Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

14.9 Disubstituted Benzenes: Ortho, Meta, and Para Substitution 659
As a result, we can expect para (or ortho) substitution to occur more rapidly than
the other two reactions (Fig. 14.67). In fact, the chlorination of anisole gives only
Reaction progress
Energy
Transition state for formation
of the meta intermediate
(higher energy)
Intermediate in para
substitution of anisole
(lower energy)
E
H
+
OCH
3
+
Intermediate in meta
substitution of anisole
(higher energy)
OCH
3
E
H
OCH
3
OCH
3
E
E
OCH
3
+
E
+
Transition state for
formation of the
para intermediate
(lower energy)
FIGURE 14.67 The intermediate
for para substitution, with four
resonance forms, is lower in energy
than the intermediate for meta
substitution (three forms) or that for
substitution of benzene (also three
forms).The transition state for para
substitution of anisole will also be
lower in energy than the transition
state for meta substitution.
o-chloroanisole and p-chloroanisole as
products (Fig. 14.68). The relatively low
energy of the intermediate formed by addi-
tion to anisole at the ortho or para substi-
tution (four resonance forms, Fig. 14.66)
means that this reaction will prevail over
meta attack (the intermediate has only three
resonance forms, Fig. 14.64). Moreover, if
we compare the rate of formation of the
lower-energy intermediate to the formation
of the benzene intermediate (also only three
resonance forms), we would expect the
lower-energy intermediate from anisole to
be formed faster, as is indeed observed.
OCH
3
Cl
2
Anisole
+
OCH
3
OCH
3
OCH
3
+
FeCl
3
p-Chloroanisole
(65.1%)
o-Chloroanisole
(34.9%)
m-Chloroanisole
(0%)
Cl
Cl
Cl
WEB 3D WEB 3D WEB 3D
PROBLEM 14.28 Provide a possible explanation for why ortho substitution is often
less favorable than para substitution, even though ortho substitution is favored
statistically by a 2:1 factor?
FIGURE 14.68 The chlorination of anisole.
660 CHAPTER 14 Substitution Reactions of Aromatic Compounds
CH
3
CH
3
E
+
E
H
+
CH
3
E
H
+
CH
3
E
H
+
CH
3
E
H
CH
2
+
There is another resonance form
for this intermediate! Remember
hyperconjugation
CH
3
CH
3
E
E
H
+
E
H
+
E
H
+
CH
3
CH
3
CH
3
E
+
The meta substitution of toluene
Toluene
Toluene
H
This resonance form
is a tertiary carbocation
All resonance forms secondary
The para substitution of toluene
E
FIGURE 14.69 The intermediates for meta
and para substitution of toluene. In the
intermediate for meta substitution, all three
resonance forms are secondary. In the
intermediate for para substitution, two
resonance forms are secondary but one
resonance form is a tertiary carbocation.
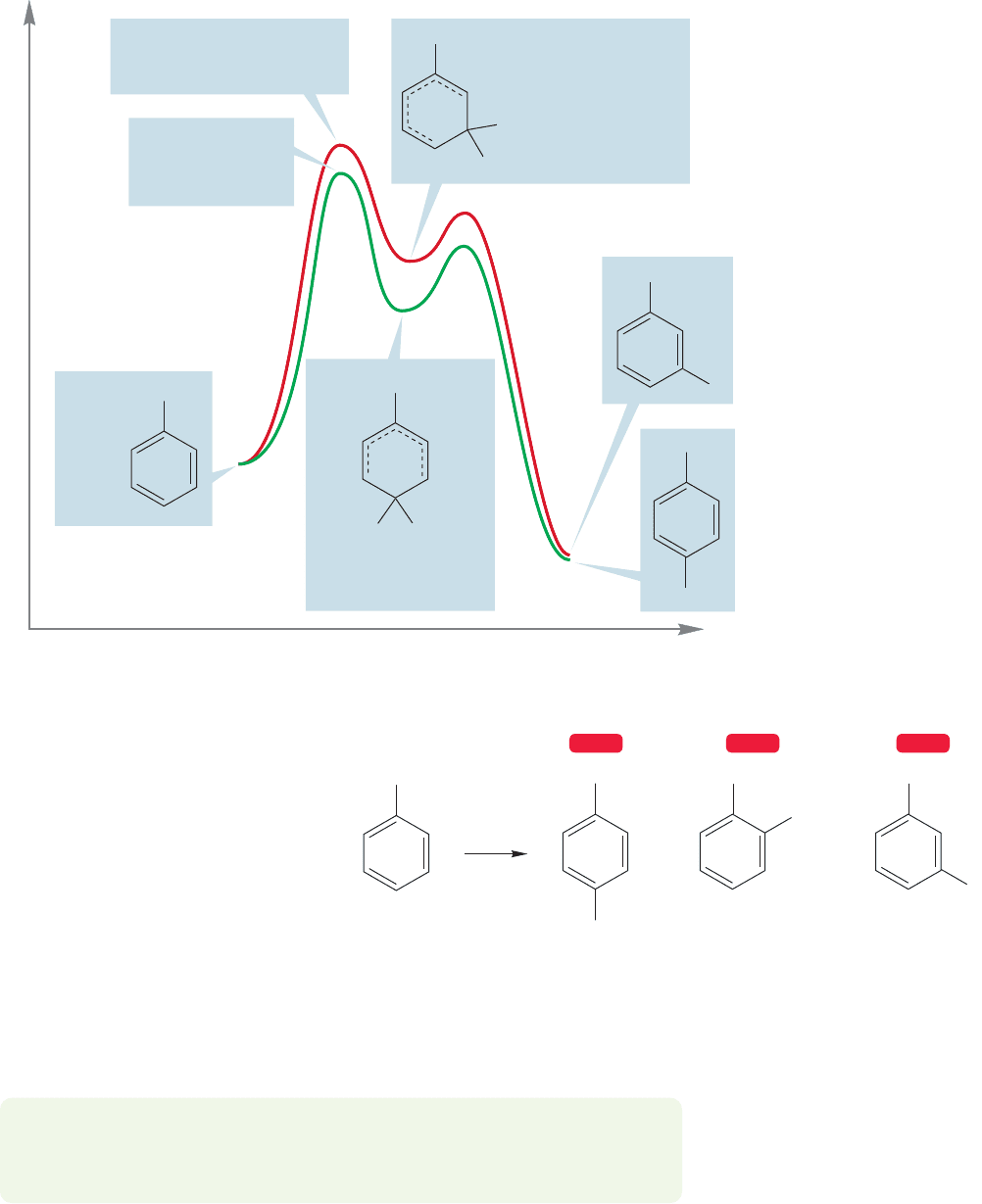
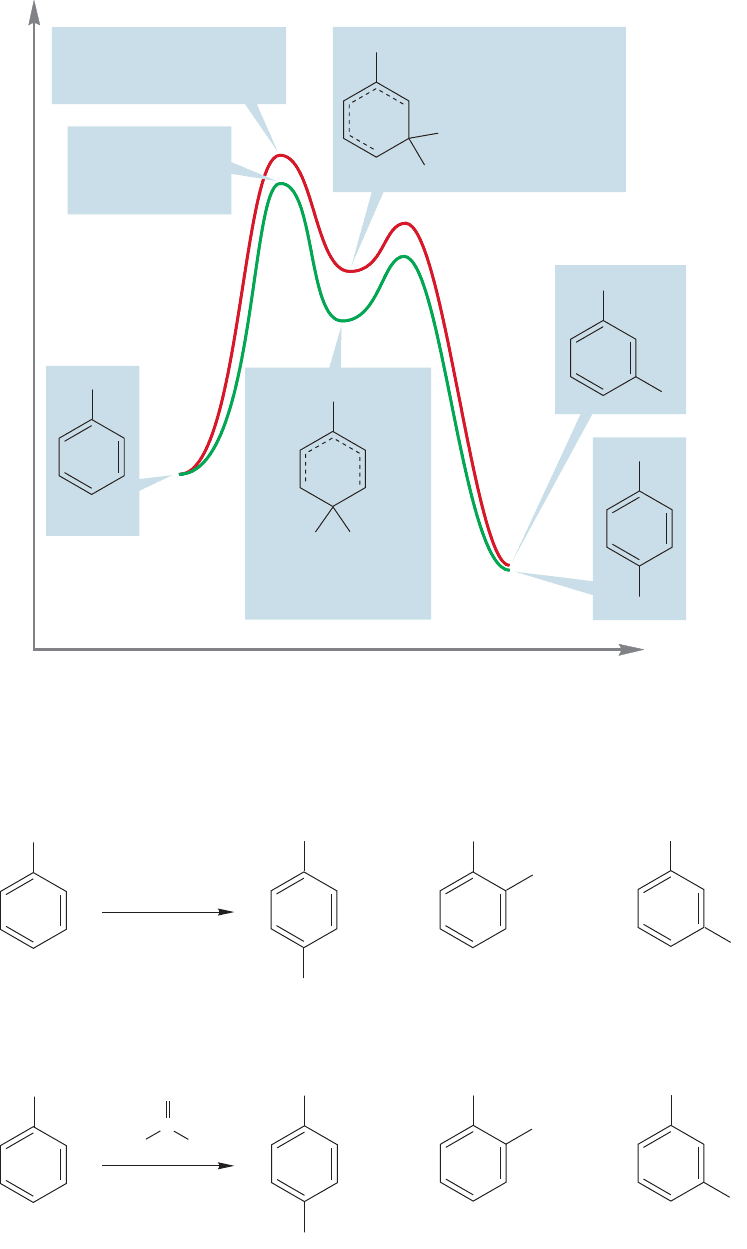
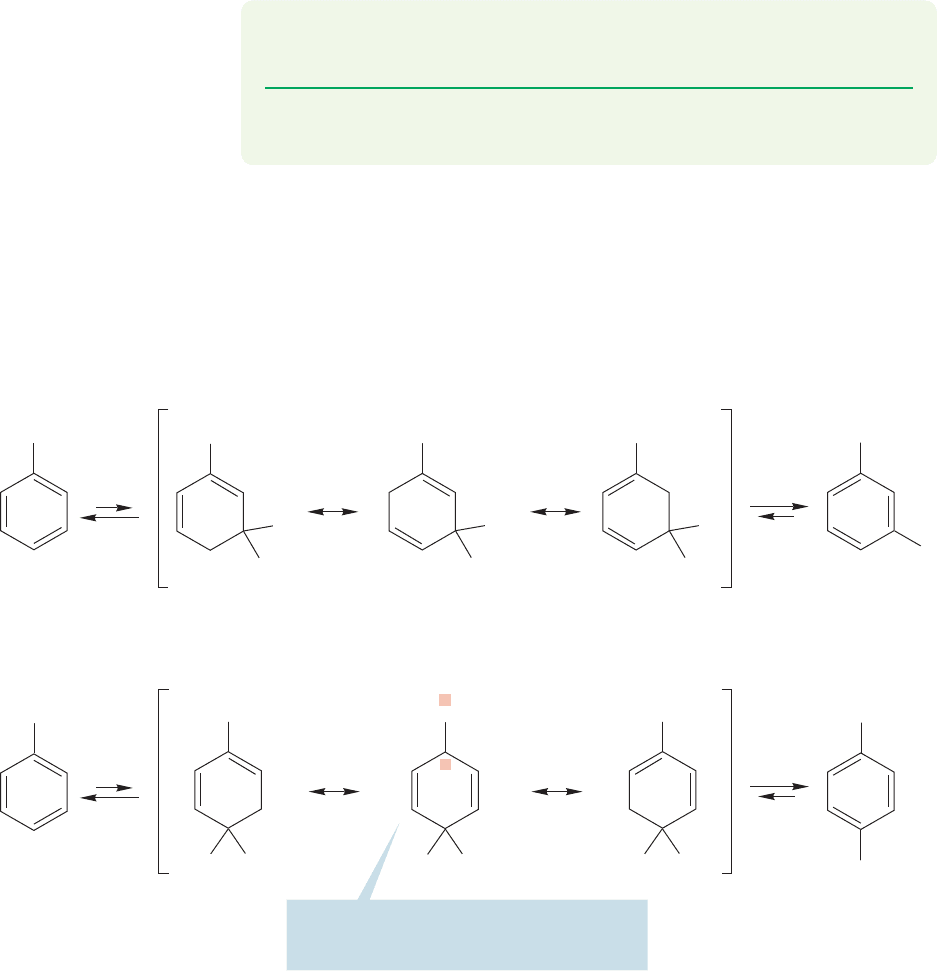
Let’s now look at the substitution of toluene. Figure 14.69 shows the interme-
diates formed by meta and para substitution using a generic electrophile, E
. This
time there is no obvious fourth resonance form,as there was in the para (and ortho)
substitution of anisole.Why is ortho/para substitution still preferred, and why does
toluene react faster than unsubstituted benzene (Table 14.2 p. 656)? Look at the
positions sharing the positive charge. In the intermediate formed from meta sub-
stitution, the three positions sharing the positive charge are all secondary. In the
intermediate formed by para substitution, one of the three positions is a tertiary
carbon and is therefore more effective in stabilizing a positive charge. So there real-
ly is a fourth resonance form! Recall the description of the stabilization of adjacent
positive charge by alkyl groups (p. 376). An alkyl group can stabilize an adjacent
positive charge in a hyperconjugative way.
PROBLEM 14.29 Hold it! Explain exactly why we “expect”that attack on anisole by
an electrophilic reagent to form a relatively low energy, resonance-stabilized inter-
mediate will be faster than attack on benzene? Are we not confusing thermody-
namics with kinetics?
14.9 Disubstituted Benzenes: Ortho, Meta, and Para Substitution 661
The intermediate for para substitution,and the transition state leading to it,are lower
in energy than those involved in meta substitution (Fig. 14.70). The transition state
Reaction progress
Energy
Transition state for formation
of the meta intermediate
(higher energy)
Intermediate in para
substitution of toluene
(lower energy)
E
H
+
CH
3
+
Intermediate in meta
substitution of toluene
(higher energy)
CH
3
E
H
CH
3
CH
3
E
E
CH
3
E
+
Transition state for
formation of the
para intermediate
(lower energy)
FIGURE 14.70 The intermediate and
transition states for para substitution
of toluene are lower in energy than
those for meta substitution.
CH
3
H
2
SO
4
+
CH
3
CH
3
CH
3
+
25 ⬚C
(59%) (36%) (4.5%)
SO
2
OH
SO
2
OH
SO
2
OH
O
C
Cl
Ph
CH
3
+
CH
3
CH
3
CH
3
+
AlCl
3
, 25 ⬚C
(89.2%) (9.3%) (1.5%)
COPh
COPh
COPh
FIGURE 14.71 Some substitution
reactions of toluene.
is also lower than the transition state for substitution of benzene. When a group on
an aromatic ring lowers the transition state compared to that for benzene, the ring
is said to be activated.This theoretical analysis is borne out in practice. Figure 14.71
shows some typical substitution reactions of toluene.
662 CHAPTER 14 Substitution Reactions of Aromatic Compounds
So, for many groups, meta substitution does not present a problem. However,
separation of ortho and para products is a problem, especially because the two are
often formed in comparable amounts. Happily, physical methods such as crystalliza-
tion and distillation can generally be used successfully to separate the products.
Electrophilic aromatic substitution is a practical source of many ortho and para
disubstituted benzenes.
N(CH
3
)
3
N(CH
3
)
3
E
+
E
H
N(CH
3
)
3
E
H
+
N(CH
3
)
3
E
H
+
N(CH
3
)
3
In this resonance form, two positive charges
are very close; this form is very high energy
and will contribute little
N(CH
3
)
3
N(CH
3
)
3
E
E
H
+
E
H
+
E
H
+
N(CH
3
)
3
N(CH
3
)
3
N(CH
3
)
3
E
+
Meta substitution of the trimethylanilinium ion
Para substitution of the trimethylanilinium ion
Trimethyl-
anilinium
ion
+
+
+
+
+
Trimethyl-
anilinium
ion
+
+
+
+
E
+
+
FIGURE 14.72 Meta and para substitution of the trimethylanilium ion. Both intermediates have three resonance
forms, but the adjacent positive charges in the intermediate for para substitution are especially destabilizing.
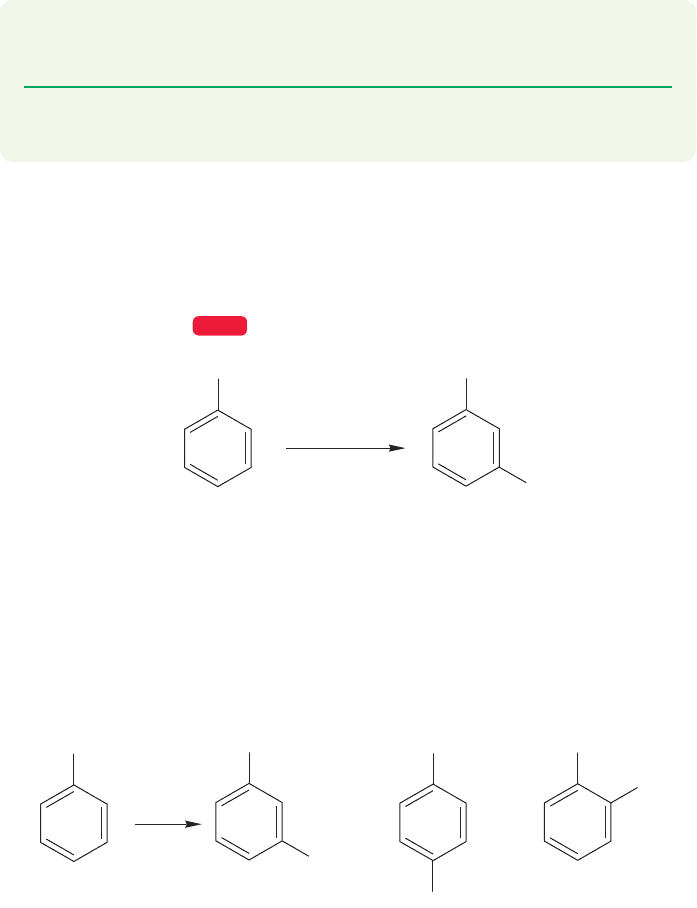
What about groups that direct substitution to the meta position and slow the
rate? Why are the effects we have seen so far not general? Once more, the way to
attack this problem is to write out the various possibilities and look for points of
difference. Let’s look first at the intermediates formed by reaction of the trimethyl-
anilinium ion in Figure 14.72.
Both meta and para substitution give an intermediate with three resonance forms,
so there is no gross difference here. Note that we are introducing a second positive
charge when we do any electrophilic aromatic substitution reaction on this molecule.
PROBLEM 14.30 Write a mechanism for the ortho substitution of toluene by an
electrophile, E
. Compare this reaction to meta substitution.
PROBLEM 14.31 Explain why bromobenzene and aniline direct electrophilic aro-
matic substitution reactions ortho/para.
14.9 Disubstituted Benzenes: Ortho, Meta, and Para Substitution 663
This second charge is bound to slow the reaction relative to substitution of benzene
itself.In this case the aromatic ring is strongly deactivated with respect to electrophilic
substitution. Now we know why this substitution reaction is especially slow, but we
can’t yet explain why the meta position is preferred. The difficulty in para substitu-
tion is that the reaction places the two positive charges especially close together. In
para substitution,the positive charge produced by reacting with the electrophile resides
partly on the carbon bearing the original substituent. When this substituent can sta-
bilize the charge through resonance, as in anisole or toluene, para substitution will
be favored. When it brings two positive charges together, as in the trimethylanilinium
ion, para substitution is decidedly disfavored for electrostatic reasons.
So the situation is clear for the trimethylanilinium ion.Meta substitution,which
does not place two like charges on adjacent carbons,is favored over para (or ortho)
substitution, which does. Figure 14.73 shows one example: bromination of the
trimethylanilinium ion.
H
2
O, 25 ⬚C
“Br
+
”
N(CH
3
)
3
N(CH
3
)
3
Br
(100%)
+
+
Trimethylanilinium
ion
WEB 3D
FIGURE 14.73 Bromination of the
trimethylanilinium ion.
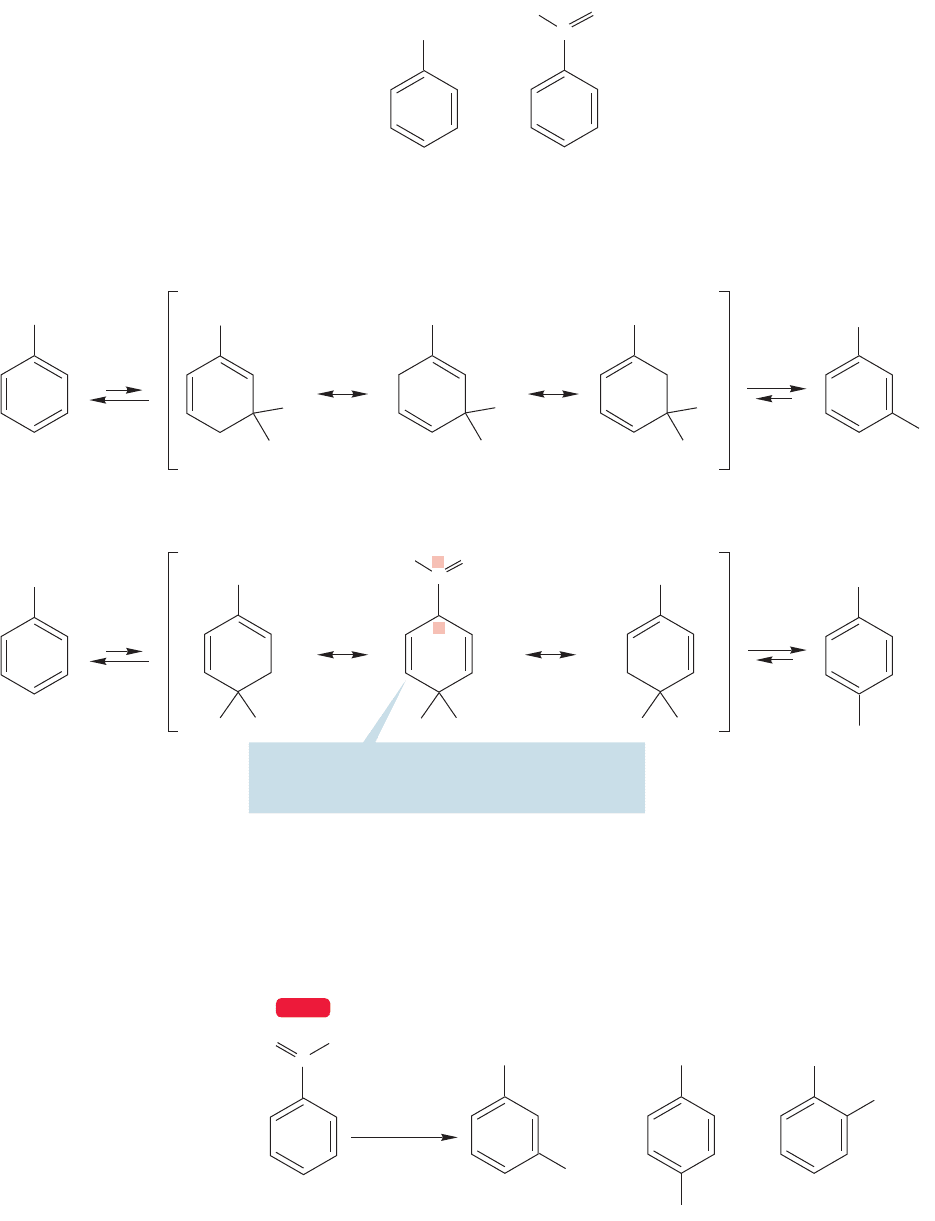
What about the other meta-directing groups? They do not bear obvious positive
charges. Nitrobenzene is a good example. Nitrobenzene is so deactivated with respect
to electrophilic substitution that it can be used as a solvent for Friedel–Crafts reactions
of other aromatic compounds and not suffer attack itself.When substitution does occur
on nitrobenzene, it is invariably largely in the meta position (Table 14.2; Fig. 14.74).
NO
2
HNO
3
,
0 ⬚C
Nitrobenzene
+
NO
2
NO
2
NO
2
+
m-Dinitrobenzene
(93.2%)
p-Dinitrobenzene
(0.3%)
o-Dinitrobenzene
(6.4%)
NO
2
NO
2
NO
2
FIGURE 14.74 Nitrobenzene nitrates
very slowly at 0 °C, and almost
entirely in the meta position.
Yet there is no obvious positive charge in the starting material, as there is in the
trimethylanilinium ion we analyzed before. However, a close look at the structure
of the nitro group reveals the problem, which has been hidden by the obfuscation
PROBLEM 14.32 Draw all of the resonance structures of the intermediate formed
by ortho substitution of the trimethylanilinium ion.
PROBLEM 14.33 Use an Energy versus Reaction progress diagram to compare
ortho and meta substitution on the trimethylanilinium ion.
664 CHAPTER 14 Substitution Reactions of Aromatic Compounds
of nitro as NO
2
. If you draw out a nitro group carefully, you will see (1) that there is
no good uncharged structure,and (2) that the nitrogen always bears a positive charge:
NO
2
NO
2
E
+
E
H
E
H
+
NO
2
E
H
+
NO
2
NO
2
NO
2
E
E
H
+
E
H
+
E
H
+
NO
2
NO
2
NO
2
E
+
Meta substitution of nitrobenzene is favored over…
Para substitution of nitrobenzene
E
N
OO
–
In this resonance form, two positive charges are
very close; this form is very high energy and will
contribute little to the stability of the intermediate
+
+
FIGURE 14.75 The resonance description for the nitro group reveals that nitrogen bears a full positive charge. Electrophilic
aromatic substitution will be directed to the meta position.The ring is deactivated.
C
HNO
3
, 0 ⬚C
+
COOEt
COOEt
COOEt
+
meta
(68.4%)
para
(3.3%)
ortho
(28.3%)
NO
2
NO
2
NO
2
O
OEt
Ethyl
benzoate
WEB 3D
FIGURE 14.76 The nitration of ethyl
benzoate gives mainly meta product.
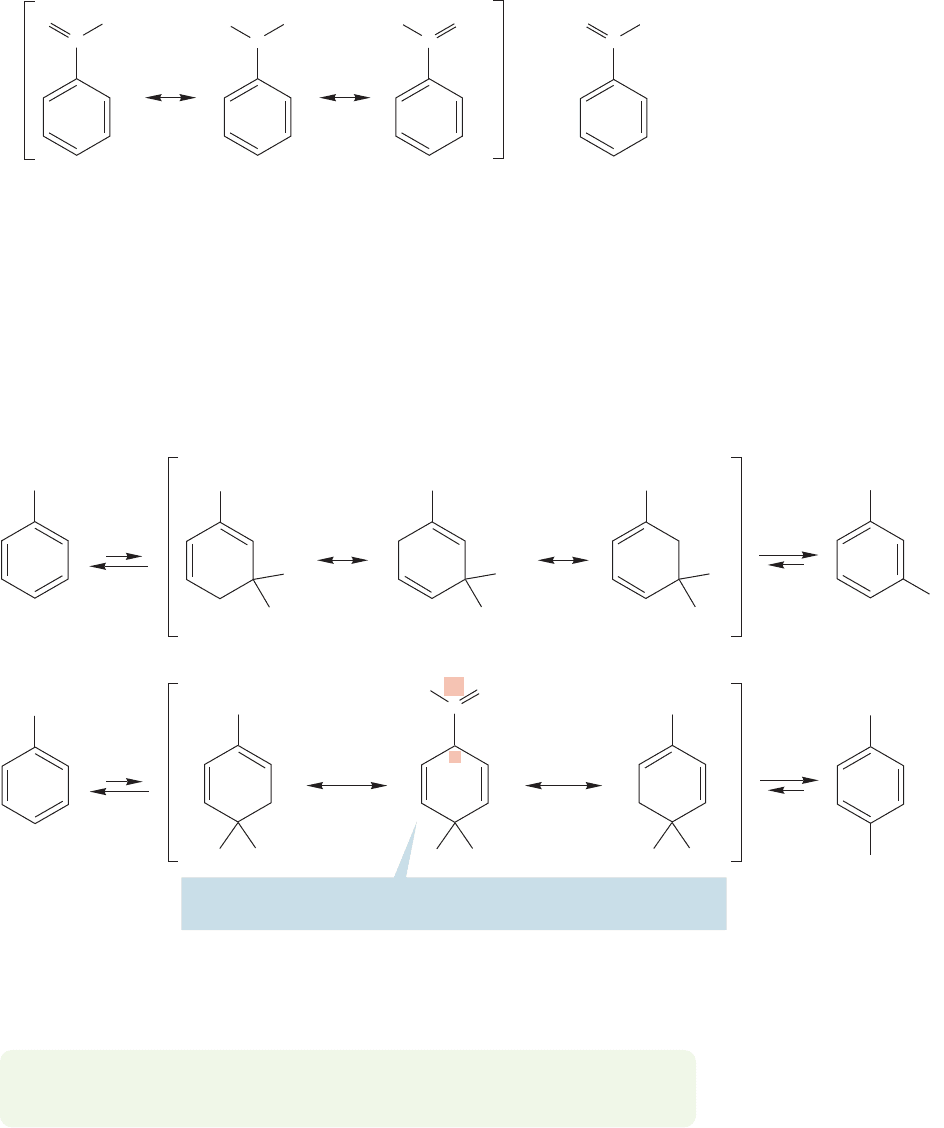
So there really is a positive charge adjacent to the ring in nitrobenzene.Therefore the
same factors that lead to meta substitution and a slow rate in the trimethylanilinium
ion will operate for nitrobenzene as well (Fig. 14.75).
We still need to discuss the other meta directors in Table 14.2, those with a car-
bonyl group attached to the ring. Ethyl benzoate (C
6
H
5
COOCH
2
CH
3
) is a good
example.It too substitutes slowly, and predominantly in the meta position (Fig. 14.76).
NO
2
N
O
O
+
=
In nitrobenzene, there
is a positive charge
adjacent to the ring
–
14.9 Disubstituted Benzenes: Ortho, Meta, and Para Substitution 665
The explanation for this behavior again comes from a close look at the struc-
ture of the group substituting the benzene ring, in this case, the carbonyl group.
Although there are no hidden full positive charges as in the nitro group, the
carbon–oxygen double bond is highly polar. Because oxygen is much more elec-
tronegative than carbon, the dipole in the bond will place a partial positive charge
on the carbonyl carbon attached to the ring (Fig. 14.77).
=
C
O
OEt
C
O
OEt
C
O
OEt
C
O
OEt
δ
+
+
–
–
+
δ
–
.. ..
..
..
..
..
..
..
..
..
....
..
..
..
..
..
FIGURE 14.77 The carbonyl group
is polar, with the carbon bearing a
partial positive charge.
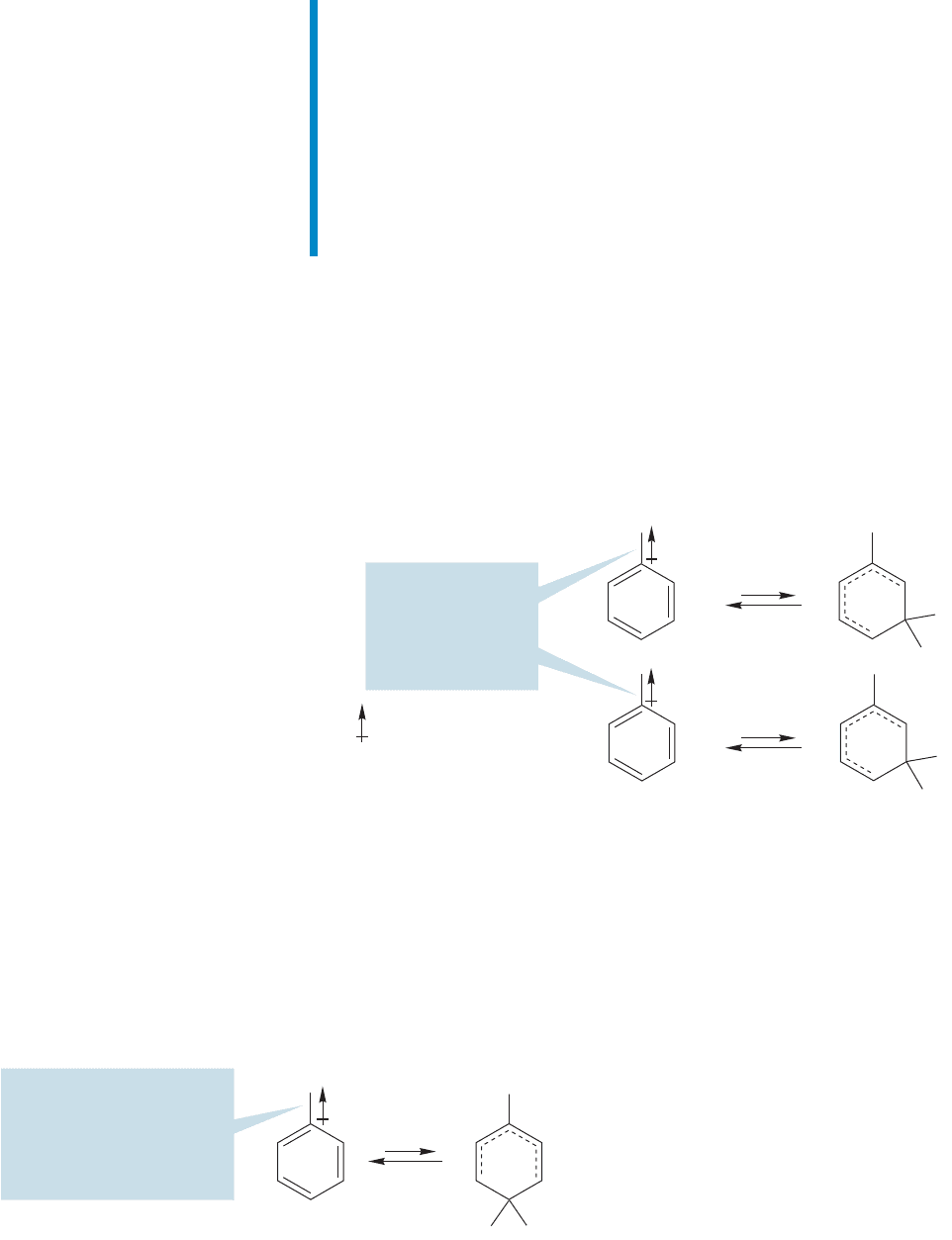
So, once again, ortho or para substitution of an electrophile results in a less sta-
ble intermediate.This time it is not two full positive charges that must be adjacent,
but a full and partial positive charge. No matter—meta substitution, which keeps
the charges further apart, will still be favored (Fig. 14.78). The rate of the reaction
will be slow relative to that for benzene. An aromatic ring with a carbonyl group
attached is deactivated.
COOEt
COOEt
E
+
E
H
E
H
+
COOEt
E
H
+
COOEt
COOEt
COOEt
E
E
H
+
E
H
+
E
H
+
COOEt
COOEt COOEt
E
+
The meta substitution of ethyl benzoate
The para substitution of ethyl benzoate
E
C
O
EtO
δ
–
+
δ
+
In this resonance form, a positive charge and a partial positive charge
are very close; this form is very high energy and will contribute little
FIGURE 14.78 Meta substitution is preferred to para substitution. In the intermediate for para (or ortho) substitution,
a full and partial positive charge are opposed on adjacent carbons.
PROBLEM 14.34 Explain why cyanobenzene (benzonitrile, C
6
H
5
CN) substitutes
preferentially in the meta position.
666 CHAPTER 14 Substitution Reactions of Aromatic Compounds
Summary
A group (G) on the benzene ring determines the position of the next electrophilic
aromatic substitution reaction. Generally, groups that direct ortho/para also
increase the rate of substitution. In such cases, the ring is said to be activated.
If substitution is in the ortho or para position (but not the meta position), G
provides additional stabilization to the cyclohexadienyl cation intermediate, and
thus increases the rate. Meta-directing groups make the best of an energetically
bad set of choices. Meta substitution is slow and wins by default—it is the least-
bad alternative for some substituents. In this case the ring is said to be deactivated
with respect to electrophilic aromatic substitution.
+
These σ bonds are
strongly polarized
so as to make
introduction of a
positive charge into
the ring difficult
N(CH
3
)
3
H
E
(CH
3
)
3
N
++
O
2
N
δ
–
δ
+
δ
–
δ
+
+
NO
2
H
E
E
+
E
+
Shows a dipole in the
direction indicated by
the
δ
+
and δ
–
FIGURE 14.79 In the
trimethylanilinium ion and
nitrobenzene, the σ bond from the
ring to the substituent is polarized
by the electron-withdrawing group.
Further substitution is difficult.
These subsituents will reduce the electron density in the ring, making the aro-
matic compound a weaker nucleophile, and thus a less active participant in any
reaction with an electrophilic reagent. More important is the destabilizing effect
of electron withdrawal on the charged intermediate and the partially charged tran-
sition state leading to the intermediate. So, it is no surprise to find that both
trimethylanilinium ion and nitrobenzene are very slow to react in electrophilic aro-
matic substitution. Similar rate-retarding effects might be expected to operate
whenever an atom more electronegative than carbon is attached to the ring.
If that is the case, what is one to make of the rate
accelerating effect of the methoxy group? Anisole reacts
much faster than benzene, and yet the methoxy group
is certainly withdrawing electrons from the ring
through induction.The dipole in the carbon–oxygen
σ bond surely points toward the electronegative
oxygen atom (Fig. 14.80).
The answer to this conundrum is simply that the
resonance effect must substantially outweigh the
inductive effect.The fourth resonance form in Figure
14.66 helps more in energy terms than the inductive
withdrawal of σ electrons by methoxy hurts.
E
+
This σ bond is also strongly
polarized so as to make
introduction of a positive
charge into the ring difficult;
yet, the reactions of anisole
are very fast
CH
3
O
E
H
+
CH
3
O
δ
–
δ
+
FIGURE 14.80 The carbon–oxygen bond in anisole is also electron-
withdrawing, yet substitution is directed ortho/para and the reactions
are fast.The resonance effect outweighs this inductive effect.
14.10 Inductive Effects in Aromatic Substitution
So far, we have mainly considered the way in which the group attached to a ben-
zene ring can affect further reaction through either resonance stabilization or elec-
trostatic destabilization of the intermediate cyclohexadienyl cation. We should also
consider the inductive effects of groups,which act by withdrawing or donating elec-
trons through the σ bonds. For example, both the trimethylammonium group and
the nitro group are strongly electron-withdrawing by induction (Fig. 14.79).
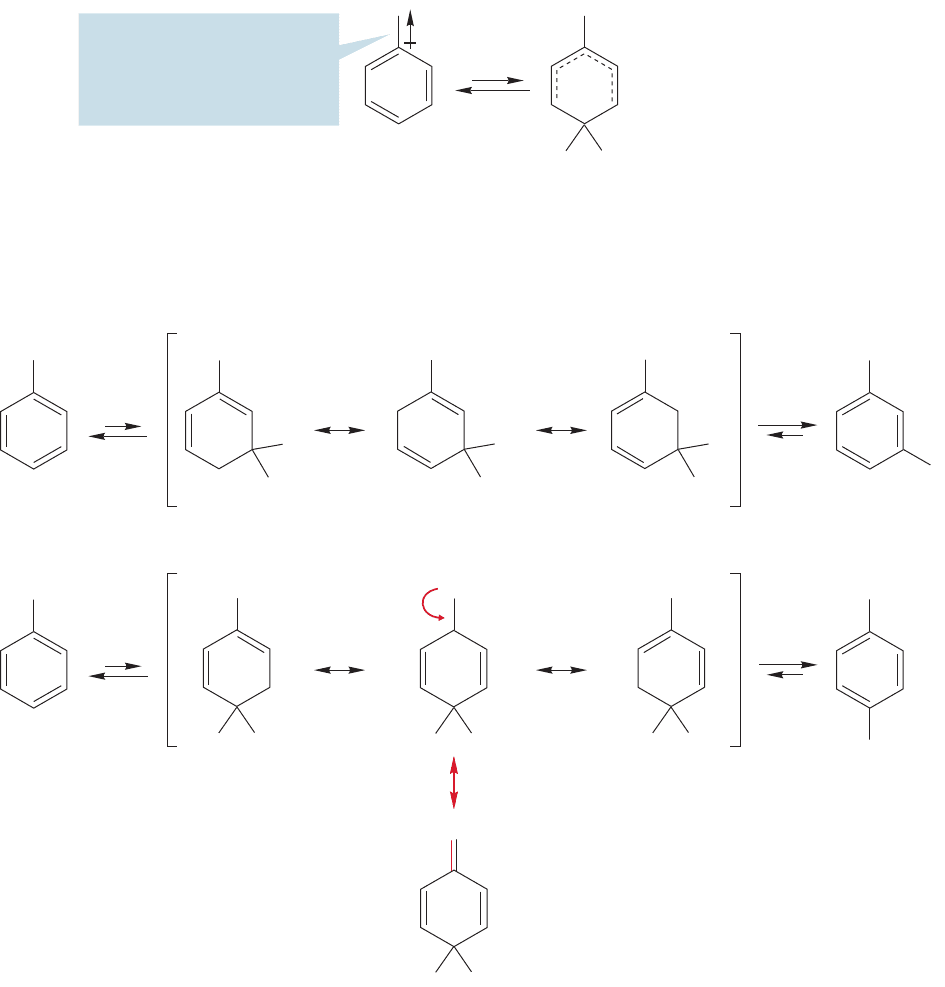
14.10 Inductive Effects in Aromatic Substitution 667
Cl Cl
+
E
+
E
H
δ
–
δ
+
This bond is strongly polarized
and the
δ
+
on carbon acts to
retard any substitution reaction
that would place more positive
charge on the ring carbons
FIGURE 14.81 Chlorine and the
other halogens are strongly electron-
withdrawing and slow the rate of
electrophilic substitution.The net
result is an overall slowing of the rate.
However, ortho/para substitution is still favored over meta because the halogen
can stabilize an adjacent positive charge by resonance (Fig. 14.82).The rate of meta
E
E
H
+
E
H
+
E
H
+
E
+
The meta substitution of chlorobenzene—three resonance forms
The para substitution of chlorobenzene—there are four forms because the chlorine can help share the positive charge
E
+
E
H
E
H
+
E
H
+
E
H
+
There is another
resonance form
for this intermediate!
Here it is
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
E
+
FIGURE 14.82 Chlorine and other halogens
can help stabilize the intermediate for para
(and ortho) substitution through a fourth
resonance form.The halogens strongly
withdraw electrons from the ring through
an inductive effect, however, and
substitution is slow relative to benzene.
Thus,often we have two effects operating in opposition. In anisole,the resonance
effect stabilizes the intermediate for ortho or para substitution and the transition
states leading to them,accelerating the reaction and directing ortho/para.The induc-
tive effect is decelerating for any substitution reaction. For anisole and most groups,
the resonance effect wins out, but we can surely find examples where the two effects
are largely in balance; where substitution is still ortho/para because of a resonance
effect, but where an inductive effect slows the reaction relative to unsubstituted
benzene. A look at Table 14.2 shows that there are such cases.For example,haloben-
zenes direct substitution to the ortho/para positions but substitution proceeds at a
slower rate than that for the reaction of benzene. In halobenzenes, all electrophilic
aromatic substitution is retarded by the strong electron-withdrawing effect of the
electronegative halogens (Fig. 14.81). As a result, the ring is deactivated.
668 CHAPTER 14 Substitution Reactions of Aromatic Compounds
Cl Cl Cl Cl
HNO
3
, 25 ⬚C
+
+
m-Chloronitrobenzene
(0.9%)
Chlorobenzene p-Chloronitrobenzene
(69.5%)
o-Chloronitrobenzene
(29.6%)
NO
2
NO
2
NO
2
nitromethane
FIGURE 14.83 The nitration of chlorobenzene gives almost entirely ortho and para products.
E
E
+
m = meta director
m = another meta director
m
m
m
m
m directs here
m directs here
FIGURE 14.84 On a benzene ring
substituted 1,3 with a pair of meta-
directing groups, both substituents
direct further substitution to the
same position.
substitution, in which the stabilizing resonance effect cannot operate, is slowest of
all, and so meta product is greatly disfavored (Fig. 14.83).
14.11 Synthesis of Polysubstituted Benzenes
Sometimes it is easy to predict the position of electrophilic attack on a polysubstituted
benzene ring. For example, if we have a ring substituted 1,3 with meta-directing groups,
further substitution will surely occur at the position meta to both groups, although the
rate will be severely retarded by the presence of a pair of deactivating groups (Fig.14.84).
+
NO
2
,
–
BF
4
NO
2
NO
2
NO
2
NO
2
O
2
N
FSO
3
H
150 ⬚C, 4 h
1,3,5-Trinitrobenzene
~ 50% yield
100% this isomer
m-Dinitrobenzene
FIGURE 14.85 Nitration of 1,3-
dinitrobenzene gives a single
product, 1,3,5-trinitrobenzene.
Further substitution of a disubstituted aromatic ring containing an ortho/para
director and a meta director at the 1- and 4-positions is similarly easy to predict.
Both groups direct the next substitution ortho to the ortho/para-directing group.
Accordingly, nitration of 1,3-dinitrobenzene gives only meta substitution (Fig. 14.85).
