Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

13.11 Introduction to the Chemistry of Benzene 609
The mechanism of the Birch reduction is similar to that for dissolving metal
reduction of alkynes (p. 452), and starts in the same way, with a transfer of an
electron from the metal to one of the empty antibonding orbitals of benzene. The
product is a resonance-stabilized radical anion (Fig. 13.67).
Na/NH
3
A resonance-stabilized radical anion
etc.
.
.
..
.
..
–
–
FIGURE 13.67 Transfer of an electron
from sodium to benzene leads to a
resonance-stabilized radical anion.
Protonation by alcohol gives a radical that can undergo a second electron
transfer–protonation sequence to complete the reduction (Fig. 13.68).
A resonance-stabilized radical
.
.
..
.
..
..
..
–
–
––
–
–
+
H OEt
H
H
H
H
H
H
..
..
..
OEt
A resonance-stabilized anion
+
H
H
H
H
H
H
H
H
H
H
..
..
..
..
..
..
OEt
Na
..
..
EtO
H
FIGURE 13.68 The radical anion is
protonated to give a resonance-
stabilized cyclohexadienyl radical
that goes on to produce the
1,4-cyclohexadiene through another
reduction–protonation sequence
(Et CH
2
CH
3
).
Substituted benzenes can also be reduced using these conditions. Electron-
releasing groups such as alkyl or alkoxy always wind up on one of the double bonds,
but electron-withdrawing groups, such as a carboxylic acid (COOH), invariably
appear on the methylene positions of the product dienes (Fig. 13.69).
CH
3
O CH
3
O
1. Na/NH
3
/EtOH
2. H
2
O/H
3
O
COOH COOH
1. Na/NH
3
/EtOH
2. H
2
O/H
3
O
+
+
FIGURE 13.69 In the Birch reduction
of substituted benzenes, electron-
releasing groups appear on one of
the double bonds, but electron-
withdrawing groups are located at
the methylene position.

610 CHAPTER 13 Conjugation and Aromaticity
13.12 The Benzyl Group and Its Reactivity
Thus far we have been looking at processes directly involving the benzene ring of
an aromatic compound. Here we sketch out some reactions in which benzene plays
a major role, but in which the action takes place on the outskirts of the ring. The
carbon adjacent to a benzene ring is called the benzylic carbon,or the benzylic posi-
tion. Remember from p. 596, the benzyl group is . Any group attached
to a benzylic carbon is referred to as being in a benzylic position. In many ways, the
reactivity of the benzylic position resembles that of the allylic position. Indeed, an
orbital at the benzylic position is stabilized by overlap with adjacent orbitals in much
the same way as is an orbital at the allylic position (Fig. 13.70).
Ph
O
CH
2
13.12a Substitution Reactions of Benzyl Compounds Benzyl compounds
are unusually active in both the S
N
1 and S
N
2 reactions. Clearly, the benzene ring
helps enormously to stabilize the carbocation formed in the rate-determining ioniza-
tion of an S
N
1 reaction. For example, the benzylic chloride in Figure 13.71 reacts
more than 100,000 times faster than isopropyl chloride in a typical S
N
1 process.
Both involve secondary cations as intermediates, but the secondary cation that is also
benzylic is significantly more stable, as is the transition state leading to it.
The benzyl group
H
H
=
The benzylic position The benzylic position
C
H
H
The allyl group
=
H
2
C
CH
2
CH
2
H
C
H
C
The allylic position
FIGURE 13.70 The position
adjacent to a benzene ring is quite
analogous to the allylic position.
CH
CH
3
CH
3
Cl
EtOH
OEt
Relative rate = 1.37 ⫻ 10
5
Resonance-stabilized
benzylic cation
Relative rate = 1
25 ⬚C
1. EtOH
2. deprotonate
EtOH
25 ⬚C
H
3
C
H
3
C
HC Cl
H
3
C
H
3
C
HC OEt
H
3
C
H
3
C
HC
+
1. EtOH
2. deprotonate
CH
H
CH
3
C
+
FIGURE 13.71 The resonance stabilization of the benzylic cation makes its formation relatively easy.
Accordingly, S
N
1 reactions of benzylic compounds containing good leaving groups are quite fast.
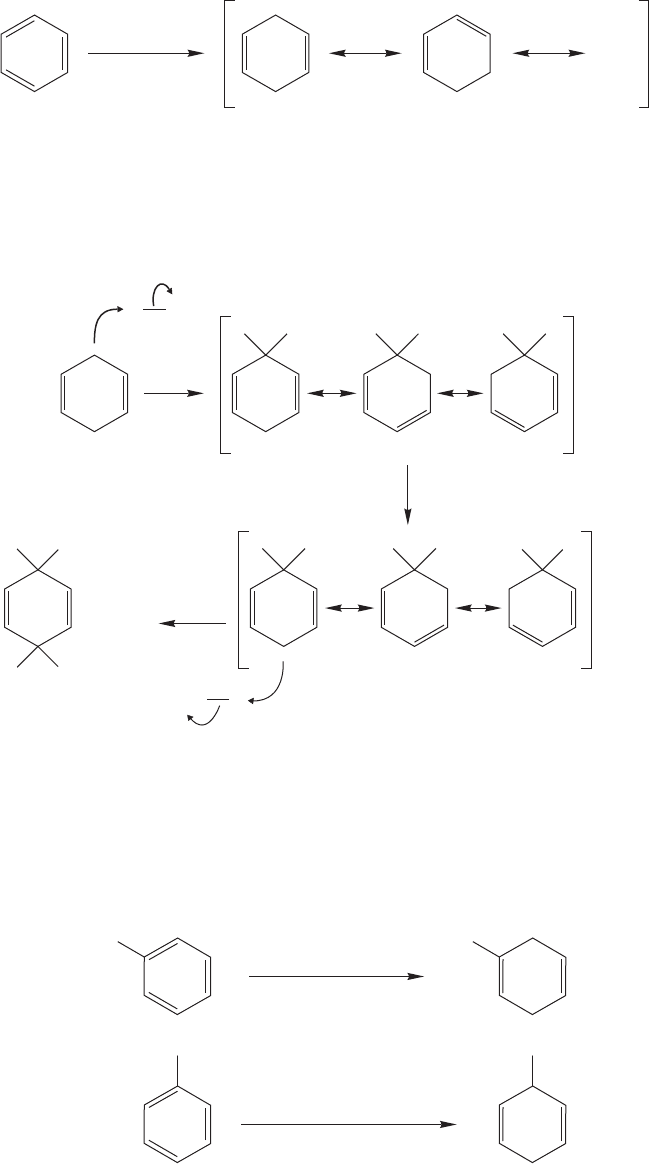
13.12 The Benzyl Group and Its Reactivity 611
In the benzyl cation itself (Fig. 13.72), the positive charge is borne by 4 carbons,
in the benzhydryl cation (Ph
2
C
H) (the benzhydryl group is Ph
2
CH) by 7 carbons,
and in the trityl cation (Ph
3
C
) by a whopping 10 carbons.
+
+
+
CH
2
+
CH
2
CH
2
The benzhydryl cation—7 resonance formsBenzhydryl chloride
The trityl cation—10 resonance formsTrityl chloride
In this composite representation
of the benzhydryl cation, the
positions sharing the positive
charge are shown as
(+)
In this composite representation of
the trityl cation, the positions sharing
the positive charge are shown as
(+)
CH
2
CH
Cl
Cl
CH
2
=
CH
2
+
(+)
(+) (+)
(+)
(+)(+)
(+)
(+)
(+)
C
Cl
HC
+
C
(+) (+)
(+)(+) (+)(+)
(+)
(+)
(+)
A composite picture in which the positions
sharing the positive charge are shown as
(+)
The benzyl cation— 4 resonance formsBenzyl chloride
+
FIGURE 13.72 Resonance
stabilization of the benzyhydryl and
trityl cations.
PROBLEM 13.25 Why is triptycenyl chloride not nearly as reactive in the S
N
1
reaction as trityl chloride?
Triptycenyl chlorideTrityl chloride
C
Cl
Cl
WEB 3D
WEB 3D

612 CHAPTER 13 Conjugation and Aromaticity
PROBLEM SOLVING
In a Problem like 13.25, in which the parts of two molecules are just about the
same (here, three benzene rings and a chlorine bonded to a tertiary carbon are
common to the two molecules), it is a good bet that the answer lies in geometry.
In most such problems it is the geometrical relationship between the various
parts that leads to the crucial difference.
CH
2
C
H
H
2
C
CH
2
S
N
2
Transition state for S
N
2
displacement of an allyl halide
Nu
Nu
–
..
Nu
–
..
S
N
2
C
H
H
2
C
CH
2
+
+
–
CH
2
Nu
C
C
C
H
H
Transition state for S
N
2
displacement of a benzyl halide
–
Nu
C
Nu
H
H
–
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
–
..
..
..
..
I
I
I
I
I
I
FIGURE 13.73 The transition state
for S
N
2 displacement at the benzylic
position is stabilized through orbital
overlap with the ring.
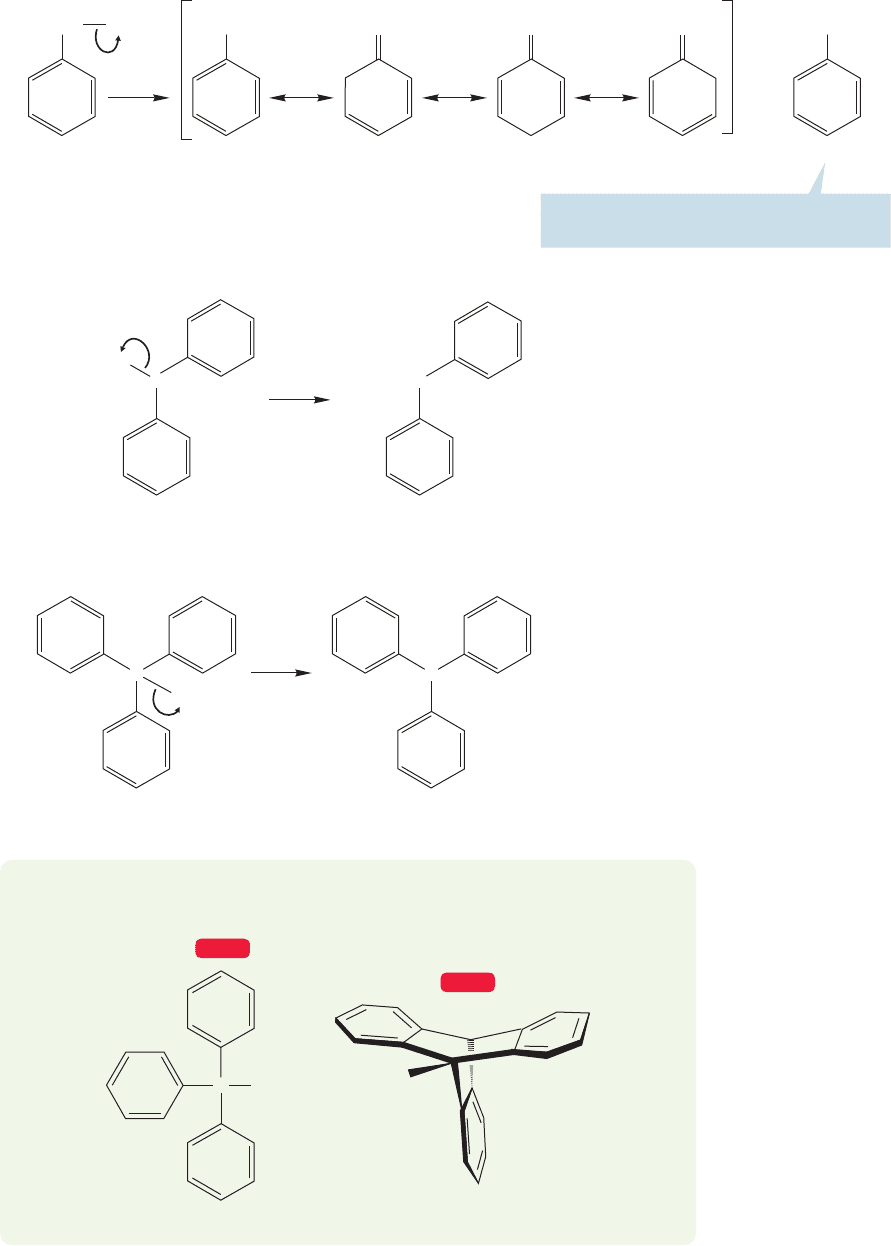
Table 13.2 compares the rates of reaction for benzyl iodide and other alkyl iodides
in an S
N
2 reaction.
13.12b Radical Reactions at the Benzylic Position A benzene ring will also
stabilize an adjacent half-filled orbital (a free radical) through resonance (Fig. 13.74).
TABLE 13.2 Some Relative
Rates of S
N
2 Displacements
in the Reaction of
with Iodide Ion at 50 °C in
Ethyl Alcohol Solvent
R Group Relative Rate
Ethyl 1.0
Propyl 0.6
Butyl 0.4
Allyl 33
Benzyl 78
R
O
I
CH
2
.
.
.
.
CH
2
CH
2
CH
2
FIGURE 13.74 The resonance forms
for benzyl radical.
Benzyl halides are also especially reactive in the S
N
2 reaction.The reason is the
same as that for the enhanced reactivity of allyl chloride (p.543).The transition state
for S
N
2 displacement is delocalized, and therefore especially stable. If the transition
state for a reaction is at relatively low energy, the activation energy for the reaction
will also be relatively low and the reaction will be fast (Fig. 13.73).

13.12 The Benzyl Group and Its Reactivity 613
CH
3
CCl
4
, hν
Toluene Benzyl
bromide
CH
2
Br
Br
N
O
O
NBS =
FIGURE 13.75 The radical bromina-
tion of toluene.
CH
2
CH
3
CHCH
3
Br
Br
2
⌬ or hν
␣
FIGURE 13.76 The thermal or
photochemical decomposition of
bromine in ethylbenzene leads to
1-bromo-1-phenylethane through
radical bromination at the benzylic
position.
In Chapter 11 (p. 497), we discussed radical reactivity at the allylic position,
and very little need be added to get a description of benzylic reactivity. Toluene,
for example, can be brominated by N-bromosuccinimide (NBS) to give benzyl
bromide (Fig. 13.75).
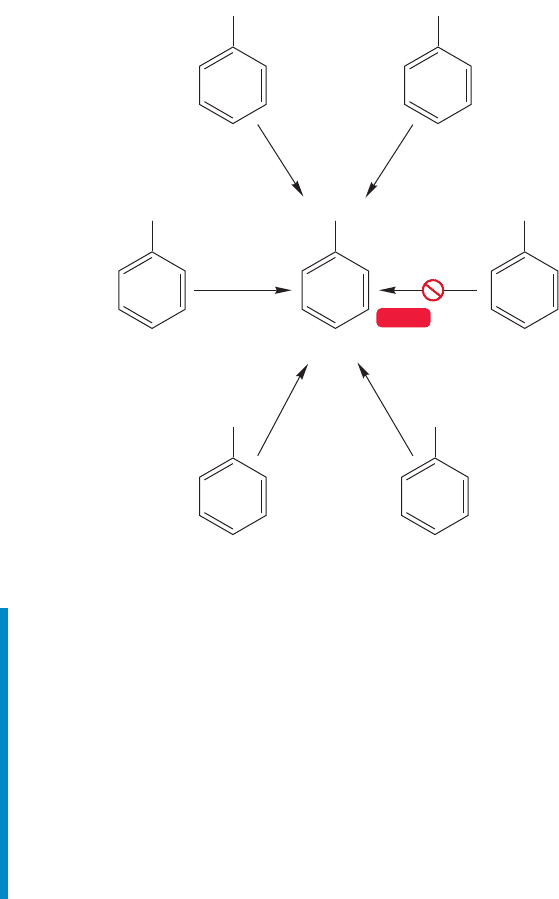
13.12c Oxidation at the Benzylic Position Both free radicals and
the special activity of the benzylic position play a role in the reaction of
alkylbenzenes with KMnO
4
or H
2
Cr
2
O
7
to give benzoic acid, which is benzene
with a carboxylic acid (COOH) attached. Side chains are “chewed down” to
carboxylic acids no matter what their length. The details are vague, but the
key observation is that tertiary side chains are untouched by oxidizing agents.
As you should be able to deduce from Problem 13.27, abstraction of hydro-
gen is especially easy at the α position of ethylbenzene. This abstraction occurs
easily because of the resonance stabilization of the benzylic radical (Fig. 13.74).
Because bromine will not attack the benzene ring without a catalyst, benzylic posi-
tions can often be brominated by simply heating or photolyzing bromine in their
presence (Fig. 13.76).
PROBLEM 13.26 Write a mechanism for the reaction between toluene and NBS.
Hint: See Chapter 11 (p. 500) for a description of the reaction of NBS with allylic
compounds.
PROBLEM 13.27 Ethylbenzene yields only the product of bromination at the α
position (the position adjacent to the ring) when treated with NBS in CCl
4
.
Explain.
Benzylic oxidation
614 CHAPTER 13 Conjugation and Aromaticity
Summary
The chemistry of benzenes is summed up by the imperative: “Preserve the circle!”
In plain English, that statement means that there is a strong thermodynamic
driving force to preserve the delocalization energy—more than 30 kcal/mol—that
the circle represents. In this chapter, the classic aromatic substitution reaction
in which an electrophile replaces (substitutes for) one of the hydrogens on the
ring is introduced. In Chapter 14, this prototypal reaction will be expanded in
many ways.
Other aromatic chemistry features a few reactions in which aromaticity is lost
(the Birch reduction is one example) and the special influence of the aromatic
ring on the benzylic position.
CH
2
CH
3
KMnO
4
KMnO
4
KMnO
4
KMnO
4
KMnO
4
KMnO
4
CH
2
CH
2
CH
3
COOH
Benzoic acid
C(CH
3
)
3
CH(CH
3
)
2
CH
3
CH
2
CH
2
CH
2
CH
3
WEB 3D
FIGURE 13.77 Oxidizing agents
convert most alkyl side chains on
benzene rings into the acid group,
COOH. However, for the oxidation
to succeed there must be at least one
benzylic hydrogen.Thus, tert-butyl-
benzene does not react.
13.13 Special Topic: The Bio-Downside,
the Mechanism of Carcinogenesis by
Polycyclic Aromatic Compounds
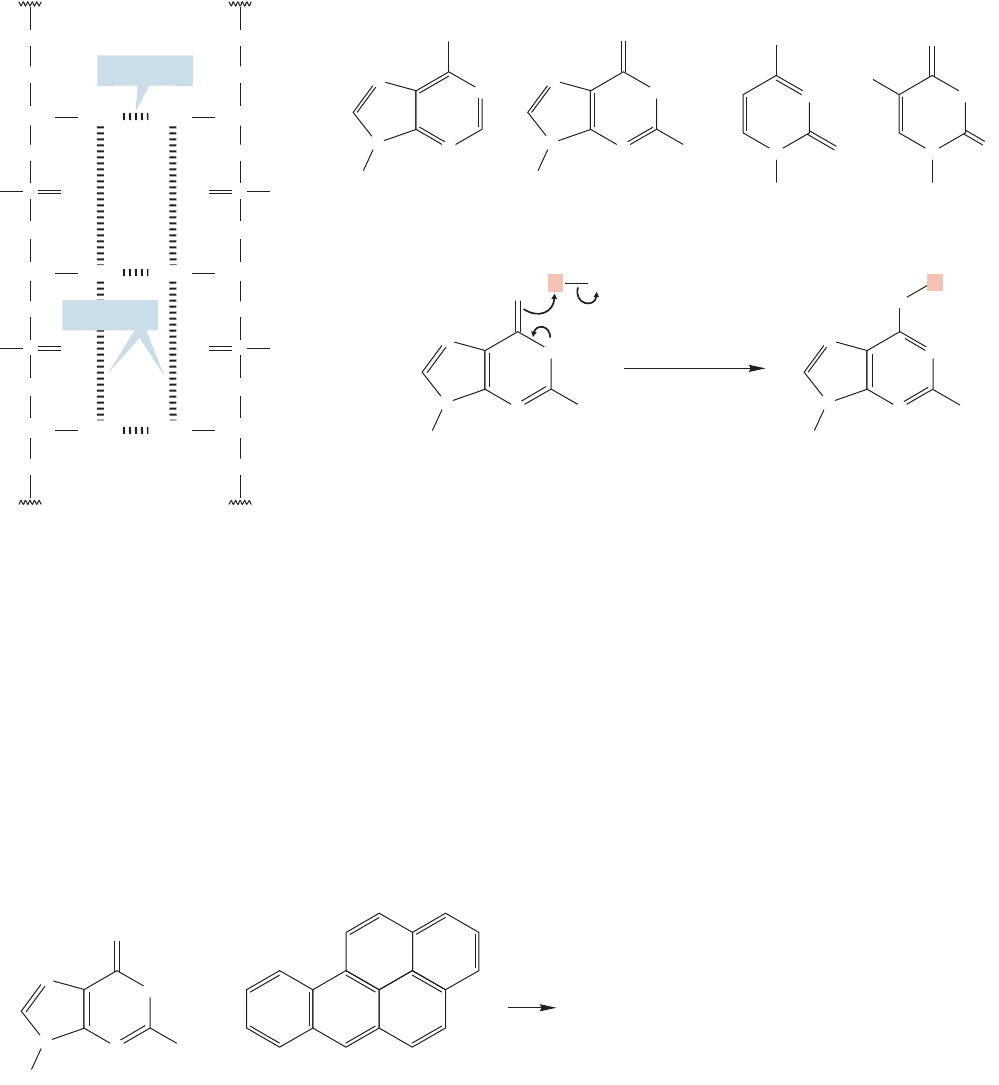
We mentioned earlier that some polycyclic aromatic hydrocarbons are strongly car-
cinogenic. Although all the details of this carcinogenesis are not worked out, the
broad outlines are known for some molecules. Deoxyribonucleic acid (DNA) and
ribonucleic acid (RNA) are huge biomolecules that contain and help transcribe
genetic information (Chapter 23). Both DNA and RNA are phosphate-linked
sequences of nucleotides, which are sugar molecules attached to heterocyclic bases.
There must be at least one hydrogen in the benzylic position for the reaction to
succeed (Fig. 13.77).
13.13 Special Topic: The Bio-Downside 615
Alkylation changes the size and shape of the DNA molecule and makes mis-
matched base pairing through hydrogen bonding more likely (see Chapter 23 for
more details of base pairing).This mismatch can lead to errors in replication and to
mutations. Most mutations are harmless to the organism, because they lead to the
death of only the individual cell. On rare occasions, however, a mutation can lead to
uncontrolled cell division and cancer.
The polyaromatic hydrocarbons (PAHs) are only indirectly responsible for the
chemical processes that lead to cancer. Aromatic hydrocarbons are not very reactive
and there is no obvious way for them to react chemically with the nucleophilic bases
in DNA or RNA (Fig. 13.79).
However, there are enzymes that can modify the PAH rings. Nonpolar mol-
ecules such as aromatic hydrocarbons accumulate in fat cells and natural methods
have been evolved to purge our bodies of such molecules. These methods gener-
ally involve making the hydrocarbons more water soluble by adding polar groups.
No reaction
Guanine Benz[a ]pyrene
+
N
..
..
..
..
N
..
N
O
..
..
N
NH
Sugar
NH
2
FIGURE 13.79 Aromatic
hydrocarbons do not react with
nucleophiles, and there is no way for
a molecule like benz[a]pyrene to
alkylate one of the bases directly.
..
NH
2
N
..
..
..
N
..
N
NH
2
..
N
N
..
..
..
N
..
..
N
O
..
NH
2
..
NH
SugarSugar
The structures of the four bases found in DNA attached to sugars
Adenine
N
..
..
..
..
N
..
N
O
..
NH
Sugar
Guanine
..
N
NH
2
..
..
N
Sugar
N
O
..
..
..
..
NH
Sugar
Cytosine Thymine
..
..
O
H
3
C
RX
N
..
..
N
..
N
..
N
N
Sugar
Alkylated
..
NH
2
O
..
..
R
O
..
..
1. S
N
2 alkylation
2. deprotonate
P
P
Base
O
O
O
O
O
O
Sugar
Base
Base
Sugar
P O
O
O
O
Sugar
–
–
P
P
Base
O
O
O
O
O
O
Sugar
Base
Base
Sugar
P
O
O
O
O
Sugar
–
–
H-bonding
π stacking
FIGURE 13.78 The DNA polymer is composed of phosphate-linked sugar-base units.Two strands of DNA
are held together by hydrogen bonding between pairs of bases and by π stacking between the aromatic
bases. Alkylation of the bases changes the size and shape of one of the units.This change can interfere with
hydrogen bonding and base pairing. In turn, this interference can lead to mutations and cancer.
These molecules bear large numbers of nucleophilic groups that can be alkylated by
other molecules (electrophiles) in what seem to be simple S
N
2 reactions (Fig.13.78).
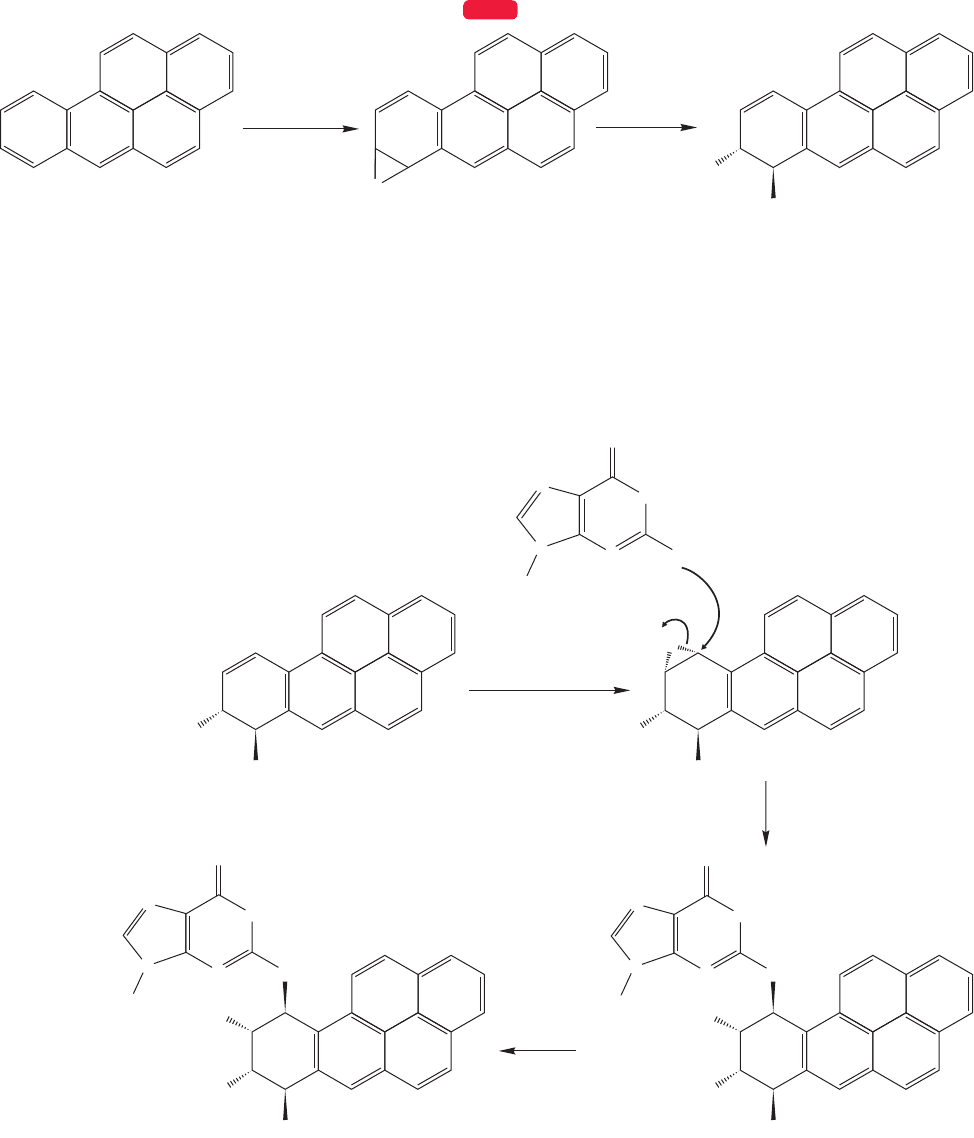
616 CHAPTER 13 Conjugation and Aromaticity
P-450
monooxygenase
Benz[a ]pyrene
epoxide
hydratase
O
OH
HO
WEB 3D
FIGURE 13.80 Benz[a]pyrene is enzymatically epoxidized and ring opened to give a polar trans 1,2-diol.
A second enzymatic epoxidation, again by P-450 monooxygenase, gives a mol-
ecule that reacts with guanine, one of the DNA bases, to give DNA that is severe-
ly encumbered by the large, alkylated guanine (Fig. 13.81). This modified base has
difficulty in maintaining normal hydrogen bonding with another complementary
base, and this mismatch can lead to mutations.
OH
HO
..
..
..
..
..
OH
HO
..
..
..
..
..
..
..
..
..
HO
..
..
..
Guanine
N
..
..
N
..
N
..
..
O
..
N
NH
Sugar
P-450
monooxygenase
S
N
2
O
+
–
NH
2
O
OH
A
HO
..
..
..
..
..
..
..
N
..
..
..
..
N
..
N
O
..
N
NH
Sugar
..
..
H
2
O
HO
NHNH
N
..
..
..
..
N
..
N
O
..
N
NH
..
NH
2
Sugar
OH
FIGURE 13.81 A second epoxide is
formed.This epoxide is opened by
guanine to give a highly encumbered
molecule, A, that has problems
maintaining proper hydrogen
bonding.
For example,benz[a]pyrene is epoxidized by the enzyme P-450 monooxygenase and
the product epoxide is then opened enzymatically to give a trans diol (Fig. 13.80).
This alkylation of guanine is not a new reaction. You already know of the open-
ing of epoxides in both base and acid (p. 426), and this process is nothing more than
a complicated version of that simple reaction.
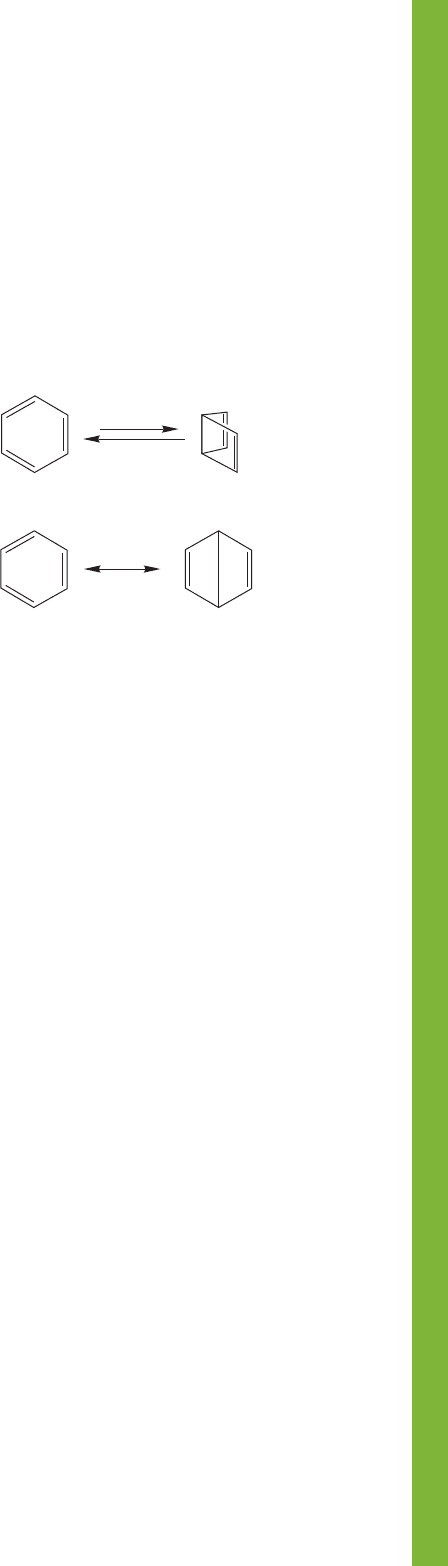
13.14 Summary 617
13.14 Summary
New Concepts
Equilibrium
Resonance
Dewar resonance
form (minor contributor)
Kekule´ resonance
form (important)
Benzene Dewar benzene
(unstable species)
FIGURE 13.82 Equilibrium versus resonance.
Reactions, Mechanisms, and Tools
equilibrating molecules have different geometries—different
arrangements of atoms in space. Benzene and Dewar
benzene are different molecules; the Kekulé and Dewar
resonance forms contribute to the real structure of benzene
(Fig. 13.82).
The material in this chapter is composed almost entirely of
concepts. There are few new reactions or synthetic procedures.
We concentrate here on the special stability of some planar,
cyclic, and fully conjugated polyenes.The special stability called
aromaticity is encountered when the cyclic polyene has a molec-
ular orbital system in which all degenerate bonding molecular
orbitals are completely filled.
These especially stable molecular orbital systems are
found in planar, cyclic, fully conjugated polyenes that contain
4n 2 π electrons (Hückel’s rule).
Heats of hydrogenation or heats of formation can be used
to calculate the magnitude of the stabilization. For benzene, the
delocalization energy or resonance energy amounts to more
than 30 kcal/mol.
It’s vital to keep clear the difference between resonance forms
and molecules in equilibrium. In this chapter, that difference is
exemplified by Dewar benzene, bicyclo[2.2.0]hexa-2,5-diene
(Problem 13.5), and the Dewar resonance forms contributing
slightly to the structure of benzene. As always, resonance forms
are related only by the movement of electrons, whereas
annulene (p. 594)
arene (p. 598)
aromatic character (p. 582)
aromaticity (p. 582)
benzene (p. 573)
benzhydryl group (p. 611)
benzoic acid (p. 613)
benzyl (p. 596)
Birch reduction (p. 608)
cycloheptatrienylium ion (p. 587)
cyclopentadienyl anion (p. 589)
delocalization energy (p. 581)
Dewar benzene (p. 573)
Dewar forms (p. 576)
Frost circle (p. 584)
furan (p. 599)
heteroaromatic compound (p. 599)
heterobenzene (p. 591)
Hückel’s rule (p. 583)
Kekulé forms (p. 575)
meta (p. 596)
ortho (p. 596)
para (p. 596)
phenyl (Ph) (p. 596)
polynuclear aromatic compound (p. 602)
pyridine (p. 598)
pyrrole (p. 592)
resonance energy (p. 581)
tropylium ion (p. 587)
Key Terms
In this chapter, the first reaction mechanism encountered is
the important and general electrophilic substitution of benzene.
A host of aromatic substitution reactions will be studied in
Chapter 14 and are exemplified here by deuterium exchange.
The aromatic ring is destroyed by an endothermic addition of
D
, but reconstituted by an exothermic loss of H
(Fig. 13.65).
There is little in the way of new synthetic procedures in this
chapter. You might remember the formation of the tropylium
ion by hydride abstraction and the Birch reduction of ben-
zenes to 1,4-cyclohexadienes. You also have a method of
Syntheses
synthesizing deuteriobenzene through the acid-catalyzed
exchange reaction of benzene. This reaction will serve as the
prototype of many similar substitution reactions to be found
in Chapter 14.
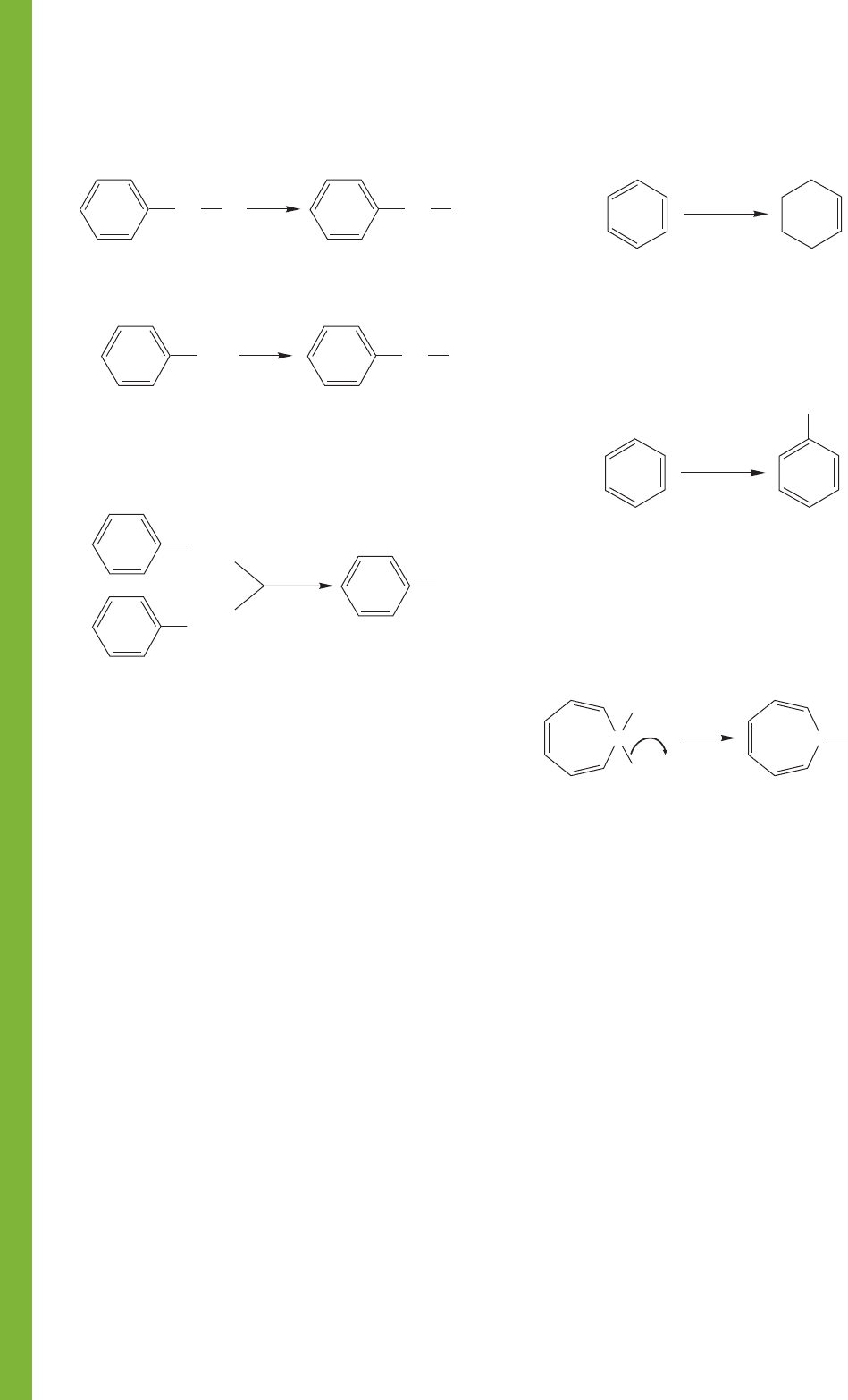
618 CHAPTER 13 Conjugation and Aromaticity
S
N
1 and S
N
2 reactions
L
CH
2
Nu
Nu
..
–
Radical bromination at the benzyl position
CH
3
NBS
Br
Oxidation of benzyl positions bearing at least one hydrogen
CH
3
CH(CH
3
)
2
COOH
KMnO
4
CH
2
CH
2
1. Benzylic Compounds
3. Deuteriobenzenes
2. Cyclohexadienes
4. Tropylium Ions
EtOH
Birch reduction of benzene
Na/NH
3
1,4-Cyclohexadiene
A
cid-catalyzed exchange of hydrogens on a benzene
ring; all hydrogens can eventually be exchanged
D
3
O/D
2
O
+
D
2
OH
+
D
+
+
–
+
Hydride transfer from cycloheptatriene
to the trityl cation (
+
CPh
3
)
C
H
BF
4
Tropylium
fluoborate
–
BF
4
H
C
H
HCPh
3
+
CPh
3
Common Errors
Certainly the most common error is to overgeneralize
Hückel’s rule. We often forget to check each potentially
aromatic molecule to be certain it satisfies all the criteria.
The molecule must be cyclic or there cannot be the fully con-
nected set of p orbitals necessary (Fig. 13.18). The molecule
must be planar so that the p orbitals can overlap effectively
(Fig. 13.16). There may be no positions in the ring at which
p/p overlap is interrupted (Fig. 13.15). Finally, there must be
4n 2 π electrons. Be certain you are counting only π electrons.
Do not include in your count electrons not in the π system.
The classic example is pyridine, which contains a pair of elec-
trons in an sp
2
orbital that are often mistakenly counted as π
electrons (Fig. 13.47). If all four of these criteria are satisfied
we find aromaticity.
Do not misapply the Frost circle device.The most common
error is to forget to inscribe the circle with the vertex of the
appropriate polygon down. This requirement is hard to remem-
ber because at this level of discussion this point is arbitrary—
there is no easy way to figure it out if you don’t remember the
proper convention.
Do not confuse absolute and relative stability. For example,
the cyclopentadienyl anion is aromatic, and therefore exception-
ally stable, but only within the family of anions. The negatively
charged cyclopentadienyl anion is not as stable as benzene.
As always, you must be clear about the difference between
resonance and equilibrium. Be certain that you know the differ-
ence between the two Kekulé resonance forms for benzene and
the hypothetical molecule 1,3,5-cyclohexatriene.
