Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

13.15 Additional Problems 619
13.15 Additional Problems
PROBLEM 13.28 Give names for the following compounds:
PROBLEM 13.29 Write structures for the following compounds:
(a) 2,4,6-trinitrotoluene (TNT)
(b) 4-bromotoluene
(c) p-bromotoluene
(d) m-diiodobenzene
(e) 3-ethylphenol
(f) m-ethylphenol
(g) 1,2,4,5-tetramethylbenzene (durene)
(h) o-deuteriobenzenesulfonic acid (Note the “i” in “deuterio.” It
is not “deutero.”)
(i) p-aminoaniline
(j) m-chlorobenzoic acid
PROBLEM 13.30 Write arrow formalisms for the conversions
of Dewar benzene, Ladenburg benzene (prismane), benzvalene,
and 3,3′-bicyclopropenyl (Fig. 13.4) into benzene. Some of
these tasks may be quite challenging.
PROBLEM 13.31 Which of the following heterocyclic
compounds might be aromatic? Explain. It will help if
PROBLEM 13.32 Pyridine could be called azabenzene. There
are three diazabenzenes. Draw these compounds. Are they
aromatic?
PROBLEM 13.33 Which of the following hydrocarbons might
be aromatic? Explain.
PROBLEM 13.34 Which of the following ions might be
aromatic? Explain.
CH
3
CH
3
H
3
C
(e)
I
Cl
(f)
Br
F
(c)
CH
3
COOH
(d)
Br
Br
F
(a)
Br
F
(b)
(a) (b) (c)
(d) (e)
BH
BH
O
N
N
N
H
H
H
H
H
H
B
B
B
NH
(a)
(b)
(c)
CH
2
CH
2
(d)
(e)
(a) (b)
–
–
–
+
2+
+
+
(c) (d) (e)
(g)(f )
you start by drawing good Lewis structures for all the
compounds.

N
2
Diazocyclopentadiene
1
R
2
R
3
R
1
N
2
R
2
R
3
R
1
N
620 CHAPTER 13 Conjugation and Aromaticity
PROBLEM 13.35 Design an aromatic heterocyclic compound that
(a) contains one boron atom.
(b) contains one boron and one oxygen atom in a six-membered
ring.
(c) contains one oxygen and one nitrogen atom in a five-
membered ring.
(d) contains two nitrogen atoms in a five-membered ring.
(e) contains three boron and three nitrogen atoms in a six-
membered ring.
PROBLEM 13.36 Try to construct an aromatic heterocycle
in which there is an oxygen and a nitrogen in a neutral six-
membered ring.
PROBLEM 13.37 The molecule 5-bromo-1,3-cyclohexadiene
is very difficult to isolate, but 1-bromo-1,3-cyclohexadiene is
much easier to obtain. Explain why there is so much difference.
PROBLEM 13.38 Carefully explain why azirines of structure 1
have never been isolated, whereas the isomeric azirines of
structure 2 are well known.
PROBLEM 13.42 Most diazo compounds are exceedingly, and
sometimes disastrously unstable. They are notorious explosives.
By contrast, the brilliant, iridescent orange compound diazocy-
clopentadiene is quite stable. Explain.
PROBLEM 13.43 Why is it that (E)-1-phenyl-2-butene reacts
only once with OsO
4
? Draw the product that is formed.
C
7
H
8
Br
2
HBr + C
7
H
7
Br
H
2
O
Ditropyl ether
O
Br
2
H
2
O
Na
2
SO
3
OsO
4
PROBLEM 13.39 The dipole moment of THF is 1.7 D, but
that of furan is only 0.7 D. Explain.
PROBLEM 13.40 The heat of hydrogenation of 1,3,5,7-
cyclooctatetraene (COT) is about 101 kcal/mol. The heat of
hydrogenation of cyclooctene is about 23 kcal/mol. Is COT
aromatic? Explain.
PROBLEM 13.41 As early as 1891 the German chemist G.
Merling showed that the bromination of 1,3,5-cycloheptatriene
leads to a liquid dibromide. When the dibromide is heated,
HBr is evolved and a yellow solid of the formula C
7
H
7
Br
(mp 203 °C) can be isolated. This compound is soluble in water,
but cannot be reisolated from water solution. Instead, ditropyl
ether is obtained. Merling could not explain what was happen-
ing, but perhaps you can.
PROBLEM 13.44 Predict the major product(s), if any, for the
bromination (Br
2
,CH
2
Cl
2
) of the following compounds:
(a) styrene
(b) 1-ethyl-3-nitrobenzene
(c) (Z)-2-phenyl-2-butene
(d) 3-phenyl-1-propene
(e) 3-phenylheptane
PROBLEM 13.45 The major product in the bromination
(1 equivalent of Br
2
in CH
2
Cl
2
) of (E,E)-1-phenyl-1,3-pentadiene
is (E)-3,4-dibromo-1-phenyl-1-pentene regardless of the reac-
tion conditions (thermodynamic or kinetic control). Show the
mechanism of the reaction and account for this selectivity.
PROBLEM 13.46 Predict the major product(s), if any, for the
reaction of HBr in THF (solvent) with each of the following:
(a) styrene
(b) (Z)-2-phenyl-2-hexene
(c) 2-methyl-1-phenyl-1-propene
(d) 3-phenyl-1-propene
(e) naphthalene
PROBLEM 13.47 Predict the product of the reaction with NBS
(CCl
4
, hν) for each of the following compounds:
(a) 3-phenyloctane
(b) 3-methyl-1-phenylbutane
(c) (Z)-1-phenyl-2-butene
(d) isopropylbenzene
(e) phenylcyclohexane
PROBLEM 13.48 Predict the major product in the reaction
between 1 equivalent of NaI and 1,5-dichloro-1-phenylhexane.
Explain the regioselectivity.
PROBLEM 13.49 Construct the π molecular orbitals for ben-
zene (p. 578) from the π molecular orbitals of allyl. Remember:
Only combinations of orbitals of comparable energy need be
considered.
13.15 Additional Problems 621
NO
2
BF
4
–
NO
2
+
Anthracene
1
2
3
1.37 A
⬚
1.42 A
⬚
PROBLEM 13.50 Vogel and Roth’s synthesis of the bridged
cyclodecapentaene (1) is outlined below. Supply the missing
reagents (a, b, c, and d) and an arrow formalism for the
last step.
PROBLEM 13.52 Draw cyclopropene. How many types of
hydrogens are there in this molecule? Which hydrogen is more
acidic? Explain why. Use the table inside the back cover of this
book to estimate the pK
a
for each type of hydrogen.
PROBLEM 13.53 Explain why the 1,2-bond of anthracene is
shorter than the 2,3-bond.
PROBLEM 13.54 The nitronium ion (
NO
2
) is known and
can react with simple benzenes to give nitrobenzenes. Write an
arrow formalism mechanism for this reaction.
PROBLEM 13.56 The compound shown below was synthesized
by Professor I. M. Dimm, who was astonished to find that it
was exceedingly stable. Prof. Dimm had expected world renown
for making what he thought would be a most unstable com-
pound. Dimm reasoned that with six double bonds and a triple
bond in the molecule, there would be 16 π electrons and thus,
no aromatic character (4n, n 4; not 4n 2). Where was his
reasoning wrong?
1
d
Cl
Br
BrBr
Br
a
b
Δ
c
Na
NH
3
H Cl
H
H
H
H
H
H
H
N
H
C(2)
N
H
Pyridine Pyrrole Imidazole
N
N
Cu(NO
3
)
2
acetic anhydride
0 ⬚C
CH
3
CH
3
CH
3
CH
3
NO
2
PROBLEM 13.51 Pyridine and imidazole are modest Brønsted
bases at nitrogen, whereas pyrrole is not.
PROBLEM 13.55 The molecule shown below undergoes reac-
tion with cupric nitrate in acetic anhydride (a source of
NO
2
)
to give a high yield of the substituted product shown. Write a
mechanism for this reaction.
In fact, pyrrole is protonated only in strong acid, and protonation
occurs at C(2), not on nitrogen. First, explain why pyridine and
imidazole are basic and pyrrole is not. Then, explain why eventu-
al protonation of pyrrole is at carbon, not nitrogen. Caution! Yo u
will have to write full Lewis structures to answer this question.
622 CHAPTER 13 Conjugation and Aromaticity
COOH
COOH
H
3
C
+
1. Na/NH
3
,
ether, –78 ⬚C
2. CH
3
I
3. H
2
O/H
3
O
PROBLEM 13.57 Provide a mechanism for the following
reaction sequence:
PROBLEM 13.58 There are many aromatic rings in nature.
You have learned about the nucleotide bases. Draw adenine,
guanine, cytosine, and thymine (p. 615). Circle any aromatic
rings in these molecules. You might need to draw resonance
structures to see the aromaticity.
PROBLEM 13.59 Even though naphthalene and anthracene
are polynuclear aromatic compounds, they undergo the
Diels–Alder reaction. For example, anthracene readily reacts
with maleic anhydride. However, the reaction of naphthalene
with maleic anhydride proceeds efficiently only under high
pressure.
100 C
9.6 kbar, 17 h
?
+
O
O
O
xylene
140 C, 10 min
?
+
O
O
O
CH
3
(a)
(b)
(c)
C(CH
3
)
3
CH
2
CH
3
CH(CH
3
)
2
CH
3
H
3
C
CH
2
CH
3
C
(a) Draw the structures of the Diels–Alder adducts formed in
these reactions.
(b) Explain why anthracene is so much more reactive than
naphthalene in the Diels–Alder reaction.
Hint: The calculat-
ed resonance energies of naphthalene and anthracene are
61 kcal/mol and 84 kcal/mol, respectively.
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 13.61 Select the reaction “Benzylic oxidation” on the
Semester 1B panel. Observe the reaction several times. The ani-
mation uses oxygen (O
2
) as the initiator of the reaction, which
also occurs in Nature. One of the reasons for the common prac-
tice of packing organic foods over nitrogen (N
2
) is to avoid the
reaction of O
2
with benzylic (and allylic) positions in natural
products present in food. Draw each step of the reaction
between O
2
and 2-phenylpropane. Include a termination reac-
tion. Label your steps as initiation, propagation, or termination.
PROBLEM 13.62 Watch the “Benzylic oxidation” reaction using
the SOMO track. What does SOMO stand for? Stop the reac-
tion as O
2
is approaching the benzylic hydrogen. Notice the
aromatic ring. What positions are showing SOMO character?
Why these carbons? Does this help you understand the reso-
nance structures? Explain.
PROBLEM 13.63 One of the final products from the benzylic
oxidation of 2-phenylpropane is phenol. See if you can show a
mechanism for the formation of phenol from the peroxide
product shown in the animation. Remember that the
oxygen–oxygen bond is very weak. The mechanism will require
a rearrangement and a second radical oxidation process.
PROBLEM 13.64 We answered questions about the “Stabilized
alkene halogenation” reaction in Chapter 10. This reaction is at
the top of the Semester 1B page. Watch it again to remind your-
self about the pathway. Bromination in this animation is shown to
occur via a syn addition. Bromination usually proceeds through an
anti addition. Why would syn addition be favored over anti addi-
tion for this reaction? Notice the benzylic-like intermediate in the
reaction. Do you suppose the napthylmethyl cation (like the one
in this animation) is more stable than a benzyl cation? Why?
PROBLEM 13.60 A certain alkylbenzene was known to have
one of the isomeric structures shown below.
Explain how the structure of the unknown compound could be
determined by oxidation with KMnO
4
.

Substitution Reactions
of Aromatic Compounds
623
14.1 Preview
14.2 Hydrogenation of Aromatic
Compounds
14.3 Diels–Alder Reactions
14.4 Substitution Reactions of
Aromatic Compounds
14.5 Carbon–Carbon Bond
Formation: Friedel–Crafts
Alkylation
14.6 Friedel–Crafts Acylation
14.7 Synthetic Reactions We Can
Do So Far
14.8 Electrophilic Aromatic
Substitution of
Heteroaromatic Compounds
14.9 Disubstituted Benzenes:
Ortho, Meta, and Para
Substitution
14.10 Inductive Effects in Aromatic
Substitution
14.11 Synthesis of Polysubstituted
Benzenes
14.12 Nucleophilic Aromatic
Substitution
14.13 Special Topic: Stable
Carbocations in “Superacid”
14.14 Special Topic: Benzyne
14.15 Special Topic: Biological
Synthesis of Aromatic Rings;
Phenylalanine
14.16 Summary
14.17 Additional Problems
14
AROMATIC SUBSTITUTION The most common reaction for aromatic compounds
is substitution.This process is much like a soccer substitution; one group (in this
case, a person) replaces another.
624 CHAPTER 14 Substitution Reactions of Aromatic Compounds
Energy
Reaction progress
Aromatic
compound
Transition
state
Nonaromatic
compound
Activation
energy
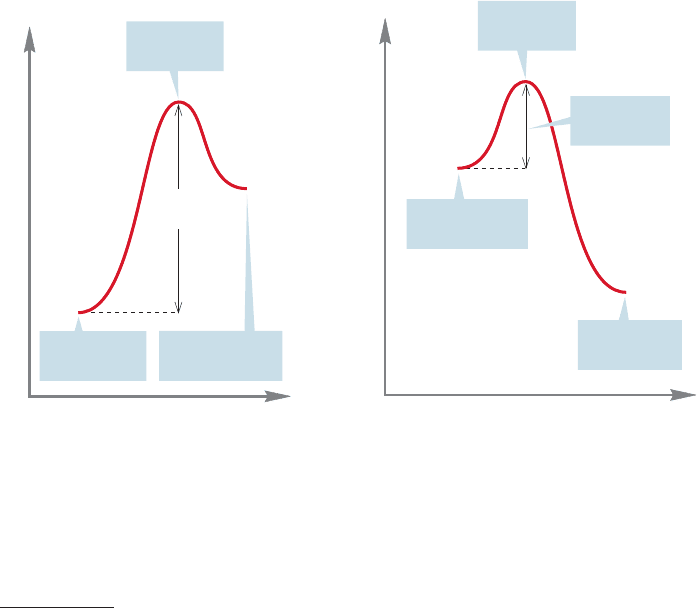
FIGURE 14.1 A typical Energy versus
Reaction progress diagram for the
conversion of an aromatic compound
into a nonaromatic compound.
Aromaticity is lost in this reaction,
and there is a high activation barrier.
Energy
Reaction progress
Aromatic
compound
Transition
state
Nonaromatic
compound
Activation
energy
FIGURE 14.2 A typical Energy versus
Reaction progress diagram for the
conversion of a nonaromatic compound into
an aromatic compound. Aromaticity is
regained in this reaction, and there is a
correspondingly low activation barrier.
Even electrons, supposedly the paragons of unpredictability, are tame and
obsequious little creatures that rush around at the speed of light, going
precisely where they are supposed to go. They make faint whistling
sounds that when apprehended in varying combinations are as pleasant as
the wind flying through a forest, and they do exactly as they are told. Of
this, one can be certain.
—MARK HELPRIN,
1
WINTER’S TALE
14.1 Preview
In Chapters 12 and 13, we examined the structural consequences of conjugation,
the overlap of 2p orbitals. This story culminates in the enormously stable aromatic
molecules, with the parent compound, benzene, being the prototype. In this chap-
ter, we will focus mostly on substitution reactions of aromatic compounds, an idea
introduced in Section 13.11a (p. 606).The chemistry of aromatic compounds is dom-
inated by a tendency to retain the stable aromatic sextet of electrons. It takes a great
deal of energy to destroy this arrangement, and it is very easily regained.
Because aromatic compounds are so stable,so low in energy, they are often sur-
rounded by especially high energy barriers, and the activation energies for their
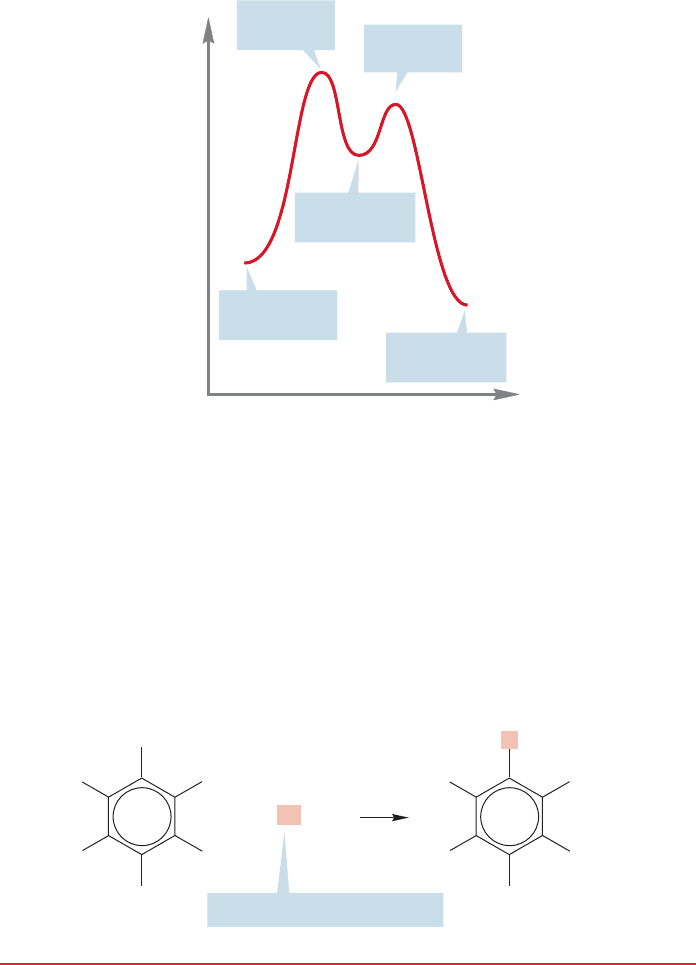
reactions are usually formidably large. The transformation of an aromatic com-
pound into a nonaromatic compound is most often highly endothermic (Fig. 14.1),
so the transition state lies close to the relatively high energy nonaromatic prod-
uct (recall the Hammond postulate,p.351). By contrast, the conversion of a nonaro-
matic compound into an aromatic molecule is likely to be very exothermic (Fig. 14.2).
1
Mark Helprin (b. 1947) is an American author. Among his wonderfully imaginative novels are Winter’s Tale,
A Soldier of the Great War, and Memoir from Antproof Case.
14.1 Preview 625
Energy
Reaction progress
Aromatic
compound 1
Aromatic
compound 2
Transition
state 2
Nonaromatic
intermediate
Transition
state 1
FIGURE 14.3 A typical Energy versus
Reaction progress diagram for the
conversion of one aromatic compound
into another. A combination of
Figures 14.1 and 14.2.
In such a case, the transition state will resemble the starting material. So, if we
again recall the Hammond postulate (and why it works), we will expect that only
small activation barriers will need to be surmounted in such reactions. A com-
plete energy diagram for a typical conversion of one aromatic compound into
another is shown in Figure 14.3. Notice that it is a combination of Figures 14.1
and 14.2.
An electrophile = Lewis acid
++
E
+
BHB
–
H
H
H
H
H
H
H
H
H
H
H
E
FIGURE 14.4 A generic aromatic
substitution reaction.
ESSENTIAL SKILLS AND DETAILS
1. The great thermodynamic and kinetic stability of benzenes results in reactions that
ultimately preserve the aromatic sextet of π electrons.The classic aromatic substitution
reaction, introduced in Chapter 13, is greatly elaborated here. Knowing the mechanism
of electrophilic aromatic substitution and manipulating the details in order to
synthesize polysubstituted compounds are the central tasks of this chapter.
In this chapter, we will see many examples of a general reaction of aromatic com-
pounds in which E
, an electrophile, a “lover of electrons” (a Lewis acid, which is
an electron-pair acceptor), substitutes for one of the hydrogens on a benzene ring.
Note that the aromatic sextet, here represented by the circle in the hexagon, is
retained in the overall reaction (Fig. 14.4). Later in this chapter,we take up the ques-
tion of how an initial substitution on the ring affects further reaction.Some groups
make a second substitution reaction easier, some make it harder.We begin with reac-
tions that are unusual for benzene rings, simple additions in which aromaticity
is lost.
626 CHAPTER 14 Substitution Reactions of Aromatic Compounds
Energy
H
2
Measured at
–28.6 kcal/mol
Delocalization or
resonance energy
=
–32.9 kcal/mol
Measured at
–49.3 kcal/mol
Estimated at
–82.2 kcal/mol
+
3 H
2
+
3 H
2
+
Energy of
cyclohexane
FIGURE 14.5 The determination of
the delocalization, or resonance
energy, of benzene. Note the
exaggerated short double bonds and
long single bonds in cyclohexatriene.
2. Some substituents increase the rate of a subsequent substitution and generally direct it
to the ortho and para positions. Other substituents slow the rate and direct further
substitution to the meta position. Understanding the dependence of substitution
position and rate on the structure of the initial substituent is essential.
3. The resonance-stabilized cyclohexadienyl cation is an intermediate in the central
reaction of this chapter. Be sure you understand why three—and only three—of the
carbons in the ring share the positive charge.
4. The nitrobenzene–aminobenzene (aniline) pair is one important key to doing aromatic
syntheses. Be sure you exit this chapter being able to interconvert these two compounds
and being able to use both nitrobenzene and aniline in synthesis.
5. Making bonds between carbon and the aromatic ring generally requires either
Friedel–Crafts alkylation or Friedel–Crafts acylation. There are subtle differences
between the two reactions, and you’ll need to know these differences to take advantage
of Friedel–Crafts reactions in synthesis.
14.2 Hydrogenation of Aromatic Compounds
If one were to try to predict the reactions of benzene from first principles, one would
probably start by examining the reactions of other π systems and applying them to
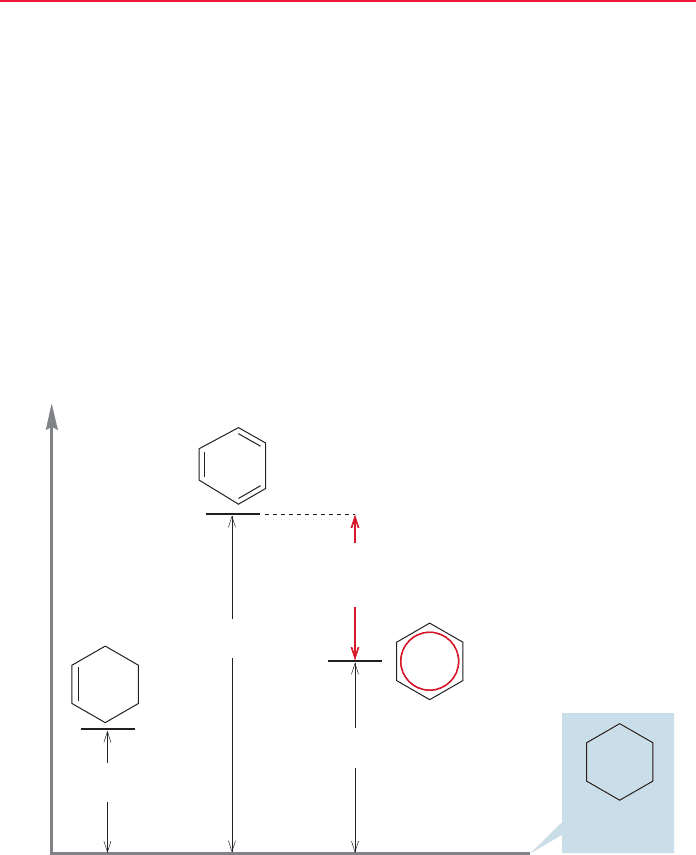
aromatic compounds. For example, we know that hydrogenation of cyclohexene is
exothermic by 28.6 kcal/mol (p. 580). It is no great surprise to find that absorption
of 3 mol of hydrogen by benzene to give cyclohexane is exothermic as well; indeed
we examined this reaction in some detail in Chapter 13.Although the reaction is very
exothermic,it is far less exothermic than a guess based on the value of 28.6 kcal/mol
for a simple alkene would suggest. The difference between the estimated value and
the measured value was taken as a measure of the delocalization energy of benzene,
approximately 33 kcal/mol (Fig. 14.5).
Often, conditions under which alkenes are swiftly transformed into alkanes are quite
without effect on benzene and other simple aromatic compounds (Fig. 14.6a). Indeed,
benzene can be used as solvent in many hydrogenation reactions. We can eventually
14.2 Hydrogenation of Aromatic Compounds 627
H
2
5% Pd/C
25 ⬚C, H
2
Rh/ Pt oxides,
CH
3
CH
2
OH
OHOH
(100%)
(b)
+
+
(a)
FIGURE 14.6 (a) Benzene does not hydrogenate under the same conditions as alkenes.
(b) More active catalysts can be used to hydrogenate the aromatic ring.
make the reaction occur by using high temperature and pressure, or by using especially
active catalysts, such as rhodium, ruthenium or platinum oxide (Fig. 14.6b).
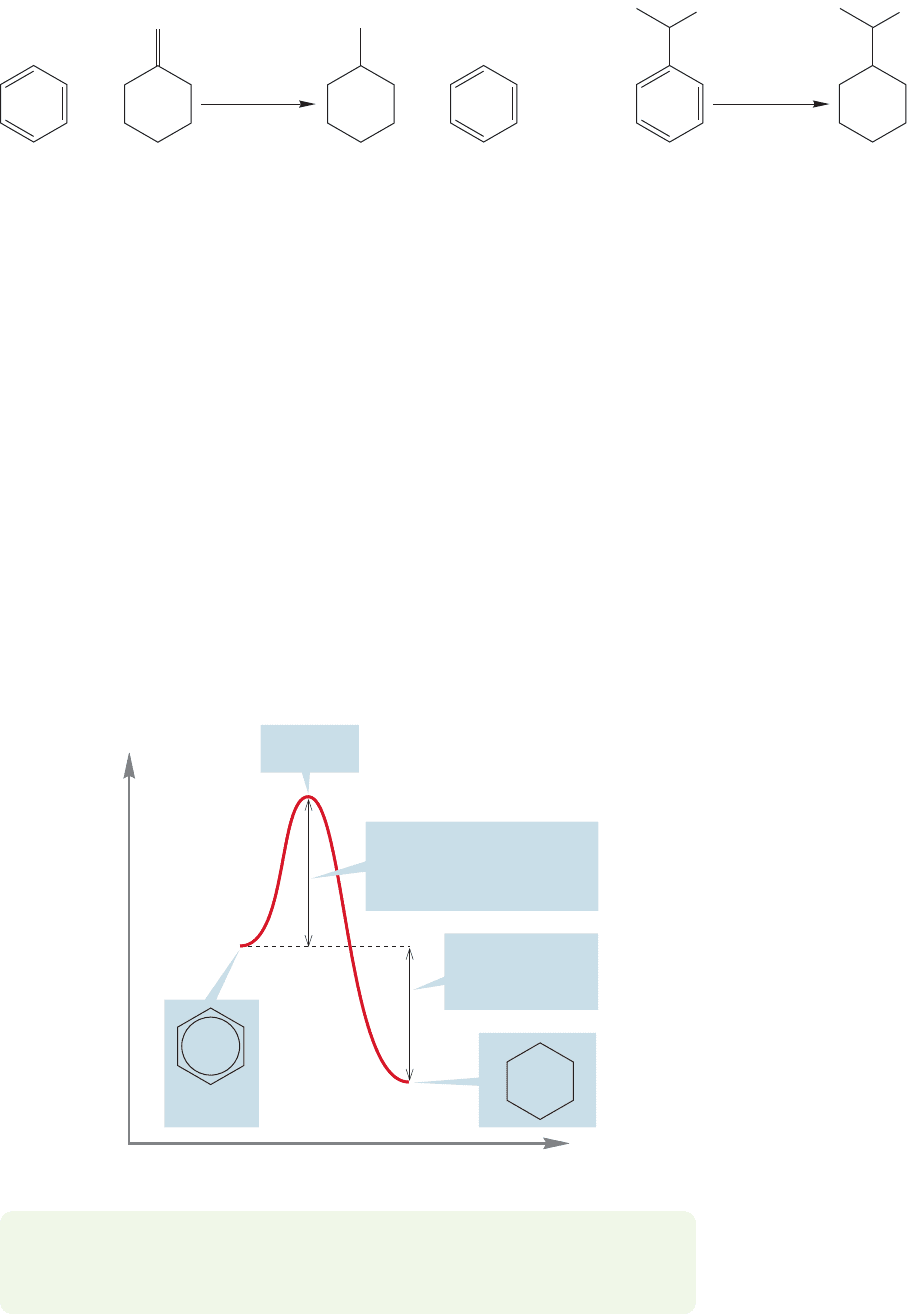
The hydrogenation reaction is highly exothermic, by 49.3 kcal/mol in the case
of the parent benzene. Even though the combination of benzene and three hydro-
gen molecules is higher in energy than cyclohexane by a substantial amount, ben-
zene is protected by high activation barriers. Heat, pressure, and/or special catalysts
are needed to help overcome these barriers. Benzene rests in a valley, separated from
other valleys—even lower energy valleys—by especially high mountains.
In the language of chemistry, we say that benzene is both thermodynamically
and kinetically very stable. The valley containing benzene by itself is low (thermo-
dynamics), and the mountains surrounding it (the activation barriers, kinetics) are
very high. Benzene is even protected by these barriers from undergoing reactions
that are, ultimately, exothermic.The example of benzene plus hydrogen nicely illus-
trates this point, as the combination of these molecules lies higher in energy than
the product of hydrogenation, cyclohexane (Fig. 14.7).
Energy
Reaction progress
Transition
state
Activation energy; this high
barrier prevents formation
of cyclohexane under usual
conditions
Cyclohexane is
more stable than
benzene 3 H
2
+
3 H
2
+
FIGURE 14.7 The activation energy
barrier for cyclohexane formation is
high and makes this reaction difficult
under normal conditions.
PROBLEM 14.1 Explain why the only product of hydrogenation of benzene is
cyclohexane. Cyclohexadienes and cyclohexene must be formed first. Why can
they never be isolated, even if the reaction is interrupted before completion?
628 CHAPTER 14 Substitution Reactions of Aromatic Compounds
200 ⬚C
CH
3
CH
3
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
CF
3
CF
3
(40%)
CF
3
CF
3
CH
3
WEB 3D
FIGURE 14.8 Benzenes will undergo
the Diels–Alder reaction, but only
with reactive dienophiles under
forcing conditions.
Energy
Reaction progress
Higher activation
energy for benzene
Lower activation energy
for a destabilized benzene
Benzene Dienophile
+
Destabilized
benzene
+
dienophile
Diels–Alder
adduct to
benzene
FIGURE 14.9 If benzene is
destabilized, the energy barrier for
reaction should be reduced.
(CH
2
)
n
[n ]Paracyclophanes
For large n, the ring
can be flat
A small n forces the
ring into a boat form
(CH
2
)
n
FIGURE 14.10 1,4-Bridged benzenes:
[n]paracyclophanes.
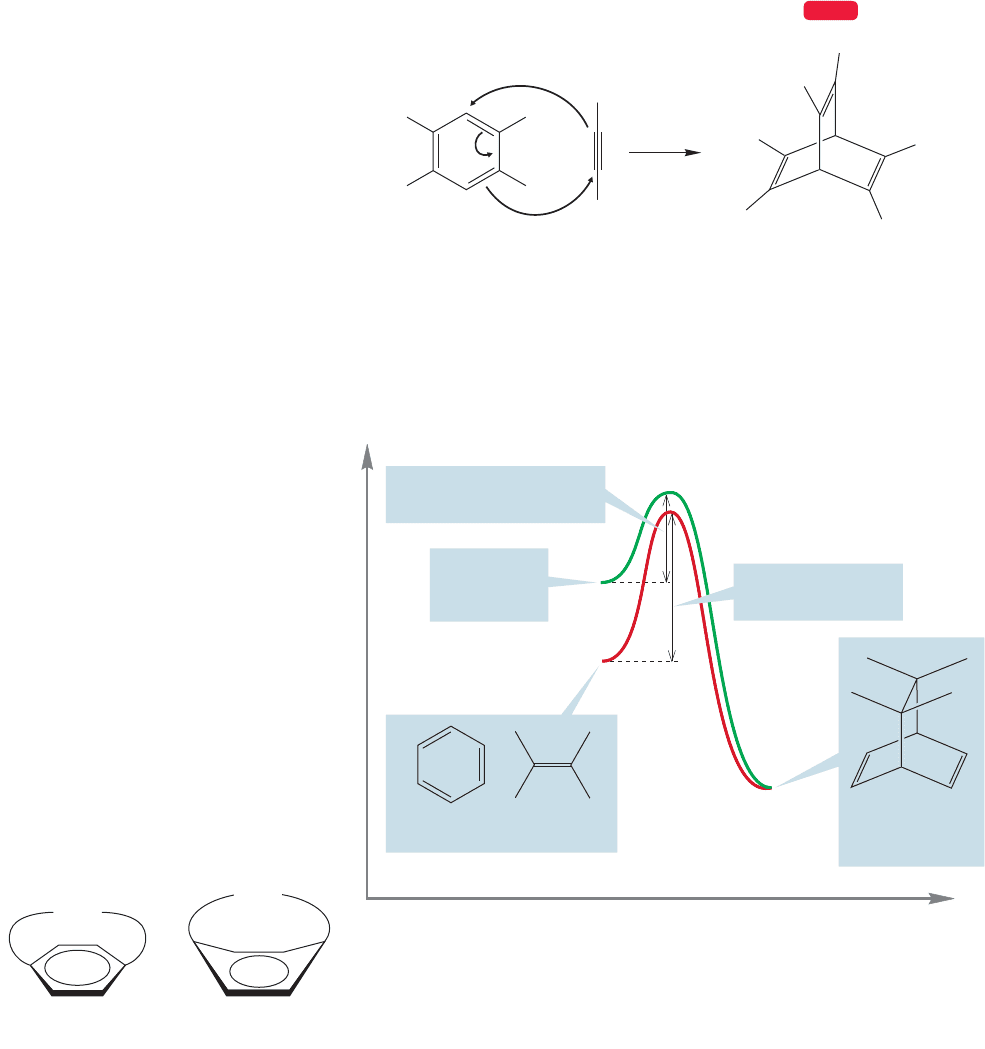
To see if all this makes sense, let’s try a thought experiment. Suppose we were
set the task of finding a way to make the Diels–Alder reaction possible for benzenes.
We might first try to use as reactive (high energy) a dienophile as possible, as in
Figure 14.8; but there is another way. We might try instead to destabilize the ben-
zene so that surmounting the activation energy barriers for Diels–Alder reaction
becomes easier. Figure 14.9 shows this idea in graphic form.
There are several nice practical examples of this idea. Imagine a benzene
ring bridged by a chain of methylene groups between the 1- and 4-positions
(Fig.14.10).Such a molecule is called an [n]paracyclophane,where n gives the
number of methylene groups in the bridge. As long as there are enough meth-
ylene groups (more than 7) the molecule behaves like a simple benzene. But as
n diminishes (molecules with n 5, 6, and 7 are known and the n 4 isomer
14.3 Diels–Alder Reactions
Benzene and other aromatic compounds contain a conjugated π system. Such mol-
ecules are known to undergo the Diels–Alder reaction (p. 544). Yet, it is extremely
difficult to induce the Diels–Alder reaction for simple benzenes,and yields are often
poor. Harsh conditions (temperature as high as 200 °C, for example) and very reactive
dienophiles such as hexafluoro-2-butyne are necessary (Fig. 14.8). The reason for
the lack of reactivity is simple: Benzene is too stable (lies in a deep energy well) and
the activation energies for this reaction are significant.
