Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.


Intergranular corrosion does not necessarily correlate with SCC in aluminum
alloys. The 6000-series (Al-Mg-Si) alloys such as 6061 can suffer intergranular
corrosion but never fail by SCC. Examination of fractured specimens following
intergranular corrosion shows crystallographic tunneling attack on either side of
the grain boundary.
Precipitation strengthening of steels, such as the PH grades of ferritic stainless
steel containing Cu, affects SCC when the operative mechanism is hydrogen
embrittlement [91]. Precipitation, without strengthening, can occur at grain
boundaries and is responsible for the most expensive form of SCC: the cracking of
412 Newman
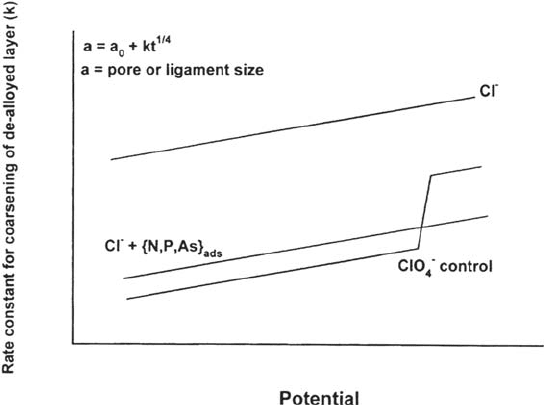
Figure 10 Direct and indirect evidence for a role of dealloying in SCC: SCC and
dealloying of Cu-Zn and Cu-Al monocrystals in cuprous ammonia solution as a function
of solute content [72].
Figure 11 SCC penetration as a function of potential for carbon steel and low-alloy Cr-Mo
steel tested in hot NaOH solution, showing a cracking regime at higher potential induced
by P segregation to grain boundaries [66]. See also Figure 22.
Copyright © 2002 Marcel Dekker, Inc.
weldsensitized austenitic stainless steel in oxygenated high-temperature water
(boiling-water reactor pipe cracking) [59]. The sensitization process in AISI type 304
stainless steel consists of the precipitation of chromium carbides at the grain
boundaries in the zone either side of a weld where the temperature has reached
about 600–800°C. This leaves narrow Cr-depleted zones that repassivate more
slowly than the matrix and therefore provide an active crack path. Effective remedies
are the reduction of carbon content to about 0.03% (the 304L grade) or the addition
of alternative carbide formers (Ti, Nb, e.g., the 321 grade). The fraction of sensitized
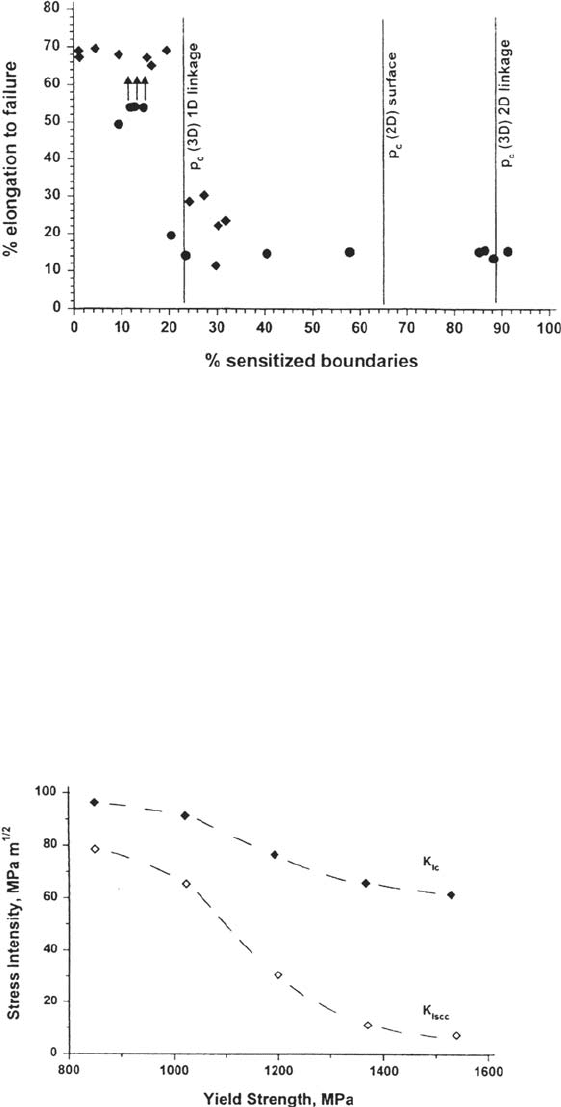
grain boundaries required to provide a continuous crack path in slow strain rate
tests has been shown to conform to a percolation model with a percolation threshold
of about 23% [92] (Fig. 13). Under static loads the required fraction of sensitized
boundaries may be higher.
Other phase transformations occuring in austenitic and duplex stainless steels
(σ phase, α′ phase) generally produce discontinuous Cr-depleted zones around
these Cr-rich phases, so crack growth is not necessarily enhanced, although pitting
resistance is greatly degraded by σ phase, so SCC is initiated much more easily
[91]. The austenite-ferrite transformation will be considered later.
Martensite is very susceptible to hydrogen embrittlement, and strong low-alloy
steels based on tempered martensite have an inescapable tendency for hydrogen-
induced SCC in saltwater or other neutral environments [93]. Bainite can be the basis
of strong steels (although not as strong as lightly tempered martensites) that are
slightly less susceptible. Depending on the flux of hydrogen into the steel (highest in
acidic H
2
S, lower in NaCl or fresh water, lower still in moist air), curves of K
ISCC
versus yield strength can be drawn for martensitic steels as shown in Figure 14.
A current challenge is to improve the hydrogen embrittlement resistance of quenched
and tempered steels in the 700–850Mpa range of yield strengths so that they can be
used in wet H
2
S environments; one strategy is to exploit the greater resistance of
Stress-Corrosion Cracking Mechanisms 413
Figure 12 Schematic drawing of the proposed mechanism of action of group 5B elements
on chloride-SCC of austenitic stainless steels [88].
Copyright © 2002 Marcel Dekker, Inc.
recrystallized ferrite that forms in some of these steels. Some specialized
high-strength steels can show superior performance, such as cold-drawn pearlite [94]
(prestressing wire or tire cord) and especially work-hardened, high-nitrogen austenite
[58], which is virtually immune to hydrogen embrittlement. Neither of these materials
is suitable as a general-purpose structural steel. Cold-drawn steel wire derives its
414 Newman
Figure 13 Dependence of SCC on the fraction of sensitized grain boundaries for slow
strain rate tests of type 304 stainless steel in sodium thiosulfate solution, showing behavior
in accordance with a 3D percolation model for linkage of sensitized grain boundary facets
with a sharp cracking threshold at 23% [92]. The thresholds at 65% and 89%, representing
growth of the crack as a continuous rumpled sheet, may be relevant in constant-load tests
where ductile ligaments can arrest the cracks.
Figure 14 Dependence of K
ISCC
and K
IC
on yield strength for AISI 4340 high-strength
steel tested in flowing seawater. (Redrawn from Refs. 93 and 152.)
Copyright © 2002 Marcel Dekker, Inc.
SCC resistance from its highly directional microstructure; the resistance of such
microstructures to transverse, intergranular SCC is well known in many systems
including aluminum alloys and steels (Fig. 15).
Duplex structures are defined as alloy microstructures in which two phases
are present in comparable proportions. Duplex (austenoferritic) stainless steels
have become very popular materials because they combine strength and SCC
resistance and can be welded with good microstructural control [91,95]. Their pitting
and crevice corrosion resistance compare favorably with those of austenitic steels
of similar Mo content.
Stress-Corrosion Cracking Mechanisms 415
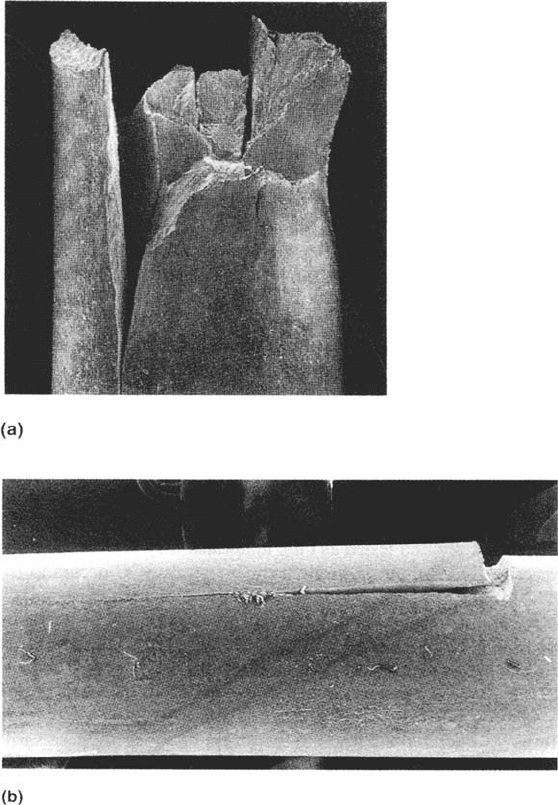
Figure 15 Example of transverse SCC resistance in a highly directional microstructure:
5-mm-thick wires of martensitic prestressing steel, notched and tested at a constant load
[higher in (a) than in (b)] in 0.6 M NaCl solution. (Courtesy of A.S. Doughty, unpublished data.)
Copyright © 2002 Marcel Dekker, Inc.
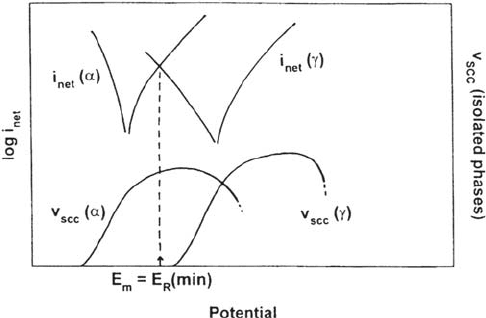
The SCC resistance of duplex stainless steels is manifested as a high value
of the threshold stress intensity, K
ISCC
, compared with austenitic steels [62] (Fig. 16).
This arises from the different chemistry of the individual phases, which gives
them, individually, different optimal ranges of potential for SCC (higher for γ,
lower for α). Because there can only be one electrode potential at the tip of the
crack, at least one of the phases must be below or above its best cracking potential
(Fig. 17). If the individual phases are synthesized and tested as bulk materials,
they both crack easily with very low K
ISCC
values [62,95], but in modern duplex
steels SCC occurs in the ferrite and is arrested by the austenite (Figs. 18a and 4c).
However, if there is a strong oxidant present, or if oxygen is supplied through a
thin boiling layer (as in evaporating seawater, which leaves behind a layer of
saturated MgCl
2
solution), then the austenite is no longer protected by the ferrite
(Fig. 18b) and K
ISCC
drops significantly. Such results are consistent with the
reported effect of anodic polarization in deaerated MgCl
2
solution [91].
Cold work reduces the ductility and fracture toughness but does not necessarily
increase the SCC velocity or reduce K
ISCC
. In austenitic stainless steels that transform
partially to martensite, the region II crack velocity in hot chloride solution is increased
by cold work, and the crack path becomes partly intergranular [57,96,97]. This
may be associated with a change of mechanism. In alloy 600 exposed to reducing
high-temperature water, the velocity of intergranular SCC increases dramatically
with increasing cold work [59,60,98]; according to Scott and Le Calvar [60],
416 Newman
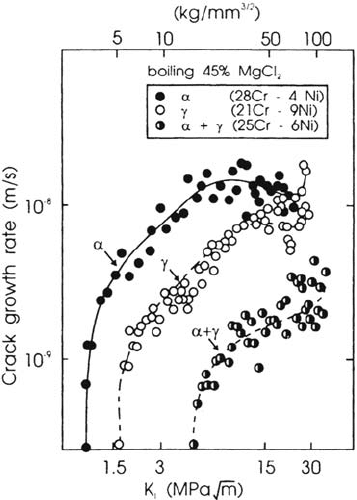
Figure 16
υ
-K curves for a duplex stainless steel and its individually synthesized phases
in hot chloride solution [62].
Copyright © 2002 Marcel Dekker, Inc.
this may be related to enhanced intergranular oxidation, a familiar effect in
high-temperature gaseous environments.
THE ELECTROCHEMISTRY AND MECHANISMS OF SCC
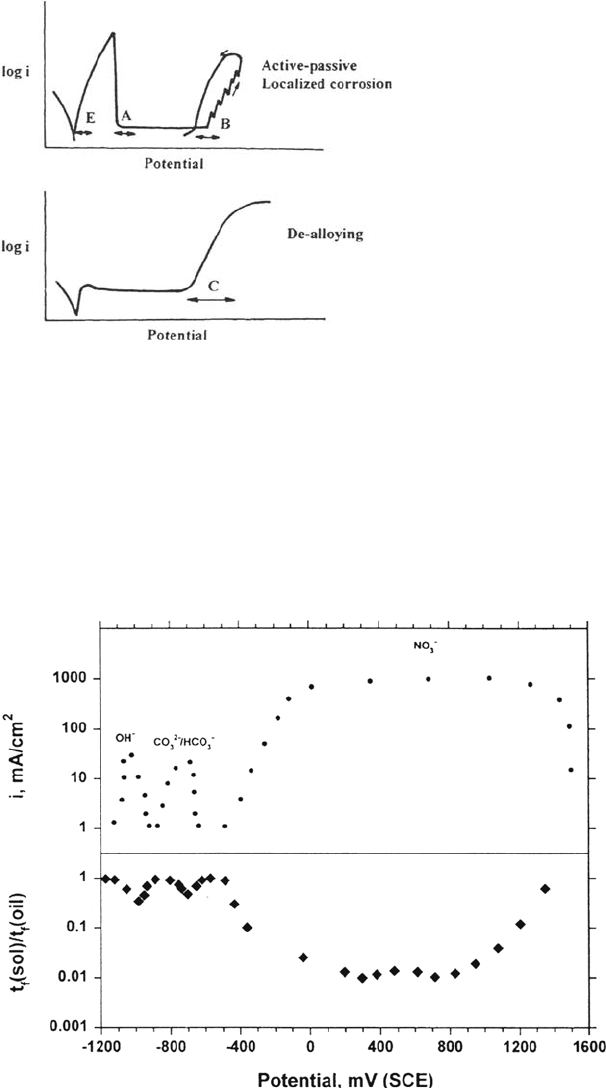
There are at least five electrochemical conditions that can lead to SCC in electrolytes,
given that the material has a susceptible metallurgy; these are shown schematically
in Figure 19:
A: A state of imperfect passivity near an active-passive transition, e.g., carbon steel
in aqueous hydroxide, nitrate, or carbonate-bicarbonate solutions [26,27].
B: Astate of slow, chloride-induced localized corrosion in stainless steels, aluminum
alloys, or titanium alloys [54,68].
C: A state of surface dealloying with no continuous oxide, e.g., gold alloys in many
aqueous solutions [75,99]. Dealloying may also occur within localized corrosion
sites in passive alloys such as austenitic stainless steels.
D: Formation of unusual surface films that may play a casual role in SCC, e.g.,
nitrides formed on steel in anhydrous ammonia [69,100]. There are a number
of related, room-temperature gaseous systems such as Zr/I
2
[101] and high-
strength steel/Cl
2
[102,103]. Where such films cause only intergranular cracking
and this occurs only at elevated temperatures, it is almost certainly due to
penetration of atoms of the gaseous species along grain boundaries [60] and
not to surface mobility as proposed by Bianchi and Galvele [104].
E: An active state leading to hydrogen-induced SCC, usually in high-strength steel
[52,93,105,106] or medium-strength steels in H
2
S media [53,107].
The most extensive mechanistic investigations have been carried out on passive
systems showing cracking of type A or type B. All SCC mechanisms rely on the
exposure of bare metal to the environment, and if this is too brief (owing to
Stress-Corrosion Cracking Mechanisms 417
Figure 17 Schematic electrochemistry of chloride-SCC of duplex stainless steel.
Copyright © 2002 Marcel Dekker, Inc.
immediate repassivation), none of the likely cracking agents, such as dissolution
or hydrogen, can operate. This explains the importance of a slow repassivation
rate [26] (Fig. 20). The kinetics of repassivation on bare metal surfaces have been
measured by numerous potentiostatic techniques such as scratching, rapid straining,
and laser illumination [108]. As well as active-passive transitions with potential,
similar transitions occur with pH under free-corrosion conditions and may lead to
critical pH requirements for SCC, notably for α-brass in near-neutral ammoniacal
solutions [109,110].
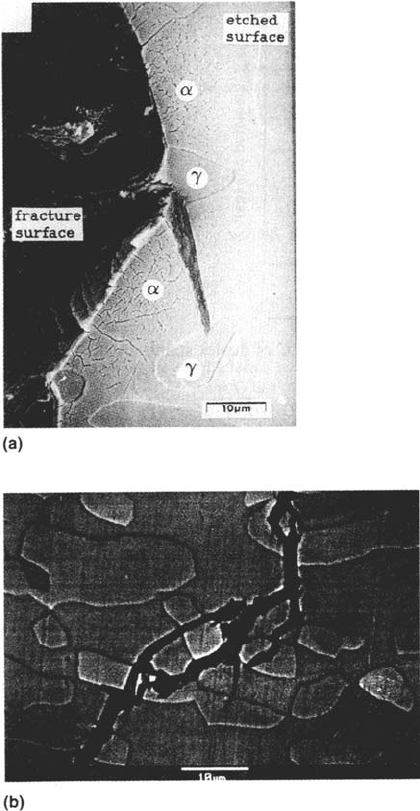
418 Newman
Figure 18 (a) Typical cracking pattern of chloride-SCC in a duplex stainless steel, showing
cracking of ferrite and partial arrest by austenite (courtesy of W. J. R. Nisbet). (b) Cracking
of a similar steel in evaporating seawater, showing little or no restraint of the crack by the
austenite under this more oxidizing condition (courtesy of H. Ezuber).
Copyright © 2002 Marcel Dekker, Inc.
This classification of SCC is useful in practice, as it can lead to remedial
measures or material developments without necessitating a complete understanding
of the atomistic mechanism. For example, in type B SCC it is sufficient to prevent the
Stress-Corrosion Cracking Mechanisms 419
Figure 19 Correlation between SCC and various types of electrochemical behavior. The
letters A, B, C and E refer to the types of SCC defined in the text. The arrows indicate
possible potential ranges of SCC.
Figure 20 Correlation between inverse passivation rate (difference between current
densities in fast and slow polarization scans) and slow strain rate SCC behavior for carbon
steel in three SCC environments [26].
Copyright © 2002 Marcel Dekker, Inc.
localized corrosion process or to ensure that it occurs too rapidly to allow crack
nucleation [68,111,112]. This topic will be discussed in detail later.
Type A SCC normally occurs by intergranular slip-dissolution and is related to
grain boundary segregation or precipitation. Most of our present understanding
has been developed by studying the passivation characteristics of bulk material
[26,27], but alloys or compounds simulating the grain boundary composition can be
useful for assessing the effect of segregation or precipitation on the repassivation
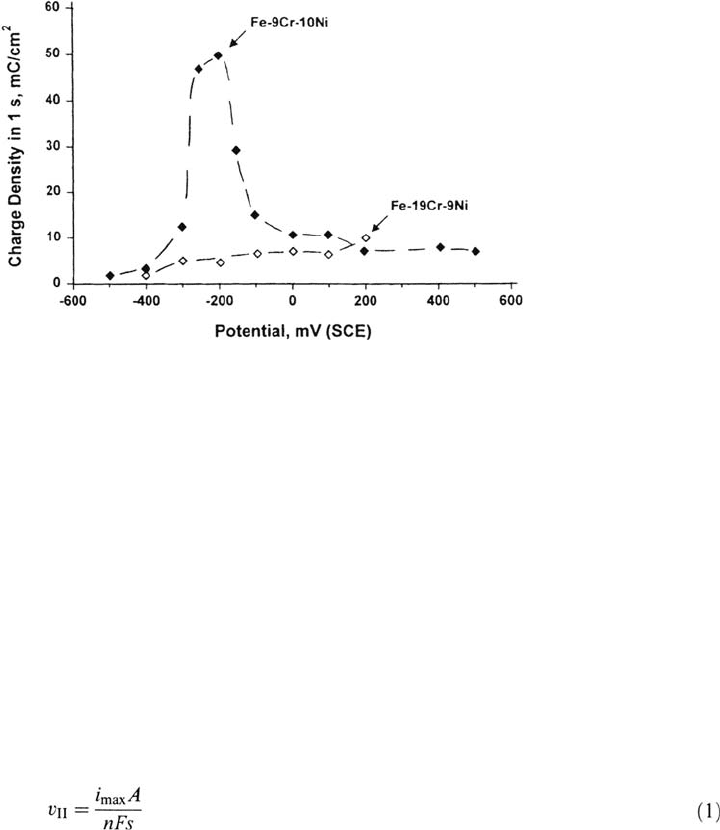
kinetics [25,84,85] (Fig. 21). Rapid solidification can be used to incorporate large
concentrations of segregants such as phosphorus [85] (Fig. 22). In a number of
systems it has been shown that the region II SCC velocity (
υ
II
) correlates with the
maximum (reasonably sustained) anodic current density on a bare surface (i
max
), via
420 Newman
Figure 21 Delayed repassivation of material simulating the chromium-depleted grain
boundary zone in sensitized stainless steel, measured in sodium thiosulfate solution using
a scratch method [32].
where A is the atomic weight (relative atomic mass) of the metal and s is its
density—that is, crack growth occurs by strain-enhanced intergranular corrosion
[26]. The role of stress is to produce plastic strain in the metal, which fractures a
brittle oxide layer.
SCC data are presented as velocity versus static stress parameter such as K
I
, yet
it is the dynamic plasticity at the crack tip that actually features the newly formed
oxide film [67,113]. This problem was considered by Vermilyea [24], who showed
that under a static stress the corrosion process could advance the crack tip into a
region that had reached an equilibrium distribution of plastic strain (ε
p
), and produce
a strain transient (Δε
p
) that could fracture a newly formed oxide (Fig. 23). Later
Sieradzki [44] showed that the depth of transient corrosion required in Vermilyea’s
original model was unreasonably large (~ μm), confirming that static slip-dissolution
models can apply only to intergranular SCC, where there is a directional active path
with a very low repassivation rate [33]. It is not clear how the shielding of the crack
Copyright © 2002 Marcel Dekker, Inc.
tip stress intensity by intact metal ligaments [39] enters into this argument—it
certainly makes real cracks much sharper than one would expect from fracture
mechanics.
Stress-Corrosion Cracking Mechanisms 421
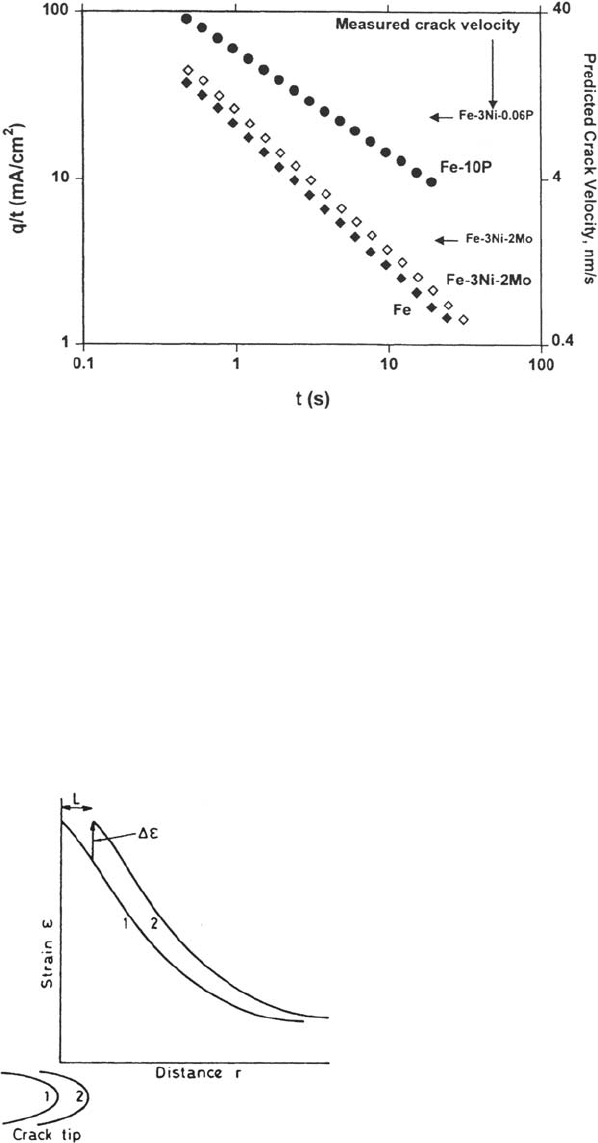
Figure 22 Anodic current decays for Fe, Fe-3Ni-2Mo, and an Fe-10P amorphous alloy
(simulating a grain boundary) in 9 M NaOH solution at 98°C and –400 mV (Hg-HgO) [85].
The original i(t) decays have been converted into q(t) (charge density versus time) and then
into q(t)/t for comparison with a slip-dissolution model of SCC where the crack velocity is
proportional to q(τ)/τ with τ being the interval between film rupture events at the crack tip.
The indicated crack velocities measured for Fe-Ni-Mo and Fe-Ni-P alloys are consistent with
a single value of τ(6 ± 3 s), giving some validity to the use of a simulated grain boundary alloy.
Figure 23 Plastic strain distribution ahead of a stress-corrosion crack tip and the effect
of corrosion, according to Vermilyea [24].
Copyright © 2002 Marcel Dekker, Inc.
