Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

14.17 Additional Problems 689
H
2
O
+
H
3
CO N
2
heat
HOCH
3
+
H
3
CO N
2
heat
Second reaction:
PROBLEM 14.52 Problem 14.18 describes the formation of an
azo compound. The dye industry is dependent on the reaction
between diazonium ions and activated aromatic rings to form
azo compounds. What aspect of the azo compounds makes them
good dyes? What diazonium salt would you use to make Para
Red (shown below)? Para Red is reported to be the first azo dye
synthesized, and has been used as a dye for both clothing and food.
OH
N
NO
2
N
CH
2
CH
2
CH
2
CH
3
(a)
NO
2
CH
2
CH
2
CH
2
CH
3
(b)
CH
2
CH
2
CH
2
CH
3
Br
(c)
(free of para)
COOH
SO
2
OH
(d)
Br
F
(b)
I
Br
(a)
Br
Br
(c)
Br
I
(a)
Br
Br
(c)
Br
(d)
Br
(b)
F
Br
PROBLEM 14.53 In the presence of very strong acid, phenol is
brominated in the meta position. Why do you suppose this is
the case? When phenol is brominated in slightly basic condi-
tions, tribromophenol is the major product. Why would basic
conditions favor polybromination?
PROBLEM 14.54 Provide syntheses for the following com-
pounds, as free of other isomers as possible. You do not need to
write mechanisms, and you may start from benzene, inorganic
reagents of your choice, organic reagents containing no more
than four carbons, and pyridine.
First reaction:
PROBLEM 14.55 Provide syntheses for the following com-
pounds, free of other isomers. You do not need to write mecha-
nisms, and you may start from benzene, inorganic reagents of
your choice, organic reagents containing no more than two car-
bons, and pyridine.
PROBLEM 14.56 How many signals would appear in the
13
C NMR spectra of the following four compounds? Try to
use an analysis of symmetry in your answer.
PROBLEM 14.57 Provide syntheses for the following com-
pounds, as free of other isomers as possible. You do not need to
write mechanisms, and you may start from benzene, inorganic
reagents of your choice, organic reagents containing no more
than three carbons, and pyridine.
OCH
2
CH
3
CH
2
CH
2
CH
3
(a)
CH
2
CH
3
D
(b)
CN
NO
2
(c)
PROBLEM 14.58 Provide structures for compounds A–E.
Mechanisms are not required, but a mechanistic analysis of
the various reactions will probably prove helpful in deducing
690 CHAPTER 14 Substitution Reactions of Aromatic Compounds
+
1. AlCl
3
O
O
O
OCH
3
2. H
2
O, H
3
O
C
11
H
12
O
4
A
1. Zn/Hg/HCl 2. H
2
O
C
11
H
14
O
3
B
SOCl
2
1. AlCl
3
2. H
2
O, H
3
O
C
11
H
13
O
2
Cl
C
C
11
H
12
O
2
D
Zn/Hg
HCl
C
11
H
14
O
E
+
+
CH
3
CH
3
CHO
CO, HCl
AlCl
3
AlCl
3
C
AB
Cl
2
C
AlCl
3
CH
3
CH
2
Cl
Zn/Hg
HCl
C
O
Cl
CH
3
hν
Na
+
(CH
3
)
3
CO
–
D
2. (CH
3
)
2
S
1. O
3
O
H
the structures. Hint: You might look at the figure in Problem
14.14 (p. 646).
PROBLEM 14.59 One substituent you do not know how to
attach to a benzene ring is the aldehyde group, . Here
is a way. The price of this knowledge is that you must write a
mechanism for the following aromatic substitution reaction.
HC
P
O
PROBLEM 14.60 Lies! Lies! Lies! We can think of at least one
way (other than the reaction in Problem 14.59) you can put an
aldehyde group on a benzene ring with your current knowledge.
It is a roundabout process, and so makes a good “roadmap” prob-
lem. Try this one. Provide structures for compounds A–D.
CH
3
OSO
2
OCH
3
NaOH/H
2
O
OH
OH
OH
OCH
3
1. KNH
2
/NH
3
(C
5
H
6
N
2
2. H
2
O
A
NaNO
2
(C
5
H
4
ClN
3
HCl
B
CuBr
)
)
(C
5
H
4
BrN)
C
N
NH
2
NH
2
NH
2
NO
2
Br
COOH
Br
Br
Br
Br
(a)
(c)
(d)
(b)
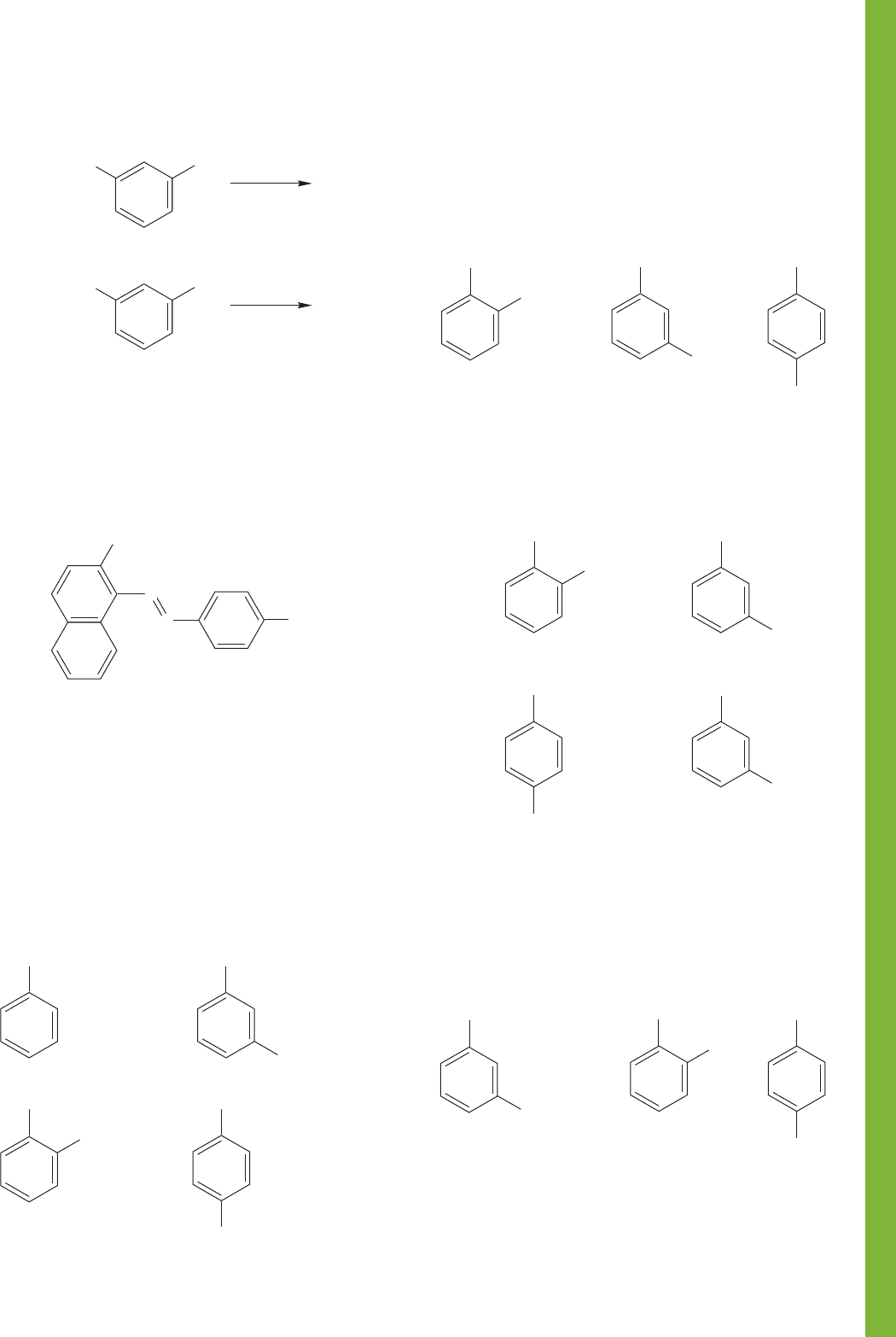
PROBLEM 14.62 Write structures for compounds A, B, and C.
PROBLEM 14.63 How would you convert aniline into each of
the following molecules? Mechanisms are not necessary.
PROBLEM 14.61 Explain mechanistically why it is possible to
alkylate a phenol selectively in the presence of an alcohol.
14.17 Additional Problems 691
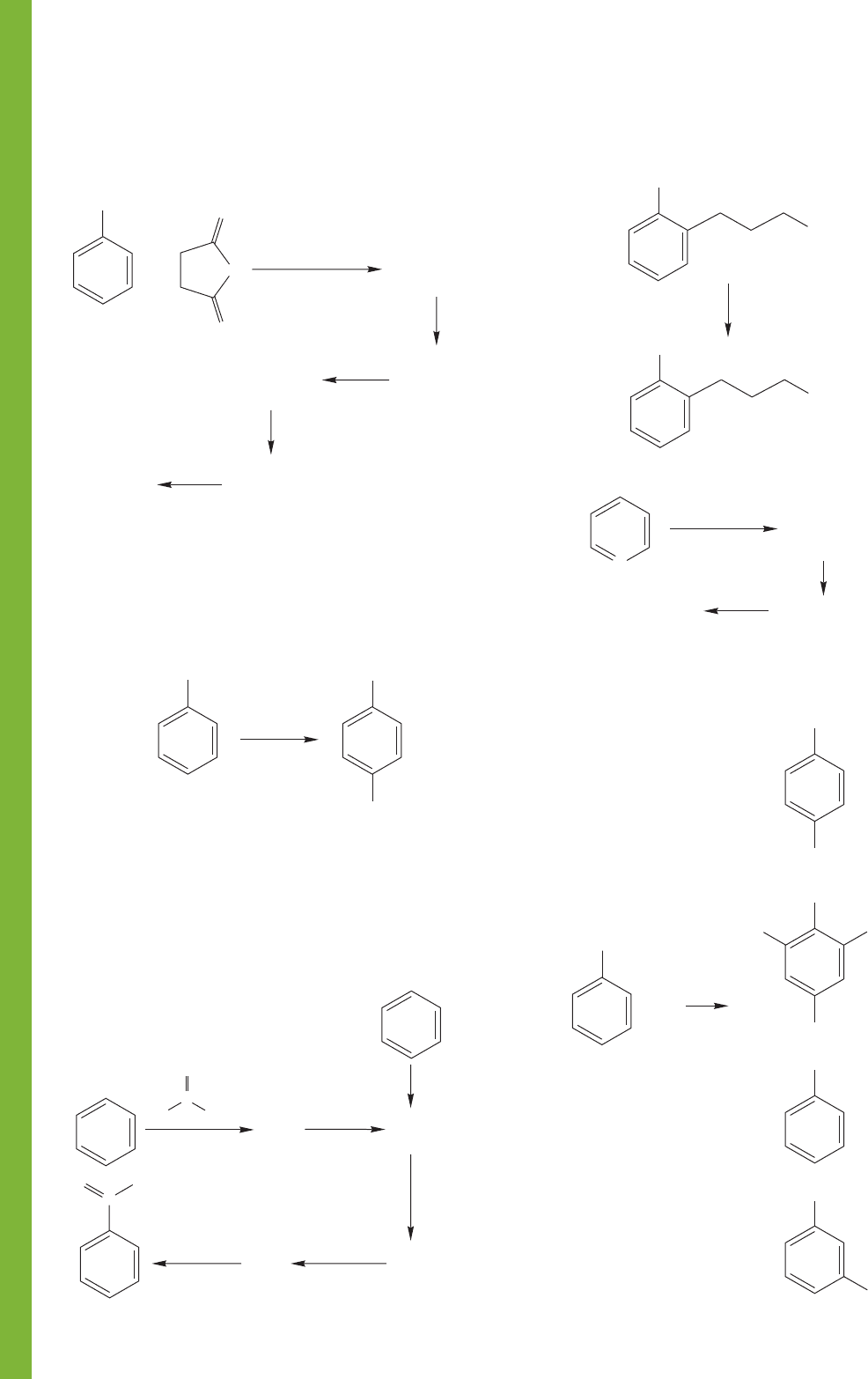
PROBLEM 14.64
Write mechanisms for the following two
related reactions:
(43%)
CH
3
CH
3
O
O
HClO
4
CH
3
CH
3
NH
N
75–80%
H
2
SO
4
N
N
(75%)
SO
2
OH
SO
2
OH
concd H
2
SO
4
(major)
40 ⬚C concd H
2
SO
4
160 ⬚C
+
(major)(minor)
(minor)
SO
2
OH
SO
2
OH
+
CH
2
Cl
H
2
C
(75%)
HCl
O
PROBLEM 14.65 Rationalize the following data. An Energy
versus Reaction progress diagram will be useful. Be sure to
explain why the low-temperature product is mainly naphtha-
lene-1-sulfonic acid, and why the high-temperature product is
mainly naphthalene-2-sulfonic acid. Note that naphthalene-2-
sulfonic acid is more stable than naphthalene-1-sulfonic acid.
You do not have to explain the relative energies of the two
naphthalene sulfonic acids, although you might guess, and the
answer will tell you.
PROBLEM 14.66 Write a mechanism for the following
chloromethylation reaction:
PROBLEM 14.67 The following wonderful question appeared
on a graduate cumulative examination at Princeton in January,
1993. With a little care you can do it easily. Write a mechanism
for this seemingly bizarre change. Hint: It is not bizarre at all.
Think simple! First consider what HCl might do if it reacts
with the starting material.
AlCl
3
/H
2
O/HCl
=
13
C
benzene,
80 ⬚C
CH
3
CH
3
H
3
C
CH
3
CH
3
CH(CH
3
)
2
CH
3
A
(48%)
(CH
3
)
2
CHCl
AlCl
3
, 70 ⬚C,
2 h
p-Xylene
B
(52%)
CH(CH
3
)
2
PROBLEM 14.68 At low temperature, only a single product
(A) is formed from the treatment of p-xylene with isopropyl
chloride and AlCl
3
, which is surely no surprise, as there is only
one position open on the ring. However, at higher temperature
some sort of rearrangement occurs, as two products are formed
in roughly equal amounts. First, write a mechanism for forma-
tion of both products. Hint: Note that HCl is a product of the
first reaction to give A. Second, explain why product A
rearranges to product B. What is the thermodynamic driving
force for this reaction?
692 CHAPTER 14 Substitution Reactions of Aromatic Compounds
C
O
hν
C
O
(a)
(CH
3
)
2
NH
CH
3
F
C
O
CH
3
N(CH
3
)
2
(b)
O
C
O
O
+
CO
2
One
product
CH
3
ONa
excess
Cl
NO
2
Cl
CH
3
OH
Δ
COOH
NH
2
ONO
COO
–
N
2
+
Δ
+
Anthracene
1
Triptycene
2
Anthranilic
acid
PROBLEM 14.69 Provide mechanisms for the following
reactions:
PROBLEM 14.70 Construct an Energy versus Reaction
progress diagram for the reaction of 1-chloro-2,4-dinitroben-
zene with sodium methoxide.
PROBLEM 14.71 When 1,2-dichloro-4-nitrobenzene is treat-
ed with an excess of sodium methoxide, three different substi-
tution products (two monosubstituted, one disubstituted)
could, in principle, be formed. In fact, only a single product is
obtained.
(a) Draw the three possible products.
(b) Carefully analyze the mechanism for this nucleophilic
aromatic substitution reaction. What single product will
be formed and why?
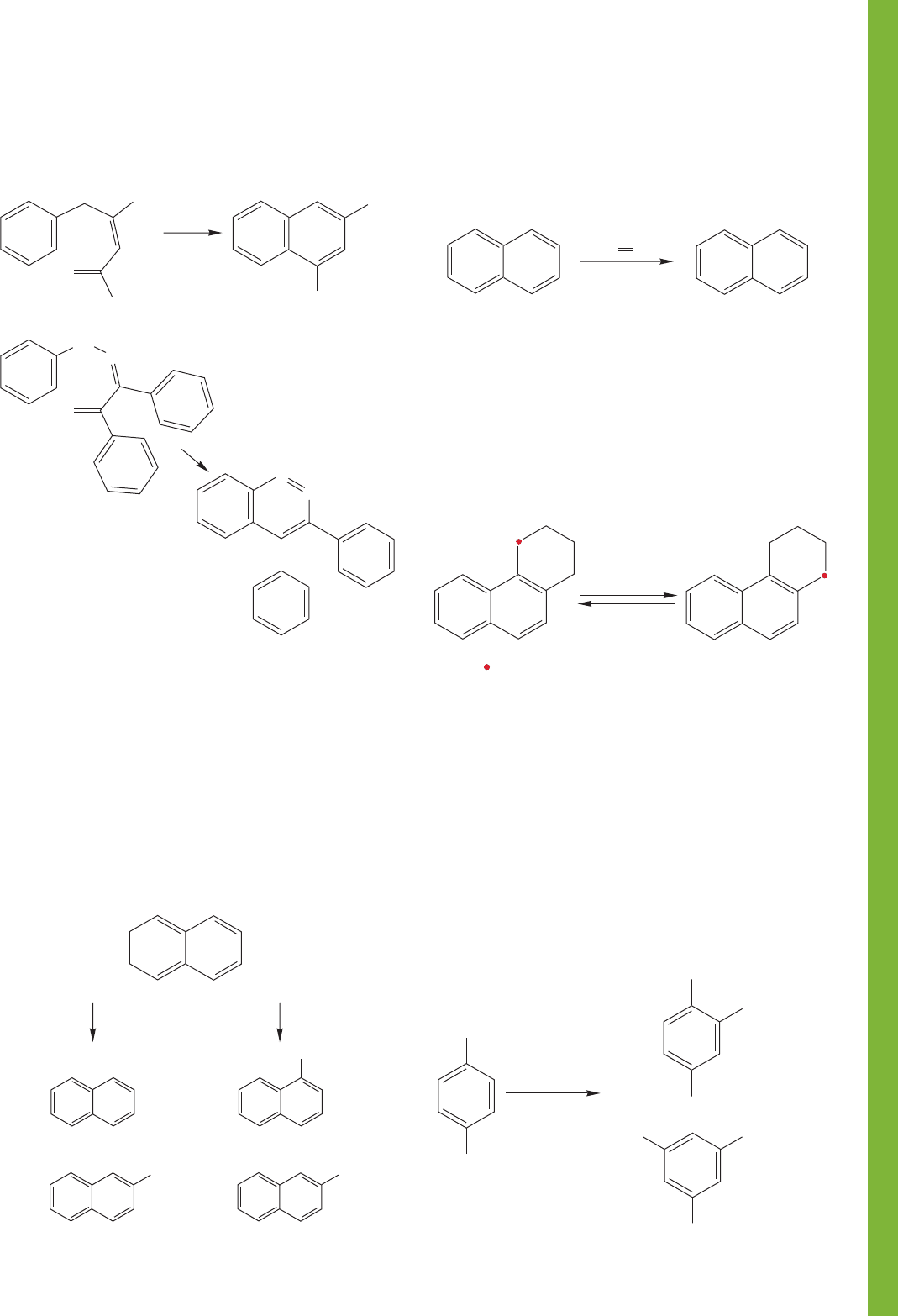
PROBLEM 14.72 A useful benzyne precursor is
benzenediazonium-2-carboxylate (1), a molecule readily
formed from anthranilic acid. Upon heating, 1 affords
benzyne, which can be trapped by anthracene to give the
architectural marvel, triptycene (2).
(a) Provide an arrow formalism for the formation of
benzyne on decomposition of benzenediazonium-
2-carboxylate.
(b) Similarly, provide an arrow formalism for the formation
of triptycene from benzyne and anthracene.
14.17 Additional Problems 693
Rationalize these cine substitutions through use of a substituted
benzyne as an intermediate. Explain why only a single isomer is
formed in the second experiment.
PROBLEM 14.74 In phenylalanine biosynthesis, the OH group
is a leaving group (see Fig. 14.118). That’s not normal. Why do
you suppose it happens in this case? How could an enzyme
make it easier?
PROBLEM 14.75 2,4-Dinitrofluorobenzene is a better elec-
trophile for S
N
Ar reactions than the corresponding chloride
(see Fig. 14.95). 2,4-Dinitrofluorobenzene reacts with amines
at room temperature to give orange-colored adducts. Show the
mechanism of the reaction between 2,4-dinitrofluorobenzene
and dimethylamine. Predict the product. Why is the fluoride
more reactive than the chloride? Why is the product highly
colored?
PROBLEM 14.73 Years before J. D. Roberts’ classic labeling
experiment (p. 681), it was known that nucleophilic aromatic
substitution occasionally resulted in what was termed cine
(from the Greek kinema meaning “movement”) substitution.
In this type of substitution reaction, the nucleophile becomes
attached to the carbon adjacent to the carbon bearing the
leaving group. Here are two examples of this kind of
substitution:
Only isomer formed
NaNH
2
NH
3
+
CH
3
CH
3
NH
2
Br
Br
CH
3
NH
2
LiNH
2
NH
3
OCH
3
OCH
3
NH
2
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 14.76 Select the reaction “Arene halogenation” on
the Semester 1B panel. Observe the reaction several times, then
select the LUMO track. As the reaction reaches the intermediate,
pause the animation. Notice that the LUMO shows the locations
sharing cationic character. Draw the resonance forms for this
intermediate. Which site has the lowest cationic character? Why?
PROBLEM 14.77 Watch the “Friedel–Crafts acylation” reaction.
What is the hybridization of the carbon of the acylium ion?
What species is animated as the weak base that deprotonates
the nonaromatic intermediate? Consider the hydrogen that is
being deprotonated in this last step of the reaction. Give a pKa
value that would reflect how acidic that hydrogen is. (Hint: It is
the most acidic hydrogen we have encountered.)
PROBLEM 14.78 We have animated the addition–elimination
version of the S
N
Ar reaction for your viewing pleasure.This
reaction has much to teach us. Select the LUMO track for the
reaction. Notice that the starting aromatic compound has the
largest LUMO character on one of the atoms. Which one is it?
Where does the nucleophile attack? The animation shows the
amine being deprotonated before the chloride is kicked out.
Why do you suppose deprotonation might be necessary prior
to the chloride leaving? Look at the final product. What is the
hybridization of the amine nitrogen? Finally, look at the
HOMO track for the second intermediate in the reaction.
It is the clearest picture of the resonance available in the
anionic intermediate. Using this visual insight, draw each
of the resonance forms.
PROBLEM 14.79 The Chapter 14 benzyne reaction is also ani-
mated. Benzyne is an intermediate in the elimination–addition
version of the S
N
Ar reaction, and is a very reactive intermediate.
Observe the reaction several times. What are the most acidic
hydrogens in the reaction mixture? What are the second most
acidic hydrogens? The animation shows benzene without dou-
ble bonds. Why are the double bonds left out? The animation
shows the amide ion (
NH
2
) attacking one carbon of the ben-
zyne. Observe the LUMO of the benzyne. Could the
NH
2
attack either carbon of the benzyne? Remember that the
LUMO tells us about cationic character. How can both carbons
of the benzyne have equal cationic character?

Analytical Chemistry:
Spectroscopy
694
15.1 Preview
15.2 Chromatography
15.3 Mass Spectrometry (MS)
15.4 Infrared Spectroscopy (IR)
15.5
1
H Nuclear Magnetic
Resonance Spectroscopy
(NMR)
15.6 NMR Measurements
15.7
13
C NMR Spectroscopy
15.8 Problem Solving: How to Use
Spectroscopy to Determine
Structure
15.9 Special Topic: Dynamic NMR
15.10 Summary
15.11 Additional Problems
15
PUZZLES Using spectroscopy to determine the structure of an unknown organic molecule
is very much like putting together the pieces of a jigsaw puzzle.

15.1 Preview 695
After a great expenditure of life and treasure a Daring Explorer had
succeeded in reaching the North Pole, when he was approached by a
Native Galeut who lived there.
“Good morning,” said the Native Galeut. “I’m very glad to see you, but
why did you come here?”
“Glory,” said the Daring Explorer, curtly.
“Yes, yes, I know,” the other persisted; “but of what benefit to man is your
discovery? To what truths does it give access which were inaccessible
before?—facts, I mean, having scientific value?”
“I’ll be Tom scatted if I know,” the great man replied frankly; “you will
have to ask the Scientist of the Expedition.”
But the Scientist of the Expedition explained that he had been so
engrossed with the care of his instruments and the study of his tables that
he had found no time to think of it.
—AMBROSE BIERCE,
1
“AT THE POLE,” FROM
FANTASTIC FABLES
1
Ambrose Bierce (1842–1914?) was an American journalist and writer of bitter, sardonic tales who vanished
during the Mexican civil war at the start of the last century.
15.1 Preview
Years ago, all universities gave one or several courses in the subject of analytical chem-
istry. Sadly, in recent times this material has sometimes been folded into courses in
physical and organic chemistry, and the subject “lost.” Sometimes also lost, we fear,
is the sense of how important the subject is. Nothing has influenced the develop-
ment of organic chemistry more strongly over the last 50 years than progress in
analytical chemistry. After a chemical reaction is run, the experimentalist is faced
with two general problems before he or she can begin to think about what the results
mean: separating the products of the reaction from one another, then identifying
those products.
Separation was a severe problem in the early days of organic chemistry. For many
compounds,the only available methods were distillation or,for solids, fractional crys-
tallization. Fractional crystallization works well (if done properly, which it rarely is),
but this method requires reasonably large amounts of material and, of course, works
only for compounds that are solids at or near room temperature. If you are taking an
organic lab along with this course, then by now your laboratory experience has likely
revealed that distillation is not often the final answer to securing large amounts of
very pure materials. Distillation works well if there is a large difference between the
boiling points of all the desired components of a mixture, and if substantial amounts
of material are available. It seems that reactions in the research laboratory are all too
prone to give several isomeric products,and distillation and fractional crystallization
are often impractical methods.The vexing problem of separation was relieved some-
what by the advent of column chromatography, then more or less solved, at least in
the research lab,by the discovery of gas and liquid chromatography. Other chromato-
graphic techniques include thin-layer, preparative-layer, and flash chromatography.
Nowadays, it is the chemist’s realistic expectation that any mixture can be separated.
Even enantiomers are now often separable by chromatography.

PROBLEM 15.1 How would a separation of enantiomers be possible? Are not all
physical properties (except, of course, for the direction of rotation of the plane of
plane-polarized light) identical for enantiomers? Can you guess the principle on
which the chromatographic separation of enantiomers must rest? Hint: What is
true for enantiomers is not necessarily true for diastereomers (p. 169).
696 CHAPTER 15 Analytical Chemistry: Spectroscopy
Even given a successful separation of a reaction mixture, the chemist is now faced
with the daunting task of working out the structures of the various components. Just
how do we find out what’s in that bottle? In the old days, structure determination was
a respected profession,and many careers were built through the chemical determination
of complex molecular structures. Diagnostic reactions were run, and the presence or
absence of functional groups inferred from the results. Gradually,the results from many
chemical experiments allowed the formulation of a reasonable structural hypothesis
that could be tested through further chemical reactions. The intellectual complexity
of such work was substantial,and the experimental skill required was prodigious.These
days, the correlation of the results of chemical reactions with chemical structure has
been largely replaced with the spectroscopic identification of compounds, and every
chemist is an analytical chemist.The profession of analytical chemist has by no means
disappeared,but rather has expanded to include us all. Ultraviolet/visible (UV/vis) spec-
troscopy (p. 525) was the earliest form of spectroscopy to gain widespread use. It was
followed by mass spectrometry (MS), which is capable of determining molecular
weights, and infrared (IR) spectroscopy, in which the vibrations of atoms in bonds are
detected. At the end of the 1950s, nuclear magnetic resonance (NMR) spectroscopy
appeared,
2
and the real revolution in the determination of the structures of organic
molecules began. In this chapter, we will briefly discuss chromatography, MS, and IR
spectroscopy, and then proceed to a more detailed investigation of NMR spectroscopy.
2
Professor Martin Saunders (b. 1931) told MJ of the arrival of the long and eagerly awaited first NMR spec-
trometer at Yale in the late 1950s. The magnet arrived in a plywood carton labeled in bold letters, “2.5 tons.”
Unfortunately, the workman who was to bring the machine into the laboratory didn’t believe that such a heavy
object could be in such a flimsy box and attempted to use his forklift, rated at 1 ton, to bring it in. The fork-
lift did its best and labored mightily to lift the object into the air. It succeeded at this task, but then, appar-
ently exhausted, gave out and dropped the magnet to the stone floor, where it burst its packing and lay blocking
the entrance.The workman then attached a chain to the magnet and dragged it about 30 feet across the cob-
blestones to the side. Naturally Professor Saunders, whose professional fate depended to a fair extent on the
performance of this instrument, was less than happy about this state of affairs. However, the story has a happy
ending. The machine functioned brilliantly after minor repairs, and Professor Saunders has had a long and
outstanding career at Yale. He claims that he advocated to Varian Associates, the manufacturer of the instru-
ment, that they add a 5-foot drop to a cobblestone floor to the manufacturing process for their magnets, in
order to bring all their magnets up to the standard of this one.Perhaps understandably, they ignored his advice.
ESSENTIAL SKILLS AND DETAILS
1. “All” there is to understanding nuclear magnetic resonance (NMR) is to know how the
chemical shift (δ) arises, how coupling between atoms works, and how intensities of
signals are compared.That’s only three things, but each requires a lot of experience
before using it becomes second nature. Understanding the chemical shift δ requires
no more than realizing that “different” hydrogens will resonate at different frequencies.
That’s not a difficult concept, but understanding what makes two hydrogens “different”
is less simple! The coupling between atoms means that hydrogens on adjacent carbons
“talk”to each other. The state of one influences the properties of the other.There is a
simple method for translating the electron–nuclear “talk” but, again, it requires an
15.2 Chromatography 697
(b)
0 5 10 15 20
(a)
Solvents;
concentrations
can be varied
as process
continues
Filter
Sample
in syringe
To collection
flasks
Time (min)
Pump
Injector
Detector
Column
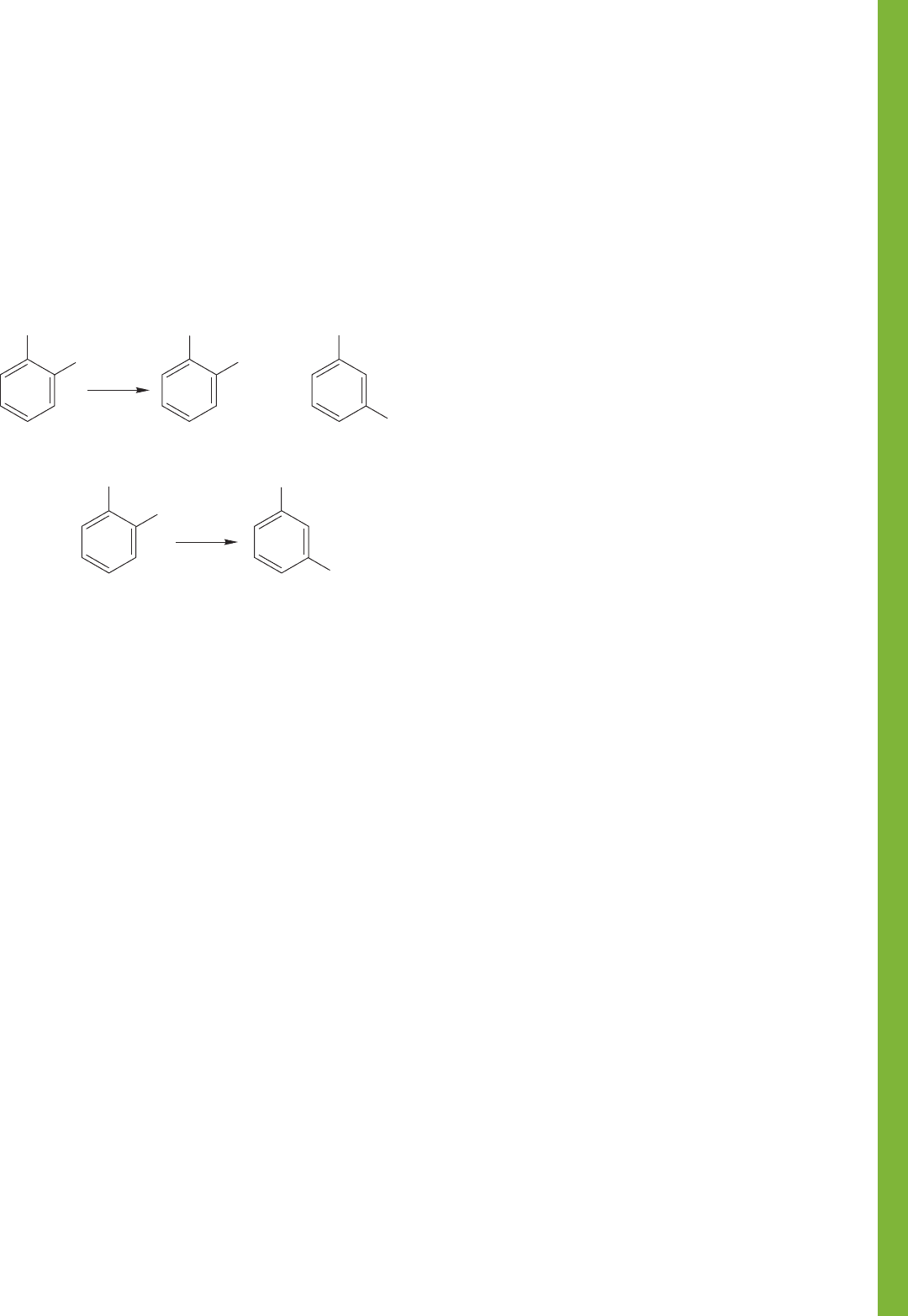
FIGURE 15.1 (a) A schematic view of a liquid chromatograph. (b) A chromatogram
showing the separation of a mixture of organic compounds into its components.
understanding of difference.The intensity of signals is determined by the technique
of integrating the signals in the spectrum.
2. Problem solving. Good spectroscopy problem solvers are good at solving spectroscopy
problems because they have solved lots of spectroscopy problems. There is no way out!
To be a decent spectroscopist you have to do many problems; the skill is highly experience-
intensive and there is only one way to gain that experience. Section 15.8 will give you one
way to approach spectroscopy problems, but it is certainly appropriate to develop your own.
For separating many organic molecules, gas chromatography (GC) is the tech-
nique of choice. Gas chromatographs are remarkably simple and it is still possible to
build one on your own that will function well in a research setting.The stationary phase
consists of a high-boiling material adsorbed on a solid support.High molecular weight
15.2 Chromatography
In Chapter 4 (p. 172), we briefly encountered column chromatography. All chro-
matography works in much the same way. The components of a mixture are forced
to equilibrate between an immobile material, the stationary phase, and a moving
phase.The more time one component spends adsorbed on the stationary phase, the
more slowly it moves. If it is possible to monitor the moving phase as it emerges
from the chromatograph, each component of the original mixture can be detected
and collected as it appears. The efficiency of column chromatography can be greatly
increased by a technique called high-performance liquid chromatography (HPLC).
In this variation, high-pressure pumps force the liquid phase through a column of
densely packed uniform microspheres, which provide an immense surface area for
adsorption. As the liquid elutes, a detector, often a small UV/vis spectrometer, mon-
itors the composition of the liquid.Figure 15.1 shows a schematic instrument as well
as a nice separation of a series of organic compounds.
698 CHAPTER 15 Analytical Chemistry: Spectroscopy
Carrier gas
Column
Helium
Oven
Injection
port
Sample in
syringe
Pure carrier
gas
Cooled
sample
receiver
Detector
Detector
filaments
Exit ports
(b)
(a)
0 4.0 5.0 6.0 7.0
Time (min)
Abundance
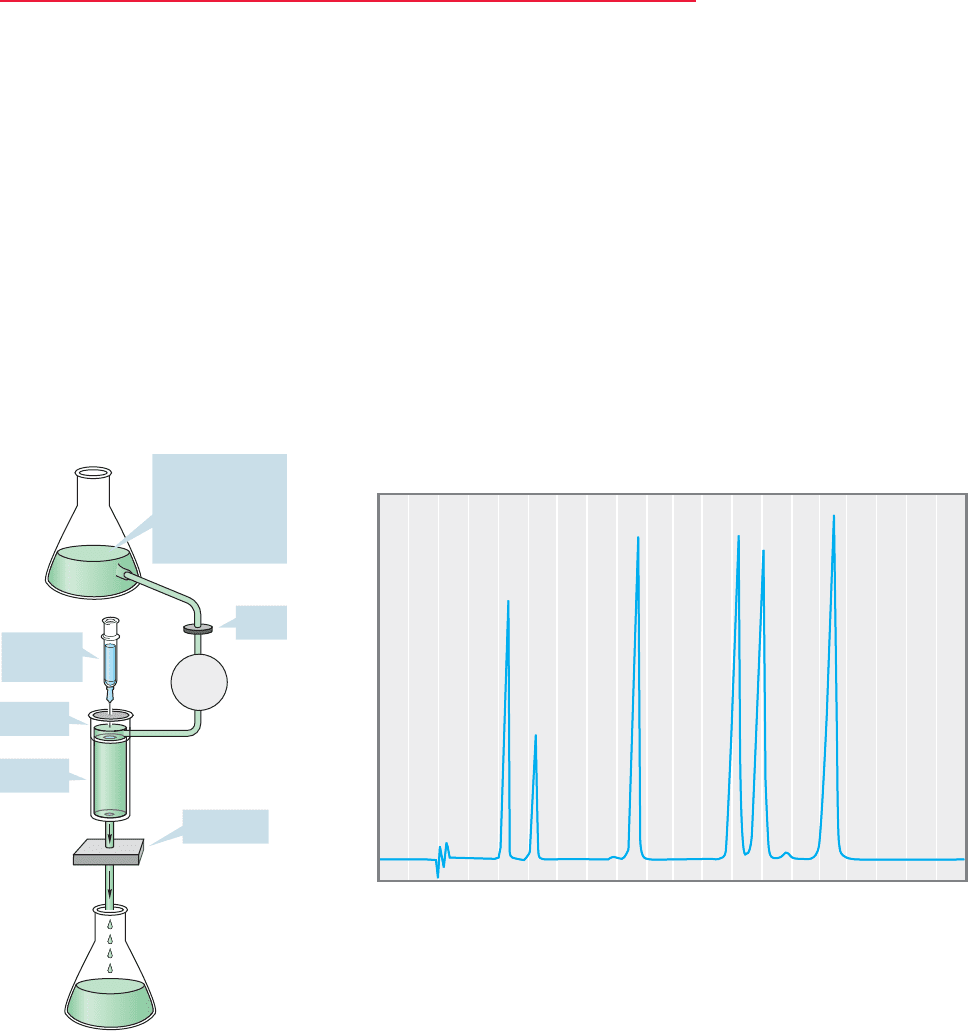
FIGURE 15.2 (a) A schematic
view of a gas chromatograph.
(b) A chromatogram showing the
separation of a mixture into its
components.
silicone oil polymers are often used,as well as many other organic chemicals.The mov-
ing phase is a gas, usually helium or hydrogen. Typically, the solid support is packed
in a coiled column through which the carrier gas flows. The mixture to be separated
is injected at one end. As the carrier gas moves the mixture through the column, the
various components are differentially adsorbed by the stationary phase and exist in an
equilibrium between the gas phase and solution. The more strongly a component is
adsorbed, the more slowly it moves through the column. At the end of the column,
there is a detector that plots the amount of material emerging as a function of time.
A schematic instrument and a typical chromatogram are shown in Figure 15.2.
There are many kinds of detectors used for gas chromatography; two common
ones are thermal conductivity and flame ionization. In a thermal conductivity detec-
tor, there are two sets of hot filaments whose temperature depends on the compo-
sition of the gas flowing over them.The detector can be set to zero for pure carrier
gas passing through the instrument over both filaments. As the components of the
mixture elute over one set of filaments, their temperature will change and the cur-
rent needed to bring them back to the zero point can be measured and plotted.
A flame ionization detector measures the current induced by ions created as the
effluent is burned. Again, a zero point for pure carrier gas is determined and devi-
ations from zero are detected and plotted as the components of the mixture elute.
All sorts of fancy bells and whistles are possible, and the art of creating effi-
cient GC columns has progressed immensely from the days when one packed large-
bore columns by hanging them down a stairwell, and pouring in the stationary
phase.The resulting columns were then tested for efficiency in the sometimes vain
hope that one would do a good enough job of separating the mixture of products.
One especially effective modification is the construction of capillary columns.Such
columns can be several hundred feet long and contain only a tiny amount of
