Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

Hydrogen Embrittlement Model
Hydrogen absorption is clearly responsible for SCC of high-strength steels in
aqueous environments [52], notably in the presence of H
2
S, where the fraction of
discharged hydrogen that is absorbed by the metal can approach 100% owing to
poisoning of the recombination reaction (H + H = H
2
) by sulfur adsorption [53].
The role of hydrogen in fcc systems remains controversial, with positive indications
in Al-Zn-Mg-Cu alloys [54,55] but waning evidence in austenitic stainless steels
and Ni alloys [56]. Transgranular SCC of stable austenitic steels at high tempera-
tures such as 300°C does not correlate with their susceptibility to hydrogen
embrittlement. Hydrogen discharge and absorption are favored when there is
acidification of the local environment by cation hydrolysis [54], especially if this
acidification damages or destroys a passive film in the crack. Once absorbed in the
metal, hydrogen can promote cleavage, intergranular separation, or a highly localized
plastic fracture. In hydride-forming metals such as titanium, formation of brittle
hydrides can be part of the fracture mechanism.
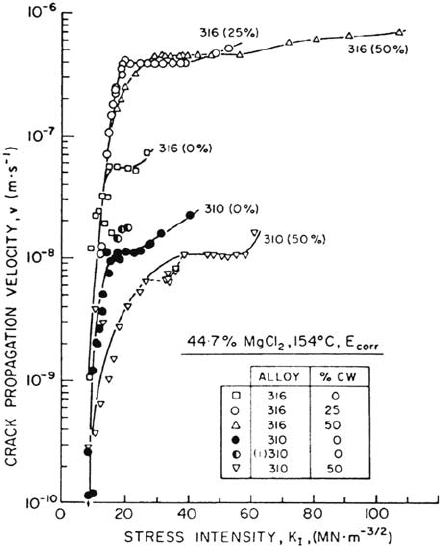
THE RATE OF SCC: EFFECTS OF STRESS INTENSITY FACTOR
“Classical” SCC phenomena, such as the cracking of brass in aqueous ammonia,
are classical because they occur at low stresses and at high rates: 10
–9
to 10
–6
m/s
or 0.1 to 100 mm/day. The crack velocity varies with the mode I stress intensity
factor (K
I
) as shown in Figure 3 [K
I
~
–
σ(πa)
1/2
, where σ is the stress and a is the
crack length] [57]. The plateau at intermediate K
I
values indicates that something
chemical rather than something mechanical is controlling the crack velocity; this
might be dissolution, diffusion, or adsorption. SCC has been detected growing
with very low velocities such as 10
–12
m/s (0.1 μm/day, or 1mm every 30 years)
[58]. Such processes, occuring in piping or steam generator tubes, threaten the
long-term integrity of nuclear power plants [59]. At these low rates and relatively
high temperatures (300°C), solid-state transport of substitutional elements becomes
a feasible part of the SCC mechanism, hence the aqueous SCC phenomenon starts
to blend with high-temperature oxidation and might be controlled by processes
such as grain boundary diffusion of atomic oxygen [60,61].
The important quantities in Figure 3 are the threshold stress intensity, K
th
or
K
ISCC
, and the region II crack velocity,
υ
II
. In strong materials that can suffer fast
fracture, the critical stress intensity or fracture toughness, K
IC
, terminates the life of
a component catastrophically, whereas ductile alloys fail by leakage or loss of cross
section. The classical SCC systems have low K
ISCC
values, especially austenitic
stainless steel in hot chloride, where values as low as 1 Mpa m
1/2
have been
reported [62,63]; there is a wide range of values in the literature, up to 12 Mpa m
1/2
.
The superior performance of duplex (austenoferritic) stainless steels in these
environments is associated with a much higher (3–10 times) K
ISCC
value [62]; thus
duplex components must be highly stressed or defective in order to fail by SCC in hot
chloride solutions. Incidentally, all the K
ISCC
values mentioned, even the lowest, are
high enough to cause yielding at the crack tip.
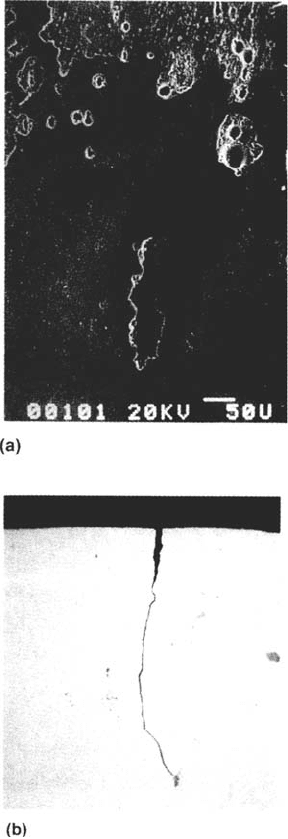
THE APPEARANCE OF SCC
SCC failures are macroscopically brittle in the sense that the ductility and load-
bearing capacity of the material are impaired. Microscopically, the cracks are either
402 Newman
Copyright © 2002 Marcel Dekker, Inc.
intergranular (sometimes along old boundaries such as those of prior austenite in
martensitic steels) or transgranular and cleavage-like (Fig. 4a and b). In duplex
microstructures, one phase often cracks more easily than the other, leading to charac-
teristic cracking patterns (Fig. 4c). Plastic deformation always accompanies crack
growth and plays a key role in most cracking mechanisms. Sometimes the fracture
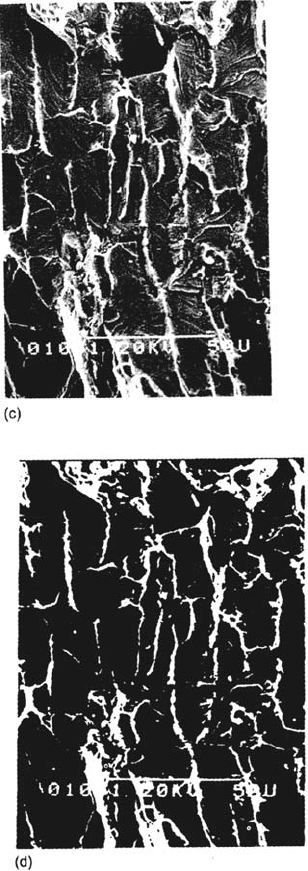
surfaces show evidence for stepwise (discontinuous) crack advance, especially in
transgranular SCC [35–39] (Fig. 5). It is possible to produce similar crack front
markings by periodic over- or underloading [37,38,64,65] and to show that the
natural crack arrest marks do indeed correspond to positions of the crack front.
The average orientations of transgranular cracks are specific, e.g., {110} in α-
brass [35], but such surfaces are sometimes composed of very fine, alternating
{111} microfacets [66]. Fracture of the ligaments between the {110} crack facets,
which produces the river lines on the fracture surface, is usually a ductile process
such as necking (in pure Cu) or crystallographic shear (in α-brass). The latter
favors crack propagation at low stresses owing to the small displacement involved
[39]. The presence of intact ligaments behind the crack tip shields the tip from
some of the applied stress intensity and explains why cracks are often much sharper
[34] than one would calculate using elastic-plastic fracture mechanics. Ligament
fracture, or its absence, can play a role in the formation of the crack arrest markings
shown in Figure 5.
Stress-Corrosion Cracking Mechanisms 403
Figure 3 Examples of crack velocity–stress intensity curves for SCC, showing the
effects of alloy composition and cold work on SCC of austenitic stainless steels in a hot
chloride solution. (From Ref. 57. Courtesy of Pergamon Press.)
Copyright © 2002 Marcel Dekker, Inc.
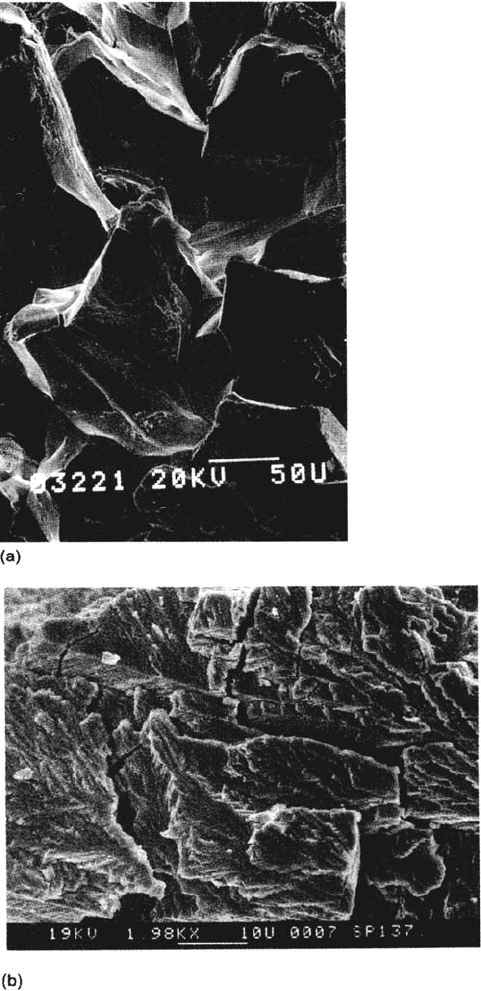
404 Newman
Figure 4 Examples of SCC fracture surfaces, all obtained with the slow strain rate test.
(a) Intergranular SCC of α-brass in Mattsson’s [109] solution, pH 7.2. (b) Transgranular
SCC of α-brass in 15 M cuprous ammonia solution equilibrated with Cu and Cu
2
O
(courtesy of T. Shahrabi). (c) SCC of 25Cr duplex stainless steel plate (50% ferrite) in
NaCl-H
2
S solution at 80°C, showing flat, cleavage-like cracking of ferrite and mainly
ductile fracture of austenite (courtesy of V. M. Salinas Bravo). (d) High-contrast schematic
of (c), showing necked austenite grains as light (high) areas.
Copyright © 2002 Marcel Dekker, Inc.
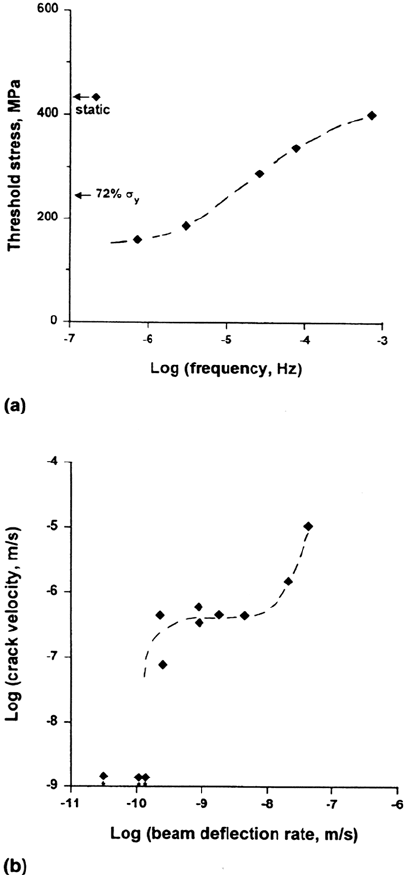
INITIATION OF SCC: THRESHOLD STRESSES AND ROLE
OF LOCALIZED CORROSION
SCC initiation at smooth surfaces shows a threshold stress (σ
th
) that varies from
20% to more than 100% of the yield stress (σ
y
), depending on the metal and the
environment. Thermal stress relief of welded or cold-worked components can
prevent SCC if σ
th
/σ
y
is fairly high, e.g., 0.7. In some systems, σ
th
can be lowered
by low-amplitude dynamic loading (Fig. 6), which is very important in practice [67].
Stress-Corrosion Cracking Mechanisms 405
Copyright © 2002 Marcel Dekker, Inc.
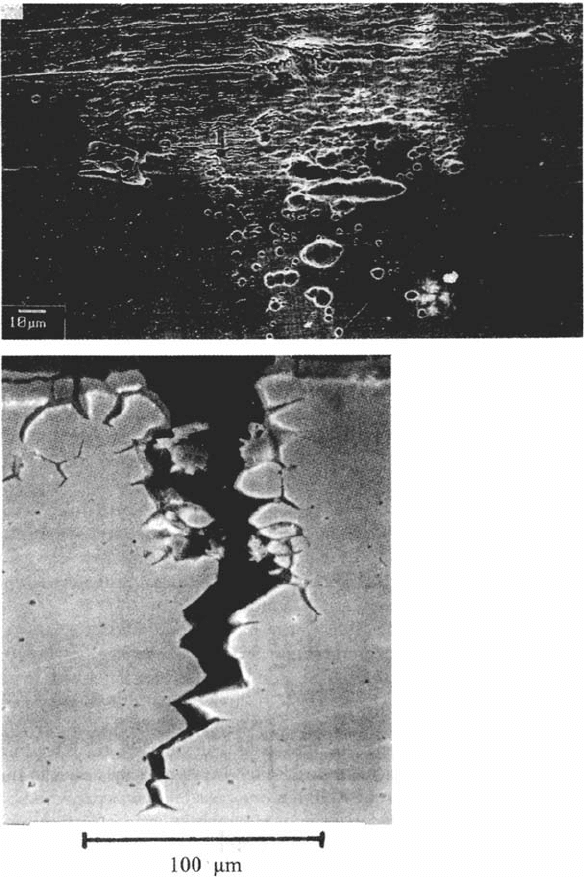
SCC is usually nucleated by some form of localized corrosion; in chloride-SCC of
stainless steels [68] or aluminum alloys [54], cracks start from areas of pitting,
intergranular corrosion, or crevice corrosion that create the stress concentration
and the acidity required for cracking (Fig. 7). In SCC of C-Mn or low-alloy steels,
intergranular corrosion occurs along segregated zones rich in carbon, nitrogen, or
phosphorous and provides a stress concentration (helps to achieve K
ISCC
) [27]
406 Newman
Figure 5 (a) Crack arrest marks on a transgranular SCC surface, observed near the overload
region on a slow strain rate specimen (∈
.
= 10
–5
s
–1
) of 70–30 brass tested in pH 7.2 Mattsson’s
[109] solution with 0.05M NaCl; (b) schematic drawing of the transgranular SCC process and
the mechanism of striation formation. (After Refs. 35–39. Courtesy of NACE.)
Copyright © 2002 Marcel Dekker, Inc.
(Fig. 8). Pure iron shows no intergranular corrosion or SCC in this kind of solution.
In mechanistic studies, one often comes across references to rupture of passive films,
but in fact SCC rarely occurs in a true (local) passive state. There is usually some
form of intergranular corrosion, pitting, crevice corrosion, or dealloying, or else SCC
occurs in a conventional active state via a hydrogen embrittlement mechanism (as in
high-strength, low-alloy steels). There are surface films in cracks, and these need to
Stress-Corrosion Cracking Mechanisms 407
Figure 6 (a) The effect of low-amplitude cyclic loading on the threshold mean stress for
SCC of C-Mn steel in carbonate-bicarbonate solution. (b) The corresponding data for
growth of single cracks [67]. (Redrawn from Ref. 164.)
Copyright © 2002 Marcel Dekker, Inc.
be ruptured to expose bare metal, but in many cases (such as carbon steel in sodium
hydroxide), the film has a precipitated or gel-like nature rather than conforming to
the conventional idea of a compact passive film a few nanometers in thickness.
408 Newman
Figure 7 Aspects of the nucleation of SCC by localized corrosion. (a) Peak aged Al-Li-Cu-Mg
alloy 8090 after unstressed preexposure in aerated 3.5% NaCl for 7 days. (b) SCC initiated
from one of the fissures shown in (a), following removal of the solution and continued
exposure to laboratory air under a short transverse tensile stress (courtesy of J. G. Craig,
unpublished data). (c) Creviced region of 316L stainless steel after a slow strain rate test
in 0.6M NaCl + 0.03M Na
2
S
2
O
3
at 80°C and an applied anodic current of 25μA, showing
unstable pitting leading to crevice corrosion and SCC initiation (courtesy of M. I. Suleiman).
Copyright © 2002 Marcel Dekker, Inc.
METALLURGY OF SCC
The main metallurgical variables in SCC are
Solid solution composition
Grain boundary segregation
Phase transformations and associated solute-depleted zones
Stress-Corrosion Cracking Mechanisms 409
Figure 8 Caustic SCC of an Fe-3Ni-1Mo pure alloy at –400 mV (Hg-HgO) in a
slow strain rate test in 9 M NaOH at 98°C [85], showing the intergranular corrosion
that initiated SCC.
Copyright © 2002 Marcel Dekker, Inc.
Duplex structures
Cold work
In the case of hydrogen-induced SCC of high-strength steels, one could add strength,
no matter how this is achieved. Environmental criteria for SCC are necessary but not
sufficient: SCC will not occur without a susceptible metallurgy. The only exceptions
to this rule are transgranular cracking processes in pure metals, e.g., iron in anhydrous
ammonia [69] or copper in sodium nitrite solution [65,70].
“Advanced” materials such as metal-matrix composites also show SCC
phenomena, but these are not distinctive and research has been quite qualitative in
this area.
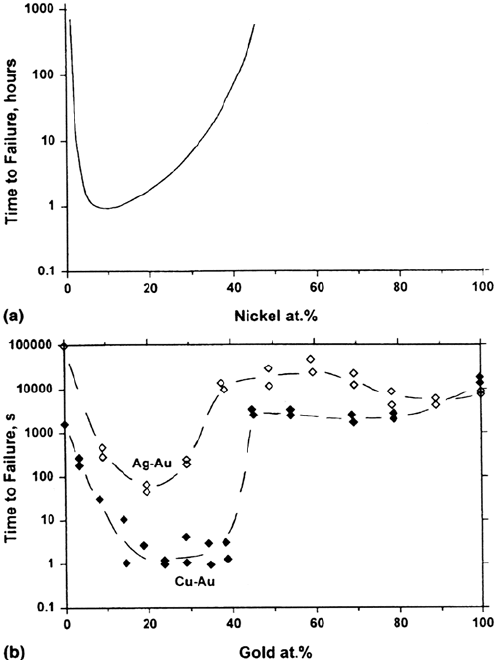
Solid solution composition classically controls the SCC of brasses [71,72],
austenitic stainless steels in hot chloride solutions [73,74], and noble-metal alloys
[75]. In all these systems there is evidence that dealloying dominates the SCC
mechanism, although this remains controversial for stainless steels. Transgranular
SCC ceases above a critical content (parting limit or dealloying threshold) of the most
noble alloying element, either 80–85% in Cu-Zn or Cu-Al or 40% in Ni-Cr-Fe or
Au-X alloys (Figs. 9 and 10). These values have been interpreted using percolation
theory [72,76,77]. In all these systems the crack walls are either oxide free or else
have thick porous oxides that allow contact of metal and electrolyte [78,79].
Flangan and co-workers [80,81] have shown that SCC of Au-Cu alloys occurs below
the critical potential for macrodealloying (E
c
) and consequently reject film-induced
cleavage for this system. However, because dealloying in Au-Cu occurs in a localized
mode resembling pitting [82,83], this conclusion may be premature. The concentration
of Cl
–
that occurs in the pit lowers E
c
locally by complexing Cu(II), and any transient
electrochemistry should be done in a CuCl
2
-rich solution, simulating the crack
environment, if it is to reproduce the behavior of a crack tip.
Minor alloying elements (C, P, N, As, …) can have several distinct effects on
the electrochemistry of SCC. In caustic intergranular SCC of carbon steel, carbon or
nitrogen segregation (and/or precipitation) at grain boundaries interferes with passive
film formation and may affect plasticity [27], and phosphorus segregation introduces
SCC in a new, more oxidizing range of potentials [84,85] (Fig. 11). In chloride-SCC
of austenitic stainless steels, the group 5B elements are so influential on transgranular
cracking that it is difficult to crack high-purity ternary alloys in the laboratory [86].
This is not understood in detail, but one possibility was proposed by the author
[87,88]: adsorption of group 5B elements is known to reduce surface self-diffusivity
in electrolytes, and dealloyed layers need extremely fine porosity to cause SCC by
film-induced cleavage, so if the 5B elements are absent there is a rapid coarsening of
the porosity and a reduced susceptibility to SCC (Fig. 12). This idea unifies the
well-known effect of arsenic in brass with that of N or P(or even As) in stainless steel.
Phase transformations are used in strong alloys to provide dispersion
(precipitation) strengthening and also occur in ductile alloys during welding or simply
as a by-product of the traditional metallurgy of the system. In strong alloys, there is
a different sequence of nucleation and growth at grain boundaries compared with the
matrix [54]. Depending on the phase(s) formed at the grain boundary, enhanced
reactivity may result, either of the phase itself or of an associated solute-depleted
zone [89]. The most important of these systems are the Al-Zn-Mg (7000 series)
aerospace alloys, which become especially susceptible to SCC when further alloyed
410 Newman
Copyright © 2002 Marcel Dekker, Inc.
with Cu. Grain boundary segregation of Cu has been detected in susceptible
conditions using analytical electron microscopy [90]. Hydrogen plays a role in this
cracking [54], and it is difficult to seperate the roles of hydrogen and dissolution,
let alone the contributions of the reactive MgZn
2
phase, the segregated Cu,
the solutedepleted zone, and the grain boundary strength. Precipitation of grain
boundary phases containing Cu-depleted (less noble) regions; this is easily
demonstrated in Al-Cu (2000 series) systems, but the narrow and deep Cu depletion
occurs only in underaged, noncommercial heat treatments, so practical problems
are relatively rare, especially as these alloys are normally used in sheet rather than
extrusion form (the arrangement of grain boundaries in unrecrystallized sheet
inhibits transverse SCC). In the 7000-series alloys the high SCC susceptibility
is shifted to the peak strength condititon—a major inconvenience to users, as it
denies them the use of the strongest condition of the alloy (it must be overaged for
most applications). Progress is being made in complex thermomechanical treatments
to improve the toughness and SCC resistance at peak strength [54].
Stress-Corrosion Cracking Mechanisms 411
Figure 9 Direct and indirect evidence for a role of dealloying in SCC. (a) The Copson
curve showing the dependence of SCC in austenitic stainless steels on their nickel content
[73]. (b) Similar behavior for gold alloys in aqua regia, where dealloying is known to control
SCC [75].
Copyright © 2002 Marcel Dekker, Inc.
