Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

14.7 Synthetic Reactions We Can Do So Far 649
(67%)
CH
3
CH
3
CN
Br
NO
2
NO
2
N
2
Cl
H
3
C
O
+
N
2
+
N
2
+
CuCN
0 ⬚C, 5 h
CuBr
water/
acetone
–25 ⬚C
CuCl
CH
3
CN,
65 ⬚C
(90%)
(98%)
H
3
C
O
FIGURE 14.54 Three Sandmeyer reactions. Decomposition of the diazonium ion with cuprous salts leads
to substitution.
Reagents other than cuprous salts are also effective. Figure 14.55 shows four
such reactions. Treatment with potassium iodide (KI) or fluoboric acid (HBF
4
)
gives the iodide or fluoride, respectively (the Schiemann reaction; G. Schiemann
1899–1969), and hydrolysis gives phenols (p. 231). Heating with hypophosphorous
acid (H
3
PO
2
) removes the diazonium group and replaces it with hydrogen.
The diazonium ion is important because it is the critical intermediate in a num-
ber of transformations lumped under the heading of the Sandmeyer reaction,named
for Traugett Sandmeyer (1854–1922). Cuprous salts convert a diazonium ion into
the related cyanide or halide.In recent times, numerous variations on the basic theme
of the Sandmeyer reaction have improved yields and reduced side products.Figure 14.54
shows three typical Sandmeyer reactions.
(75%)
(85%)
N
2
+
N
2
+
I
N
2
+
F
F
KI
25–100 ⬚C
NO
2
+
NO
2
H
2
SO
4
H
2
O
100 ⬚C
(80%)
N
2
+
CH
3
CH
3
H
3
PO
2
excess
HBF
4
0 ⬚C
Schiemann reaction
(68%)
N
2
OH
FIGURE 14.55 Other substitution
reactions of diazonium ions.
650 CHAPTER 14 Substitution Reactions of Aromatic Compounds
+
CuCl
–
+
HCl H CuCl
2
Dichlorocuprate
ion
FIGURE 14.56 Formation of the
dichlorocuprate ion from CuCl
and HCl.
+
–
CuCl
2
+
.
CuCl
2
+
N
2
+
CuCl
2
+
CuCl
.
.
.
N
2
N
2
Cl
Dichlorocuprate
ion
Benzenediazonium
ion
Phenyl
radical
Chlorobenzene
FIGURE 14.57 The mechanism of chlorobenzene formation.
PROBLEM 14.16 We reject out of hand the notion that any of these net displace-
ment reactions could go by something as simple as an S
N
2 reaction. Why?
+
S
N
1
BF
4
–
N
2
N
2
+
F
Phenyl
cation
Fluorobenzene
FIGURE 14.58 Some reactions of
benzenediazonium chloride involve
a phenyl cation.
Some Sandmeyer reactions apparently involve a phenyl cation formed by the irre-
versible S
N
1 ionization of benzenediazonium chloride (Fig. 14.58).
In the Sandmeyer reactions involving copper, electron-transfer-mediated radi-
cal reactions are certainly involved. In HCl, cuprous chloride (CuCl) is in equilib-
rium with the dichlorocuprate ion
CuCl
2
(Fig.14.56).The dichlorocuprate ion can
transfer an electron to the diazonium ion, which can then lose molecular nitrogen
to give a phenyl radical. In turn, the phenyl radical can pluck a chlorine atom from
a copper chloride molecule to give the chlorobenzene and a molecule of cuprous
chloride.The mechanism for the formation of chlorobenzene in the Sandmeyer reac-
tion is shown in Figure 14.57.
Of course,you are now clamoring for the mechanisms for all these changes. Sadly,
only the outlines of the mechanisms are known, and much remains to be filled in.
The details of many reactions are imperfectly understood,and there remains a delight-
ful (to some) atmosphere of magic about parts of organic chemistry. What are we to
make of the admonition in one procedure for the Sandmeyer reaction to “stir the mix-
ture with a lead rod.”What happens if we use a glass rod? Although one would think
that the reaction might still succeed, there are examples to the contrary, and perhaps
microscopic pieces of lead do affect the success of the reaction. We’d use lead.
3
3
Speaking of lead and magic, two of MJ’s chemical relatives, Milton Farber (b.1925) and Adnan Abdul-rida
Sayigh (~1922–1980), once discovered a useful reaction of lead dioxide, and the work was duly published as
an effective way to make alkenes from 1,2-diacids. However, the reaction only worked when one particular
bottle of lead oxide was used, and once that bottle was used up no one could reproduce the yields achieved
earlier. All other sources of lead dioxide failed! Apparently, the particle size of the lead dioxide was critical.
(What’s a “chemical relation”? That’s someone who did his or her graduate work with the same professor as
you. Farber and Sayigh both worked with the same person as MJ and hence are his chemical brothers.)
14.7 Synthetic Reactions We Can Do So Far 651
NaNO
2
/HCl
+
An azo compound
N
2
+
NH
2
N
N
NH
2
WEB 3D
It doesn’t matter that some mechanistic details are sketchy; the formation of the
diazonium ion from aniline (and therefore ultimately from the easily made nitroben-
zene) and its further transformations give you the ability to make a great number
of substituted benzenes from very simple precursors.
PROBLEM 14.19 Suggest synthetic procedures leading to each of these compounds.
You may start with benzene, any inorganic reagent, and organic compounds con-
taining no more than four carbon atoms.
(a)
SO
2
OH
(e)
CH
2
CH
2
CH
2
CH
3
(f)
NH
2
(g)
F
(h)
I
(i)
OH
(b)
C(CH
3
)
3
(c)
Cl
(d)
O
CCH
2
CH
2
CH
3
PROBLEM 14.20 Phenols made by the Sandmeyer reaction can be used further
in synthesis. First, explain why phenol (pK
a
10) is a much stronger acid than
cyclohexanol (pK
a
16).
PROBLEM 14.17 Draw resonance forms for the phenyl radical, Ph (be careful).
PROBLEM 14.18 In the formation of diazonium ions from aromatic amines, side
products called azo compounds are occasionally encountered, as shown below.
Give a mechanism for the formation of these compounds.
#
652 CHAPTER 14 Substitution Reactions of Aromatic Compounds
NaH
0 ⬚C, THF
Br
27 ⬚C, CH
3
OCH
2
CH
2
OCH
3
Phenoxide 3-Phenoxypropene
(allyl phenyl ether)
(100%)
Phenol
OH
O
–
O
PROBLEM 14.21 Aryl ethers can be made by the following reaction. Write an
arrow formalism mechanism for each step.
Pyridine Pyrrole
N
..
..
N
..
N
..
N
2-Substitution 3-Substitution 4-Substitution
2-Substitution 3-Substitution
H H
..
N
H
..
N
..
N
E
+
E
+
E
E
E
E
E
5
6
3
4
1
2
34
25
1
WEB 3DWEB 3D
FIGURE 14.59 Possible positions for electrophilic aromatic substitution in pyridine and pyrrole.
PROBLEM 14.22 A side product can be formed in the reaction in Problem
14.21, depending on the conditions. Explain the formation of the compound
A shown below in the reaction of phenoxide with allyl bromide. You will need
the additional information that in basic conditions ketones are transformed
into enolates and, after protonation, enols.
OH
O
1. base
2. protonation
A Ketone Enol
OH
14.8 Electrophilic Aromatic Substitution
of Heteroaromatic Compounds
Although heteroaromatic compounds generally have lower resonance energies than
benzene (Table 14.1, p. 629), they also undergo electrophilic aromatic substitution
rather than addition.
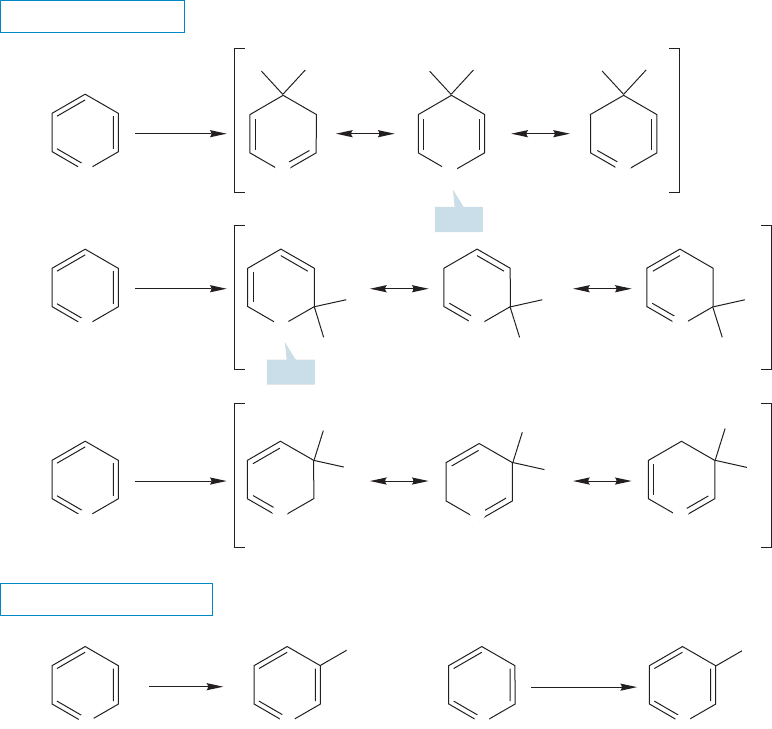
14.8a Reactions of Pyridine and Pyrrole The heteroatom introduces a
complication in aromatic substitution not present in benzene. Benzene has six
equivalent positions, but substitution could occur at two positions in pyrrole and
three in pyridine (Fig. 14.59).
14.8 Electrophilic Aromatic Substitution of Heteroaromatic Compounds 653
GENERAL CASES
SPECIFIC EXAMPLES
N
..
E
+
4-Position
2-Position
3-Position
+
+
+
..
N
N
E
H
E
H
E
H
..
..
N
E
H
E
H
E
H
N
..
E
+
+
..
N
..
N
+ +
E
H
N
..
N
..
N
Cl
..
E
+
..
N
E
H
..
N
Favored: No
+
on N!
+
+
E
H
+
Cl
2
AlCl
3
(30%)
(90%)
N
..
N
Br
..
Br
2
H
2
SO
4
/SO
3
..
N
..
N
Bad!
Bad!
FIGURE 14.60 In pyridine, substitution at the 3-position is favored, although the reaction is
slower than with benzene.
As with benzene, electrophilic attack on pyridine gives an intermediate in which
three atoms share the positive charge (Fig. 14.60). Substitution at the 2- or 4-position
(ortho or para to the nitrogen in pyridine) places a partial positive charge on the
relatively electronegative nitrogen atom. Substitution at the 3-position (meta to the
nitrogen) does not and is accordingly favored.
This rate-diminishing effect is accentuated if the nitrogen is protonated, as it
very often is under the strongly acid conditions necessary to carry out substitution
on pyridine. Therefore it is not surprising that substitution of pyridine takes place
more slowly than analogous reactions involving benzene. Pyridine itself substitutes
about as rapidly as nitrobenzene, 10
6
times more slowly than benzene. The pyri-
dinium ion substitutes an impressive 10
18
times more slowly than benzene.
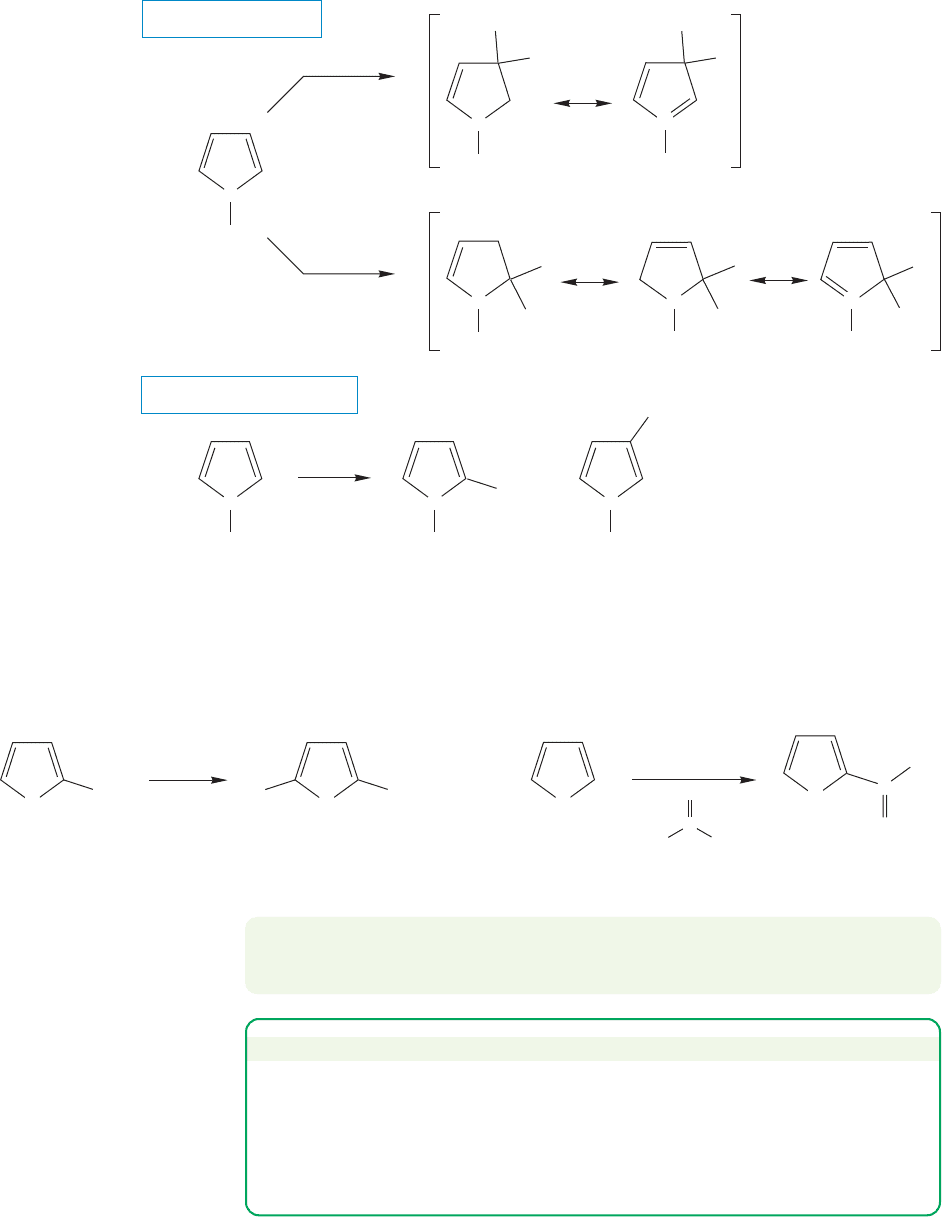
A similar analysis serves to locate the more favorable position for substitution
on pyrrole. Addition at either carbon 2 or 3 (Fig. 14.59) allows the nitrogen to help
bear the positive charge. However, in the intermediate produced by substitution at
carbon 2, two ring carbons share the charge with the nitrogen, whereas substitution
at carbon 3 leads to an intermediate in which only one carbon shares the charge with
654 CHAPTER 14 Substitution Reactions of Aromatic Compounds
..
N
H
..
N
..
N
..
N
H
E
+
+
..
N
H
H H H
+
attack at
C(3)
E
+
attack at
C(2)
E
H
N
H
+
E
H
..
N
H
+
EE
H
H
N
H
+
E
H
HNO
3
Ac
2
O
NO
2
..
N
NO
2
(80%) (20%)
+
A SPECIFIC EXAMPLE
GENERAL CASES
FIGURE 14.61 In pyrrole,
substitution at the 2-position
is favored.
Cl
2
C
ThiopheneFurfural
..
..
O
O
..
..
..
..
..
O
CH
3
AlCl
3
..
..
S
(90%)
(43%)
S
..
..
CHO
O
..
CHO
Cl
..
..
..
..
..
..
H
3
C
Cl
C
FIGURE 14.62 Electrophilic substitution reactions of furan and thiophene.
PROBLEM 14.23 Explain why 2-substitution is preferred to 3-substitution in
electrophilic aromatic substitution of furan.
the nitrogen. Addition to the 2-position is favored, although the formation of mix-
tures is commonly observed (Fig. 14.61).
Pyrrole is more reactive than benzene or pyridine,because pyrrole is less well sta-
bilized by resonance than the six-membered ring molecules (Table 14.1).
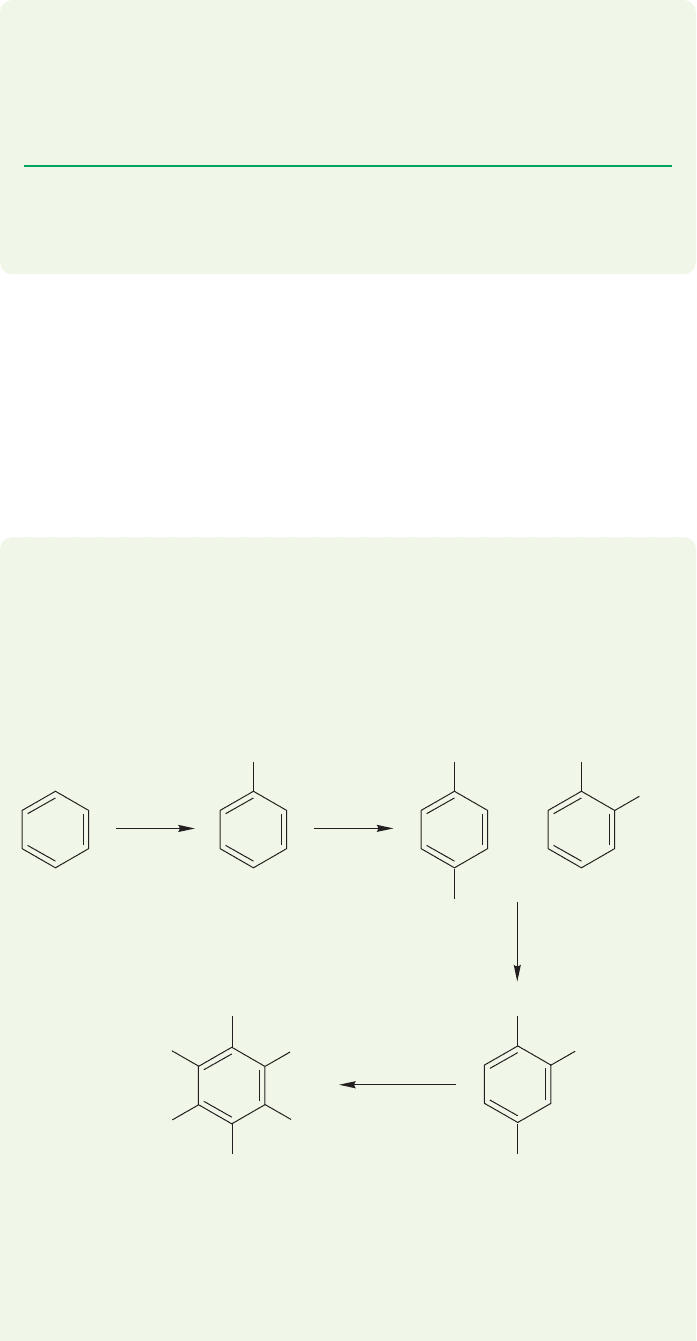
14.8b Reactions of Furan and Thiophene These compounds are very
reactive toward electrophiles, E
. As in pyrrole, aromatic substitution is directed
first to the 2-position (Fig. 14.62), although mixtures are sometimes obtained in
which some 3-substitution appears.
PROBLEM SOLVING
All problems of this kind ask you to make comparisons, but they usually require
you to write mechanisms so that you can see the molecules that must be
compared. It may seem obvious, but attacking such problems is best done by
carefully writing out the mechanisms in parallel so that you can see the possible
points of comparison. Don’t just think, write.
14.9 Disubstituted Benzenes: Ortho, Meta, and Para Substitution 655
PROBLEM 14.24 In addition to the mechanism following the path shown for
pyrrole in Figure 14.61, there is another reasonable mechanism for aromatic
substitution of furan. Suggest one. Hint: Think simple! What are the possible
reactions between electrophiles and double bonds (Chapters 9 and 10)? Use the
chlorination of furan as an example.
PROBLEM 14.25 There is a difficulty with the answer to Problem 14.24. Find what
is wrong (or unlikely) and suggest an alternative mechanism. Hint: Recall what you
know of the stereoelectronic requirements of the E2 reaction (Section 7.9, p. 301).
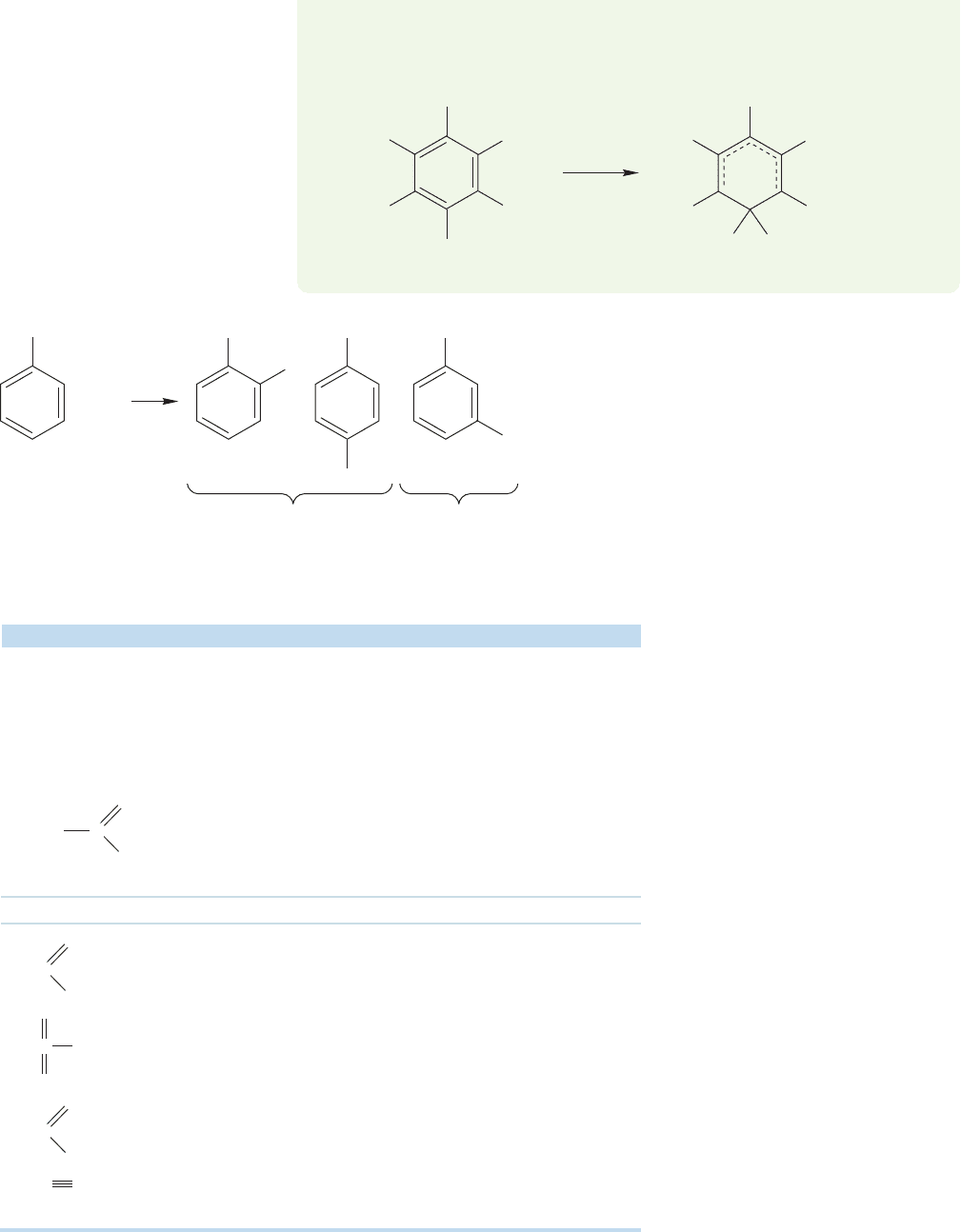
14.9 Disubstituted Benzenes: Ortho, Meta,
and Para Substitution
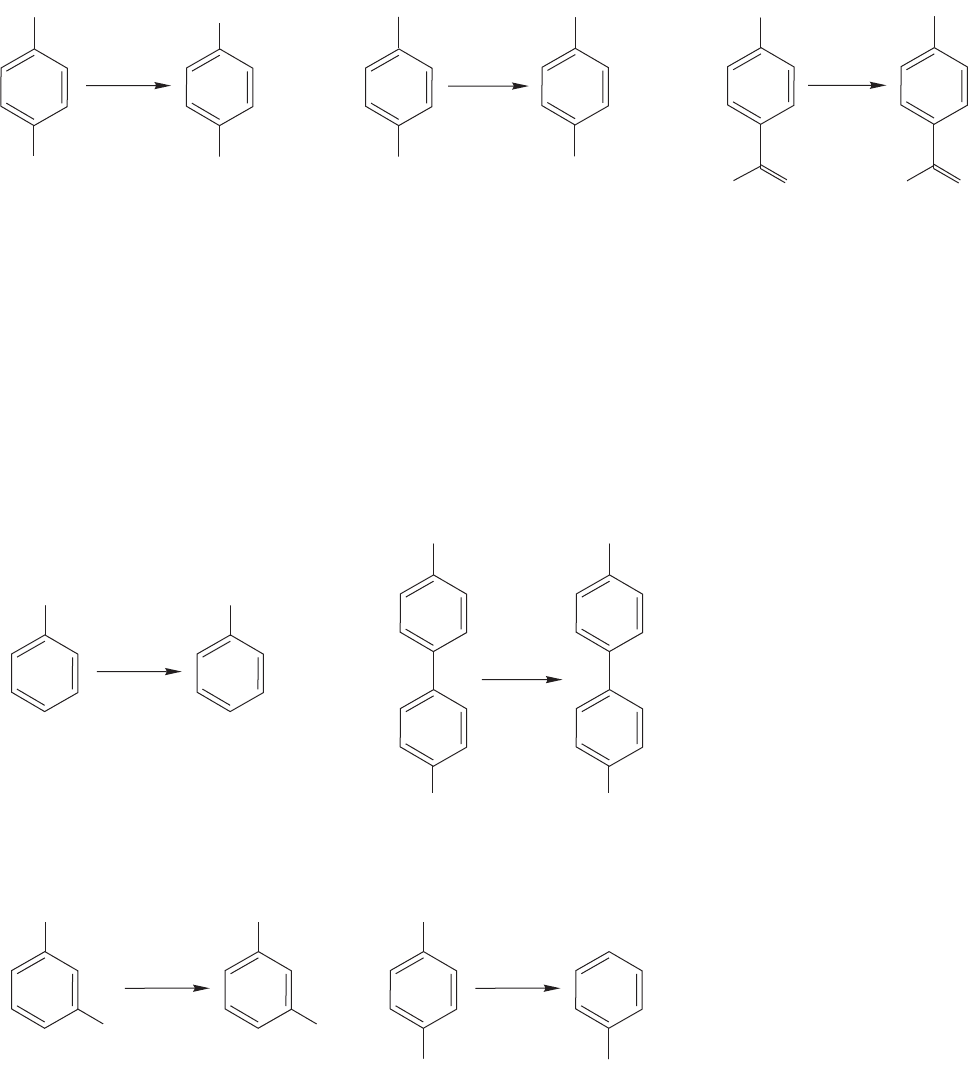
We have already mentioned (Problem 14.9) that the synthesis of toluene from benzene
is made difficult because once a significant amount of toluene is formed, it competes
successfully with the unsubstituted benzene in further methylation reactions.Unless one
is careful to use a vast excess of benzene, the result is a horrible mixture of benzene,
toluene, dimethylbenzenes (xylenes), and even tri- and tetra-substituted molecules.
CH
3
Cl
CH
3
CH
3
CH
3
CH
3
H
3
C
H
3
C
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
AlCl
3
CH
3
Cl
AlCl
3
+
CH
3
Cl
AlCl
3
repeat
three times
Toluene
Xylenes
Hexamethylbenzene
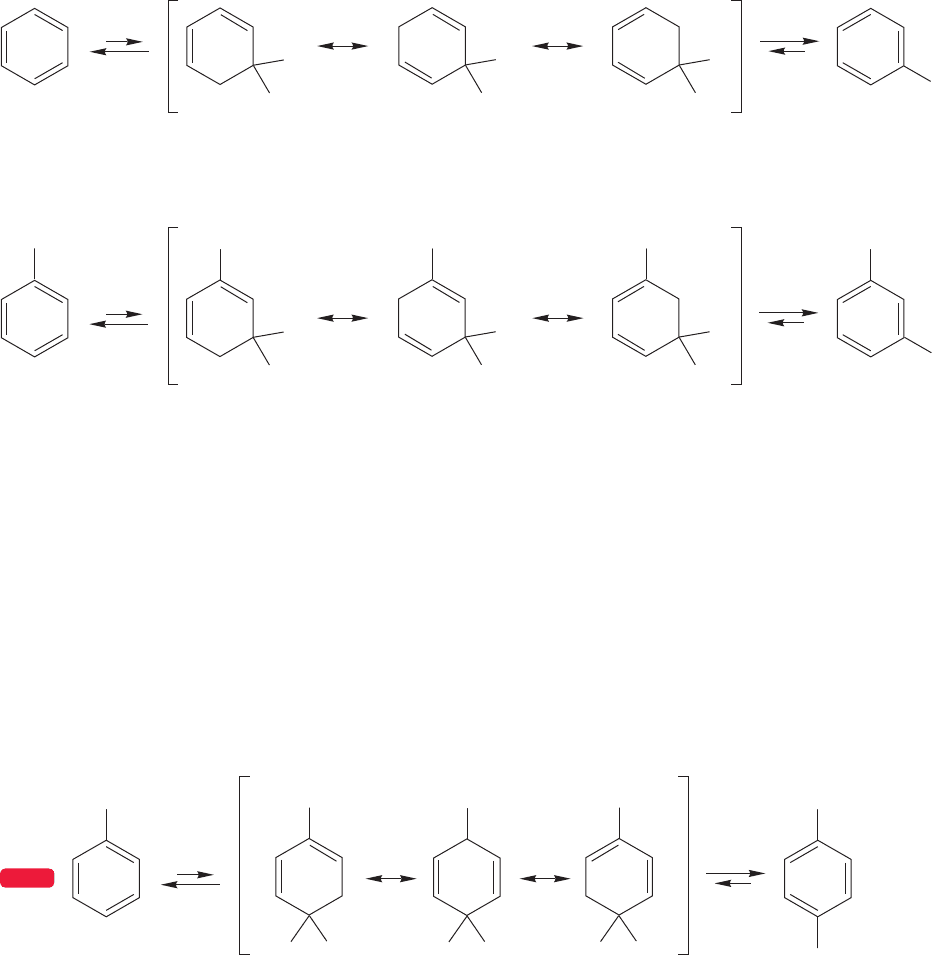
WORKED PROBLEM 14.26 If one attempts to methylate benzene completely with
CH
3
Cl/AlCl
3
, the product is a greenish solid of the formula C
13
H
21
AlCl
4
.
Propose a structure for the compound and a mechanism for its formation.
ANSWER This problem involves a sequence of Friedel–Crafts alkylations.Toluene
is made first, then the xylenes, then trimethylbenzenes, and so on, until hexa-
methylbenzene is formed.
What happens if another Friedel–Crafts reaction occurs? The product is the
heptamethylcyclohexadienyl cation tetrachloroaluminate salt, and this material
is stable under the reaction conditions. Why? The resonance forms contributing
(continued)
656 CHAPTER 14 Substitution Reactions of Aromatic Compounds
The general question now arises of how the
substituted benzenes we have learned to make will
react with electrophilic reagents. What are the
possibilities? There are three isomers of disubsti-
tuted benzene, ortho, meta, and para, as shown in
Figure 14.63.
Substituents influence the distribution of the
three isomeric disubstituted products dramati-
cally. Further substitution of monosubstituted
benzenes by no means proceeds to give a statisti-
cal distribution of two parts ortho
product, two parts meta product,
and one part para product. In
fact, if one looks at the reactions
of a number of monosubstituted
benzenes, a pattern emerges. Some
groups (G) substitute predominant-
ly ortho and para, while for other
groups meta is dominant. There
is also a correlation between the
position of further substitution
and the rate of the reaction.
Monosubstituted benzenes that
give mainly ortho and para prod-
ucts usually react faster than ben-
zene. Monosubstituted benzenes
that give mainly meta product
react more slowly than benzene
(Table 14.2).
A look at the general mecha-
nism of substitution shows how
different groups influence the posi-
tion of subsequent reaction. We
can also see how these orientation
(regiochemical) effects are con-
nected to the rates of the reactions.
Let’s look first at methoxybenzene
(anisole). Methoxy (CH
3
O) is one
TABLE 14.2 Patterns of Further Reactions of Substituted Benzenes
G Position
a
o/p
Relative Rate
Very fast
o/p
Very fast
o/p
Very fast
o/p
Fast
m
Slow
m
a
Ortho = o, para = p, meta = m.
Slow
o/p
Fast
1
NH
2
, NHR, NR
2
OH
OR
R (alkyl)
o/p
Slow
I, Cl, Br, F
H (benzene)
(R´ = OH, OR, NH2,
Cl, alkyl, aryl)
NH C
O
R´
C
O
R´
m
Slow
O
SOH
O
m
Slow
CN
+
NR
3
m
Slow
N
O
O
+
–
ortho + para
G
G
E
G
E
G
E
+
+
E
meta
FIGURE 14.63 The three possible disubstituted benzenes.
to the structure are all tertiary carbocations, and there is no proton that can
be lost to give a new aromatic system. All positions are occupied by methyl
groups.
CH
3
CH
3
CH
3
CH
3
H
3
C
H
3
C
CH
3
CH
3
CH
3
H
3
C
H
3
C
H
3
C
CH
3
+
CH
3
Cl
AlCl
3
AlCl
4
–
C
13
H
21
AlCl
4
14.9 Disubstituted Benzenes: Ortho, Meta, and Para Substitution 657
OCH
3
OCH
3
E
E
H
+
E
H
+
E
H
+
OCH
3
OCH
3
OCH
3
E
+
Meta substitution of anisole
Anisole
Substitution of benzene
E
+
E
E
H
+
E
H
+
E
H
+
Benzene
FIGURE 14.64 The intermediate cyclohexadienyl cations involved in the substitution of benzene and
the meta substitution of anisole.
of the groups most effective at directing further substitution to the ortho and
para positions and at increasing the rate of reaction. We will work through the
mechanism for meta and para substitution and look for a point of difference.
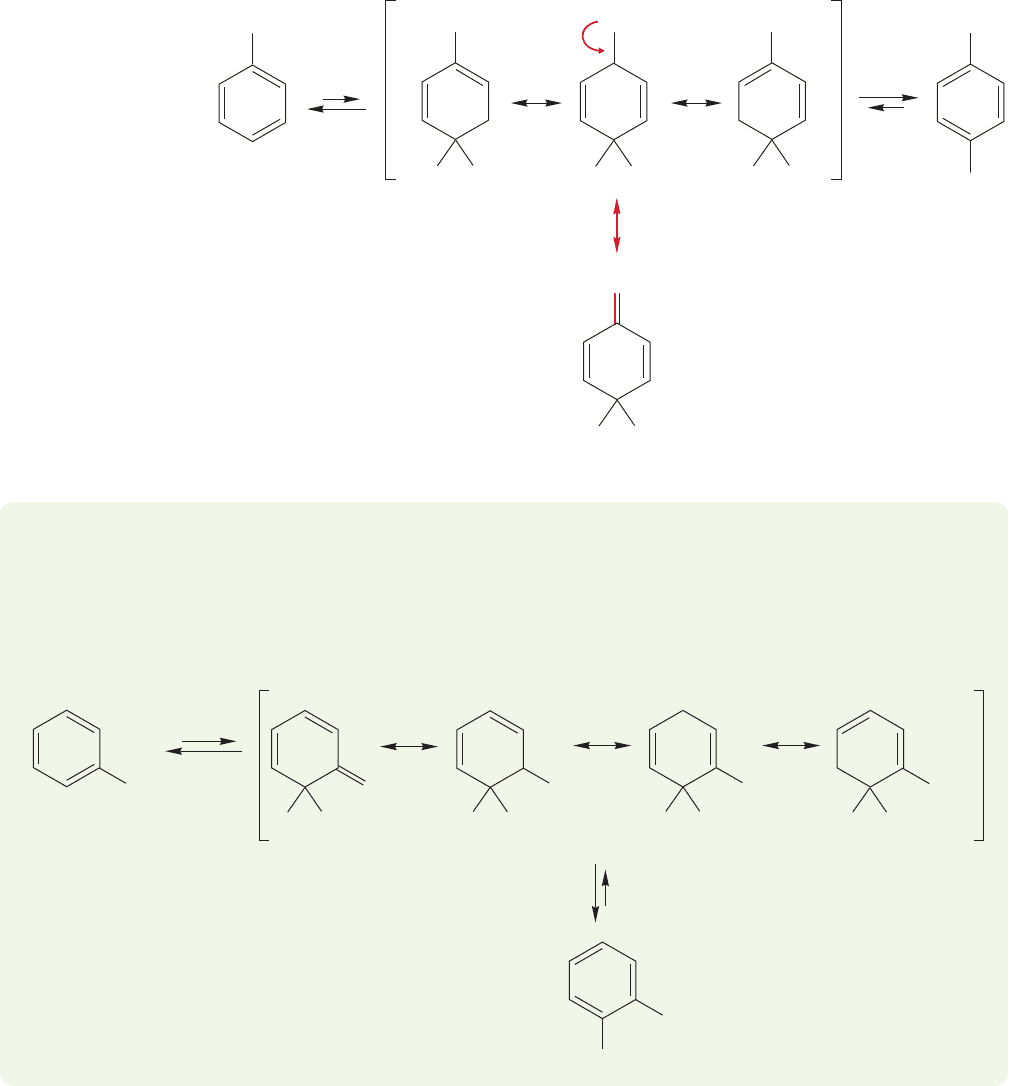
Figure 14.64 shows the mechanism for meta substitution of anisole by a generic
E
reagent, and compares the reaction to that of benzene. As usual, a resonance-
stabilized cyclohexadienyl cation is formed first, and then deprotonated to give
the substituted product.
At first glance, substitution at the para position of anisole seems similar to
meta substitution (Fig. 14.65). But look carefully at the intermediate in para
substitution and remember our discussion of the stabilization of carbocations that
are adjacent to an oxygen (p. 378). In the intermediate from para substitution,
there is a fourth resonance form in which oxygen shares the positive charge
OCH
3
OCH
3
E
+
Para substitution of anisole
Anisole
E
H
+
OCH
3
E
H
+
OCH
3
E
H
+
OCH
3
E
WEB 3D
FIGURE 14.65 The intermediate cyclohexadienyl cation involved in para substitution
of anisole.
658 CHAPTER 14 Substitution Reactions of Aromatic Compounds
OCH
3
OCH
3
E
+
Anisole
EH
+
OCH
3
EH
+
OCH
3
EH
+
OCH
3
..
..
..
..
..
..
..
..
..
..
E
H
OCH
3
..
+
There is another resonance form
for this intermediate; here it is!
E
FIGURE 14.66 In the intermediate for
para substitution of anisole, there is a
fourth resonance form in which
oxygen bears the positive charge.
(Fig. 14.66). This fourth form stabilizes the cation relative to that formed in the
meta substitution or in the substitution of benzene.
WORKED PROBLEM 14.27 Draw the resonance structures for the intermediate formed
by substitution of anisole at the ortho position.
ANSWER The figure shows the four resonance forms of the cyclohexadienyl cation
intermediate. As in the para case, the methoxy group is in a position to help share the
positive charge.
OCH
3
..
..
+
E
+
E
H
OCH
3
..
+
E
H
OCH
3
..
..
+
E
H
OCH
3
..
+
E
H
OCH
3
..
..
..
E
OCH
3
..
..
deprotonate
Because the intermediate formed in para (or ortho) substitution of anisole is more
stable than that formed in meta substitution of anisole or in substitution of benzene,
the transition state leading to the para (or ortho) intermediate is more stable than
the transition states for meta substitution of anisole or substitution of benzene itself.
