy to write a general mechanism. A complex
is first formed with AlCl
3
. But which complex? Unlike alkyl chlorides, acid chlo-
rides contain two nucleophilic sites, the oxygen and chlorine atoms. The evi-
dence is that both complexes are formed, and that the two are in equilibrium.
The resonance-stabilized acylium ion can be formed by dissociation of the
complex (Fig. 14.43).
The acylium ion
C
(a)
path a
(b)
path b
R
O
..
..
Cl
..
..
..
C
RC
O
..
..
Cl
..
..
δ
+
δ
+
+
δ
–
AlCl
3
AlCl
3
AlCl
3
AlCl
4
δ
–
–
AlCl
3
C
R
R
O
..
Cl
..
..
O
..
..
..
+
R
C
+
O
..
FIGURE 14.43 Two acid chloride–AlCl
3
complexes can form; dissociation gives
the acylium ion.
The acylium ion can then add to a benzene ring to generate an intermediate cyclo-
hexadienyl cation.Deprotonation by a Lewis base completes the reaction and produces
644 CHAPTER 14 Substitution Reactions of Aromatic Compounds
–
δ
+
δ
–
AlCl
3
AlCl
3
AlCl
3
used up
in this step
+
O
..
..
RC
+
+
O
..
..
R
H
2
O
O
..
..
R
C
H C
O
..
R
C
O
..
..
R
Cl
..
..
..
..
HCl
..
..
..
HCl
..
..
..
C
++
Al(OH)
3
O
..
..
R
A final hydrolysis step liberates
the ketone, an acyl benzene
C
FIGURE 14.44 The mechanism of
Friedel–Crafts acylation. Aromatic
ketones can form complexes with
AlCl
3
, which is why a full equivalent
of AlCl
3
is necessary for
Friedel–Crafts acylation.
H
3
C
AlCl
3
(97%)
H
3
C
O
C
Cl
O
C
AlCl
3
(77%)
O
Cl
O
WEB 3D
FIGURE 14.45 Two typical
Friedel–Crafts acylations.
AlCl
3
O
1-Phenylbutanone
(phenyl propyl ketone)
(butyrophenone)
1-Phenylbutane
(butylbenzene)
Cl
O
?
FIGURE 14.46 Friedel–Crafts
acylation is not complicated by
rearrangements.
acyl benzenes (Fig. 14.44). As mentioned above, unlike Friedel–Crafts alkylation,
Friedel–Crafts acylation is not a catalytic reaction. The acyl benzene is also reactive
toward AlCl
3
and forms its own 1:1 complex as it is produced. So, for every molecule
of acyl benzene formed in the reaction, one molecule of AlCl
3
is used up in complex
formation. A stoichiometric amount, or one equivalent, of AlCl
3
is therefore required
in this reaction. At the end of the reaction, addition of water destroys the complex and
generates the free, acylated benzene, as shown in the last step in Figure 14.44.
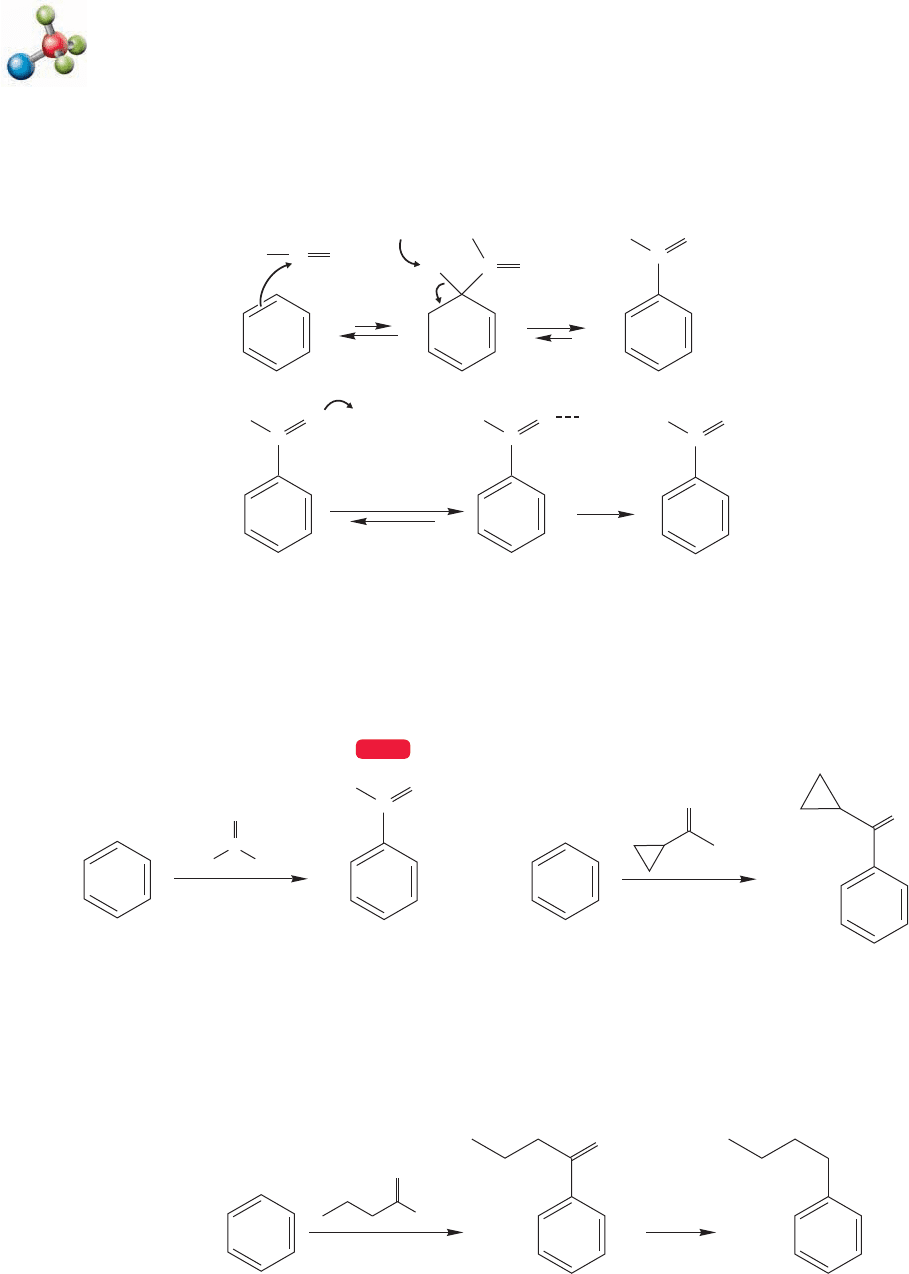
Figure 14.46 shows another successful Friedel-Crafts acylation, but this reaction
contains a bonus.The reaction of the four-carbon acid chloride with benzene yields
the unrearranged ketone. This result implies that acyl complexes are not prone to
rearrangement (Fig. 14.46).
Despite the mechanistic complexity, in practice the Friedel–Crafts acylation reac-
tion works very well,and a variety of phenyl-substituted ketones can be produced in good
yield.Be certain to add this reaction to your collection of synthesis file cards (Fig.14.45).
Friedel–Crafts acylation
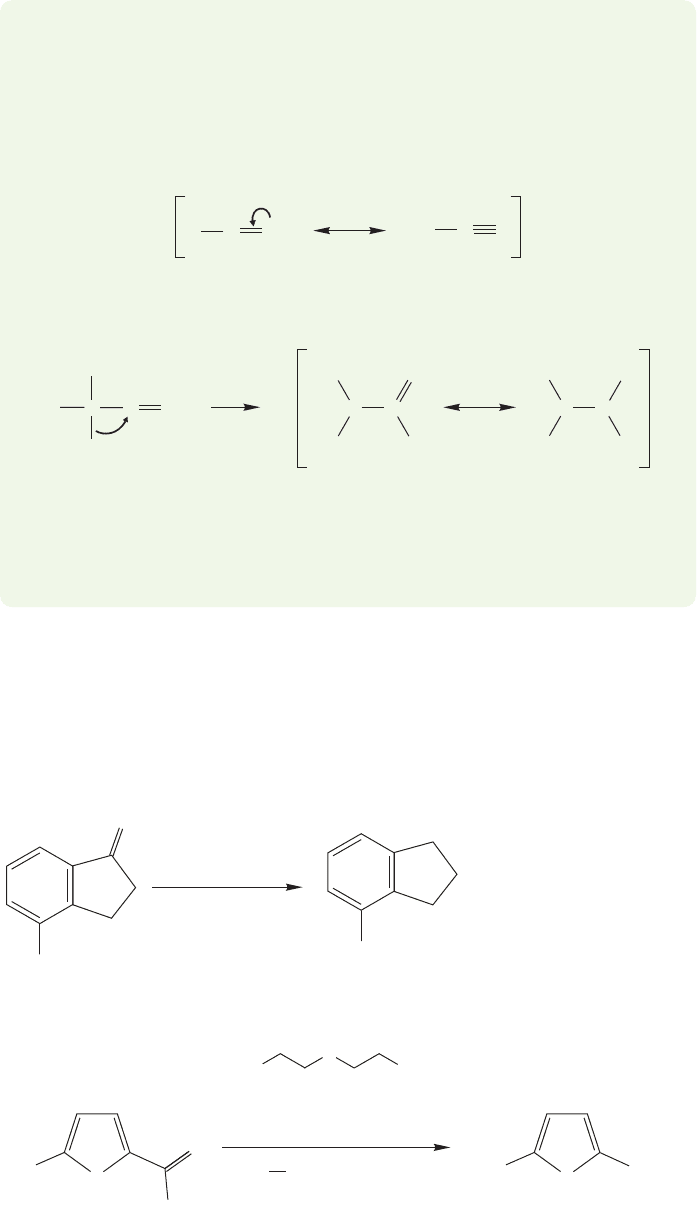
14.6 Friedel–Crafts Acylation 645
An acylium ion
O
..
..
C
+
R
O
..
C
+
R
WORKED PROBLEM 14.13 Explain why an acylium ion (or acyl complex) is not
likely to rearrange. Two reasons should be apparent. First, what differentiates the
acylium ion from alkyl carbocations? Next, look closely at the product of
rearrangement and consider the polarity of the carbon–oxygen double bond.
ANSWER The first reason has to do with the stability of the acylium ion,which is well
stabilized by resonance and is therefore less likely to rearrange than alkyl carbocations.
In fact, there are many such ways to convert a carbonyl into a methylene group,
two of which are shown in Figure 14.47.The Clemmensen reduction,named for its
discoverer E. C. Clemmensen (1876–1941), takes place under strongly acidic
conditions, whereas the Wolff–Kishner reduction (Ludwig Wolff, 1857–1919;
N. Kishner, 1867–1935) requires strong base.
C
H
3
C
H
O
H
C
H
H
C
+
O
..
..
H
3
C
C
+
..
..
C
H
3
C
H
O
H
C
++
–
..
..
..
Second, consider the structure of the putative rearranged cation.
EtOH, 79 ⬚C, 24 h
H
2
N NH
2
, KOH, 200 ⬚C
Clemmensen reduction
(a high-boiling-point
alcohol used to facilitate
a high temperature)
Wolff–Kishner reduction
Zn/Hg, HCl
(93%)
(~90%)
O
O
HO
OH
CH
3
CH
3
S
O
CH
3
H
3
C
S
CH
2
CH
3
H
3
C
FIGURE 14.47 Two methods for
converting a carbonyl group into a
methylene group.
The four-carbon chain is intact in the product, and if we had a way to transform
the carbon–oxygen double bond into a methylene group, we would have a way to
make butylbenzene without the complications of rearrangement that come from the
Friedel–Crafts alkylation.
The carbonyl group is strongly polarized in the sense shown in the drawing. The
carbon is δ
and the oxygen is δ
. Rearrangement generates a full and partial
positive charge on adjacent atoms.This situation is very bad energetically and will
act to retard migration of hydrogen or an alkyl group.
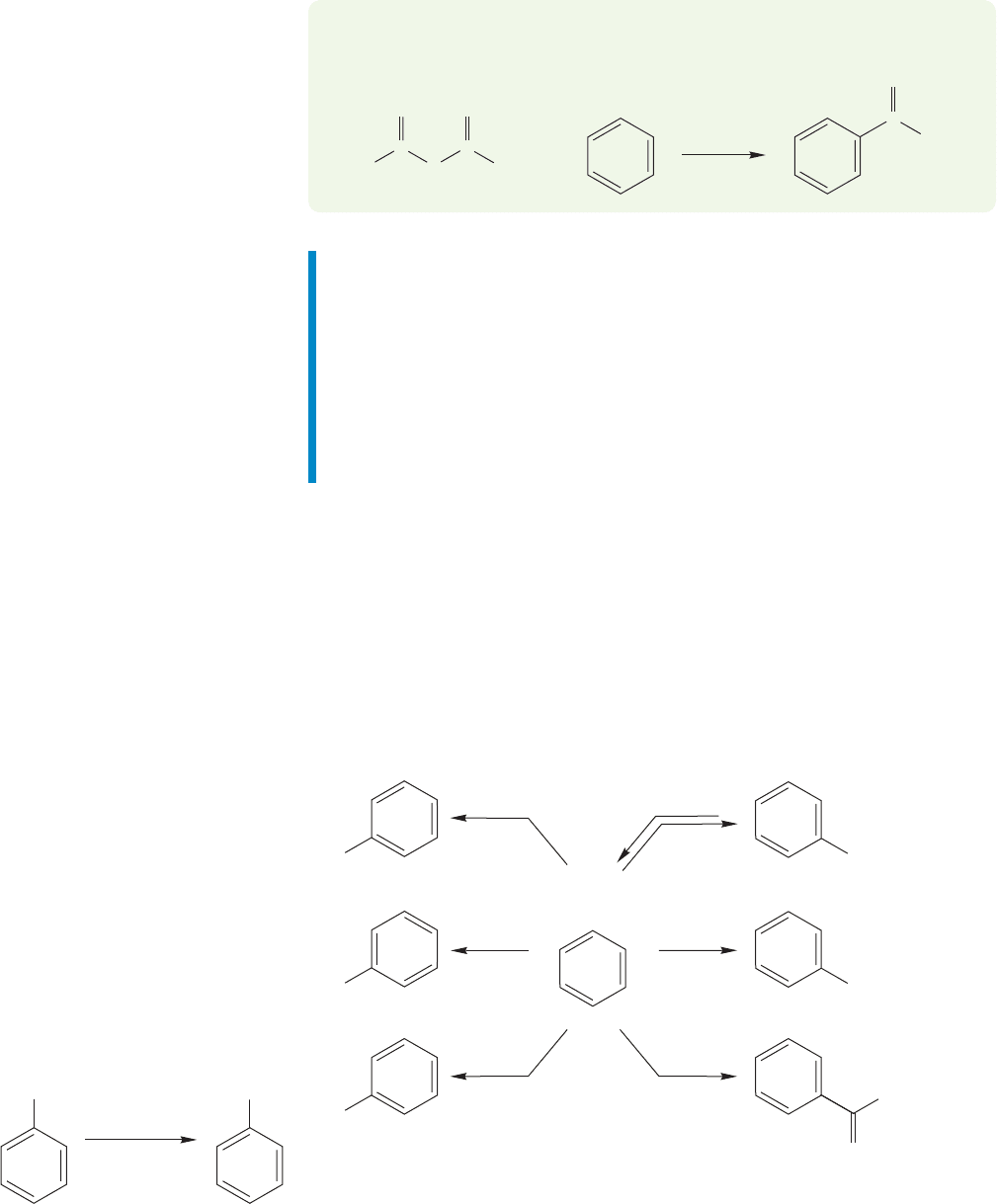
Nitro-
benzene
NO
2
..
NH
2
H
2
/Pd/C,
EtOH
1. Sn/HCl
2.
–
OH/H
2
O
Aniline
or
FIGURE 14.49 The reduction of
nitrobenzene to aniline.
646 CHAPTER 14 Substitution Reactions of Aromatic Compounds
1. AlBr
3
2. H
2
O
O
C
O
C
O
O
C
CH
3
CH
3
H
3
C
+
PROBLEM 14.14 Other reagents besides acid chlorides can lead to acylation reactions.
Write a mechanism for the following reaction:
Summary
A Friedel–Crafts alkylation is an electrophilic aromatic substitution reaction that
attaches a carbon–carbon bond to the ring.The electrophile is R
,which will add
to the aromatic ring to produce a cyclohexadienyl cation. Aromaticity is regained
when that intermediate cyclohexadienyl cation is deprotonated. That’s all there
is to it—all the rest is details.Remember to watch out for rearrangements,because
this Friedel–Crafts alkylation is especially prone to them. In the Friedel–Crafts
acylation, an acid chloride is used to generate the acylium ion which is the reac-
tive electrophile. No rearrangements are observed in Friedel–Crafts acylation.
(watch out for
rearrangements
in R)
O
2
N
Br
Cl
SO
2
OH
R
Cl
2
FeCl
3
H
2
SO
4
HNO
3
AlCl
3
RCOCl
H
2
SO
4
SO
3
FeBr
3
Br
2
AlCl
3
RCl
O
R
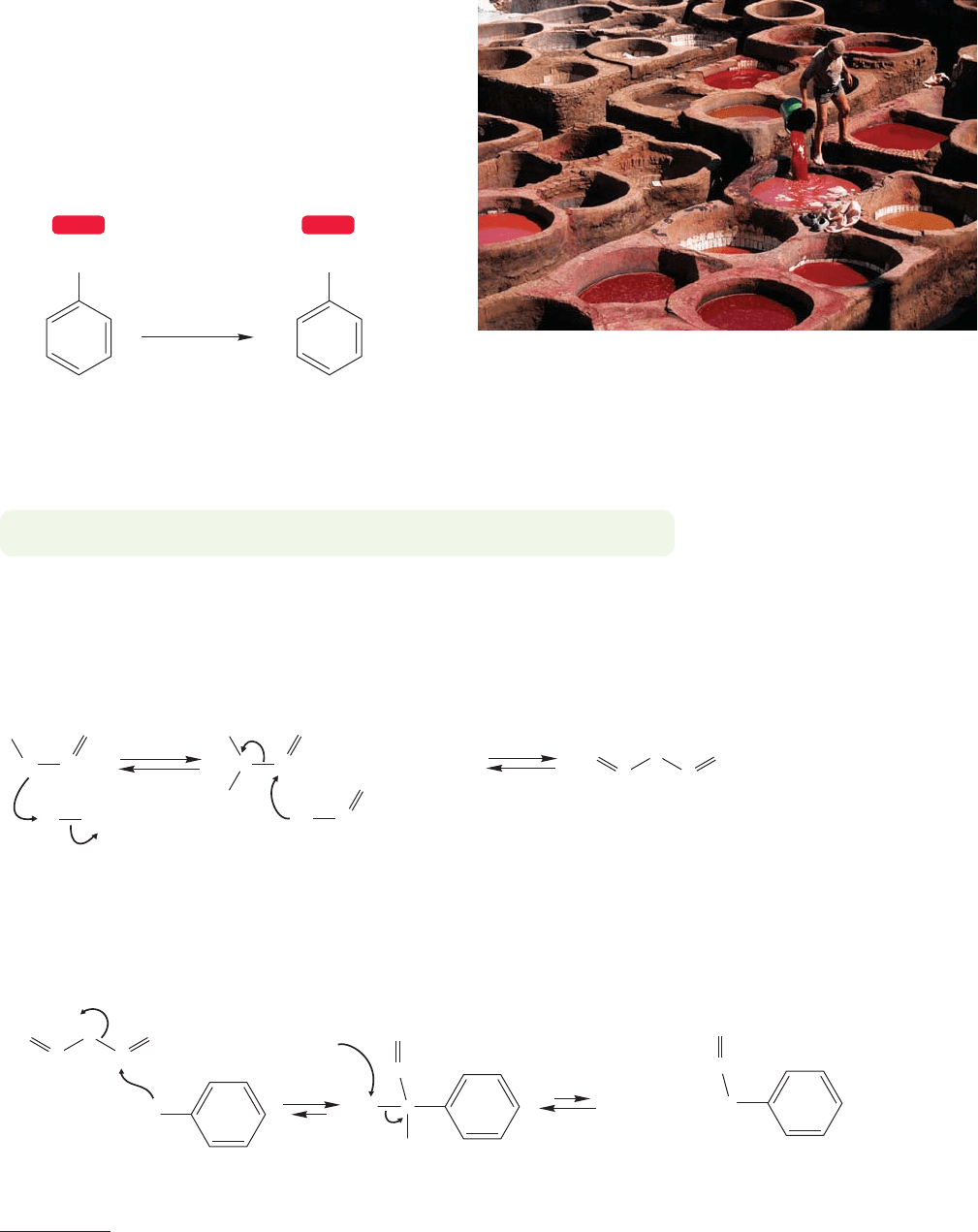
FIGURE 14.48 Aromatic substitution
reactions encountered so far.
Although there are relatively few groups we can attach to a benzene ring directly, we
can often transform one group into another through chemical reactions. It is nitroben-
zene that is the key to many of these transformations.Nitrobenzene itself is relatively unre-
active,but the nitro group can be reduced in many ways to the amino group (Fig.14.49).
This transformation opens the way for the synthesis of a host of new molecules.
14.7 Synthetic Reactions We Can Do So Far
We have uncovered seven synthetically important reactions in this chapter so far.
We can (1) brominate and (2) chlorinate benzene.We can (3) nitrate and reversibly
(4 and 5) sulfonate benzene. The two Friedel–Crafts reactions (6 and 7) allow the
construction of carbon–carbon bonds to give some alkyl benzenes and give you
another synthetic route to compounds containing carbon–oxygen double bonds.
Figure 14.48 summarizes the situation.
14.7 Synthetic Reactions We Can Do So Far 647
Aminobenzene, generally known by its common name
aniline (p. 243),can be treated with nitrous acid (HONO)
or its sodium salt (Na
ONO) and HCl, to produce
benzenediazonium chloride,which is explosive when dry,
but a relatively safe material when wet (Fig. 14.50). A
molecule of the structure is called a diazonium
ion. We will soon use them in a number of important
reactions.
R
O
N
+
2
Benzenediazonium
chloride
N
2
..
NH
2
NaONO/HCl
Aniline
–
..
..
..
..
Cl
+
WEB 3DWEB 3D
FIGURE 14.50 Reaction of aniline with nitrous
acid to give benzenediazonium chloride.
Aniline derivatives are used to make azo dyes. Almost all dyes,
including azo dyes and these natural dyes from the Fez Tannery in
Morocco, are colored because they contain highly conjugated
aromatic amines.
PROBLEM 14.15 Draw resonance forms for benzenediazonium chloride.
The mechanism for the reaction that converts aniline into the diazonium salt
contains many steps, but is really within our range now. A critical intermediate is
dinitrogen trioxide, formed in equilibrium with nitrous acid by loss of water. When
nitrous acid is protonated, a good leaving group, water, is created and can be dis-
placed by a nitrite ion (Fig. 14.51).
2
+
Dinitrogen trioxide
(N
2
O
3
)
ON
H
O
ON
H
..
H
+
ON
O
–
OH
2
H
..
..
..
..
..
..
O
..
..
..
..
..
..
..
..
..
+
N
N
O
O
..
..
..
..
..
..
+
H
2
O
..
..
H
2
O
..
..
O
..
..
FIGURE 14.51 The formation of dinitrogen trioxide, N
2
O
3
.
2
This reaction (Fig. 14.51) is actually a bit more complicated than is shown in the figure. It involves addition
of nitrite to the nitrogen–oxygen double bond followed by loss of water. We will explore this kind of mecha-
nism in detail beginning in Chapter 16.
+
N
O
N
O
O
..
..
..
..
..
..
..
..
ONO
..
H
2
N
–
+
H
H
O
..
..
..
..
ONOH
..
N
N
HN
N
N-NitrosoanilineAniline
O
..
..
FIGURE 14.52 Formation of a nitrosamine by displacement of nitrite from N
2
O
3
.
The nucleophilic nitrogen of aniline can displace the good leaving group nitrite
(
ONO) from N
2
O
3
, which leads, after removal of a proton, to N-nitrosoaniline
(Fig. 14.52).
648 CHAPTER 14 Substitution Reactions of Aromatic Compounds
A diazotic acidN-Nitrosoaniline
H
2
O
H
2
O
protonation
+
H
OH + Cl
–
..
..
N
N
..
O
..
..
..
..
..
..
HN
N
Cl
H
+
..
..
..
Cl
H
..
OH
2
..
..
OH
..
..
N
N
..
deprotonation
..
protonation
..
..
..
N
N
OH
2
+
–
Cl
..
..
..
..
lose
..
N
N
+
++
–
Cl
..
..
..
..
..
..
Benzenediazonium ion
..
..
H
2
O
..
..
WEB 3D
FIGURE 14.53 Loss of water
from the protonated diazotic
acid gives the diazonium ion,
benzenediazonium chloride.
ANILINE
acid
Pseudomauvine
N
N
N
+
H
2
N
NH
2
H
Cl
–
Aniline featured prominantly in the development of the syn-
thetic dye industry. In 1856, the 17-year-old William Henry
Perkin, on holiday from the Royal College of Chemistry where
he was studying with August Wilhelm Hofmann, was working
in his home lab (isn’t this what you do on your vacations?). As
an unexpected outcome of a failed attempt to make quinine,
Perkin obtained a black goop that stained his laboratory rag a
beautiful mauve.This dye is the molecule shown above,
pseudomauvine. Over Hofmann’s objections, Perkin and his
family set out to commercialize this dye. It was not a vast
financial success, as colorfastness became a problem, and
mauve was very expensive, being roughly the same price per
pound as platinum at the time. It was overtaken by other, relat-
ed dyes made in similar ways from substituted anilines.
Nonetheless, the serendipitous synthesis of mauve marks the
beginnings of a great chemical industry.
It shouldn’t be difficult to find the four molecules of
aniline within the structure of pseudomauvine. It is harder
to map out a general mechanism through which the four
molecules come together; keep in mind that the necessary
oxidations would be easy to accomplish under Perkin’s crude
reaction conditions.
The N-nitrosoaniline equilibrates with a molecule called a diazotic acid, which loses
water in acid to generate the diazonium ion (Fig.14.53).These transformations are prob-
ably bewilderingly complex right now,even though they are made up of very simple reac-
tions in sequence. It certainly takes some practice to get them right. However, many
reaction mechanisms involve similar sequences of protonations,deprotonations,and addi-
tions and losses of water molecules. Now is the time to start practicing them. We will
encounter many more when we discuss carbonyl chemistry in Chapters 16 through 19.
