Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

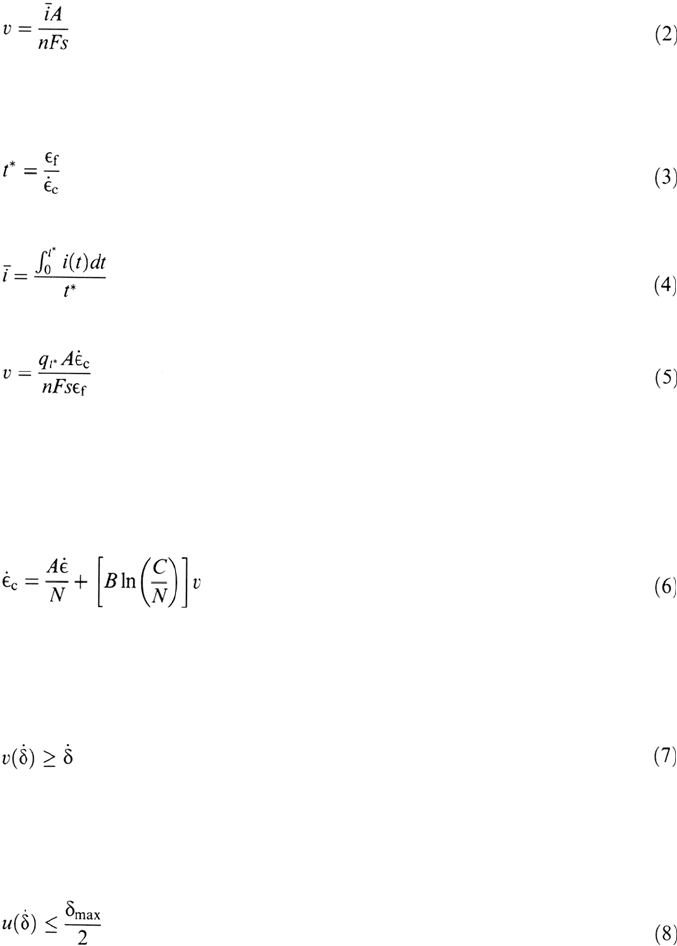
The introduction of a crack tip strain rate (∈
.
c
) enables a wide range of SCC
information to be rationalized [30]. For example, Eq. (1) can be generalized to
422 Newman
where i is the time-averaged anodic current density at the crack tip. For a cyclical
process in which the bare surface is exposed by film rupture, partially repassivated,
and then exposed again, the interval t
*
between the film rupture events is given by
where ∈
f
is the fracture strain of the newly formed oxide film; thus i is given by
Writing the integral as q
t
, this gives
The film rupture interval t
*
decreases with increasing strain rate until eventually
i = i
max
and
υ
approaches the maximum or region II velocity
υ
II
. For cyclic loads
or slow strain rate tests,
∈
.
c
is relatively easy to estimate, and one can test the
slip-dissolution model as a function of strain rate and potential [30–33]. More
generally, one can include a continuum version of Vermilyea’s concept [114]:
where ∈
.
is the bulk strain rate, N is the number of identical circumferential cracks
per unit length, and A, B, and C are constants.
The disappearance of SCC above a critical applied strain rate (mechanical
blunting) is rationalized by recognizing that, to maintain a sharp crack.
where δ
.
is the rate of increase of the crack tip opening displacement (δ) due to the
applied strain rate [33]. Sometimes SCC also vanishes below a particular strain
rate, especially in systems that show extremely slow repassivation (chemical
blunting); now a possible condition for crack stability is
where u(= t*
υ
) is the depth of material dissolved between film rupture events and
δ
max
is the maximum value of the crack tip opening displacement consistent with
continued crack growth. This might correspond to the transition from small-scale
to general yielding, given δ
max
~
–
2–3μm for carbon steel in typical test geometries
[see Eq. (9) below]. That is, the amount of corrosion between film rupture events
must not enlarge the mechanical crack opening to such an extent that the crack
degenerates into a blunt slot. Because t
*
is a function of δ
.
this leads to another equation
Copyright © 2002 Marcel Dekker, Inc.
similar to Eq. (7); the regions of SCC, chemical blunting, and mechanical blunting
can then be mapped for various functions i(t) (Fig. 24). The main uncertainties
in such a procedure are the composition of the material that is reaching at the
crack tip [this obviously affects i(t)] and the restricted crack geometry, which
alters the environment and increases both the solution resistance and the effective
diffusion length. However, the wedge-shaped geometry of a real crack [33,115,116]
significantly reduces the solution resistance and dissolved cation concentration
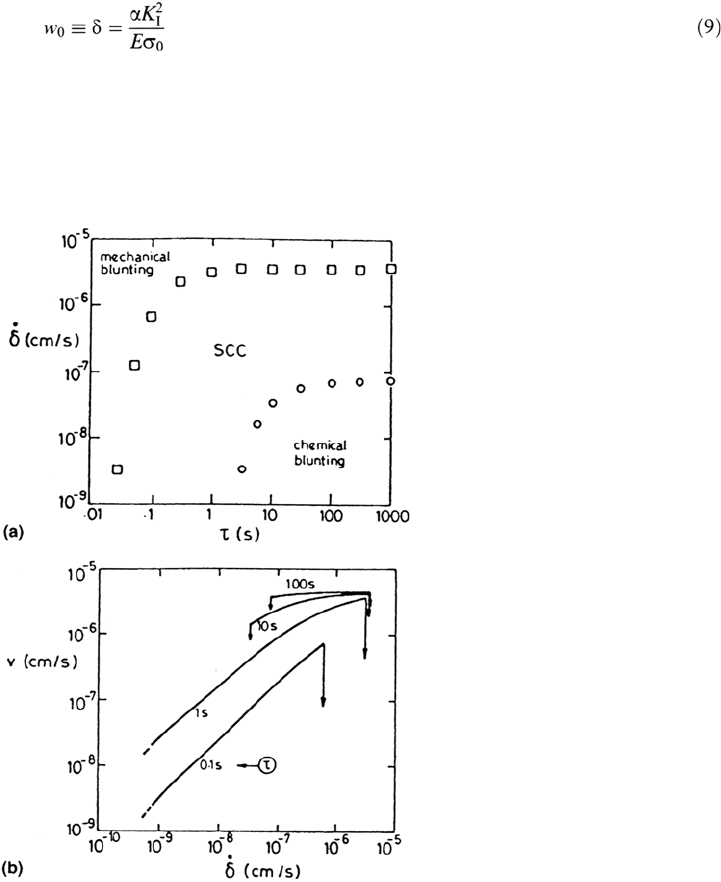
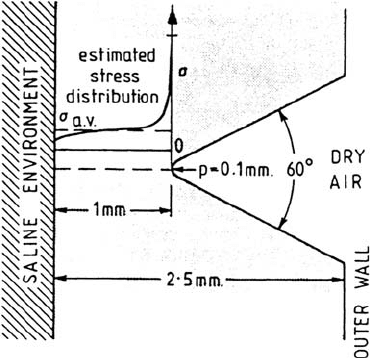
compared with a one-dimensional slot. The crack tip opening displacement w
0
(≡δ,
see Fig. 25) is given by
Stress-Corrosion Cracking Mechanisms 423
where E is Young’s modulus and σ
0
is the flow stress (roughly the average of the
yield and ultimate stresses); α is a constant that varies with the stress state and strain
hardening coefficient and is about 0.3 for carbon steel under plane strain [117].
Figure 24 The effect of strain rate on SCC velocity by slip-dissolution for a system showing
a t
–1
current decay on a fresh surface, for various values of the decay time constant [33].
Copyright © 2002 Marcel Dekker, Inc.
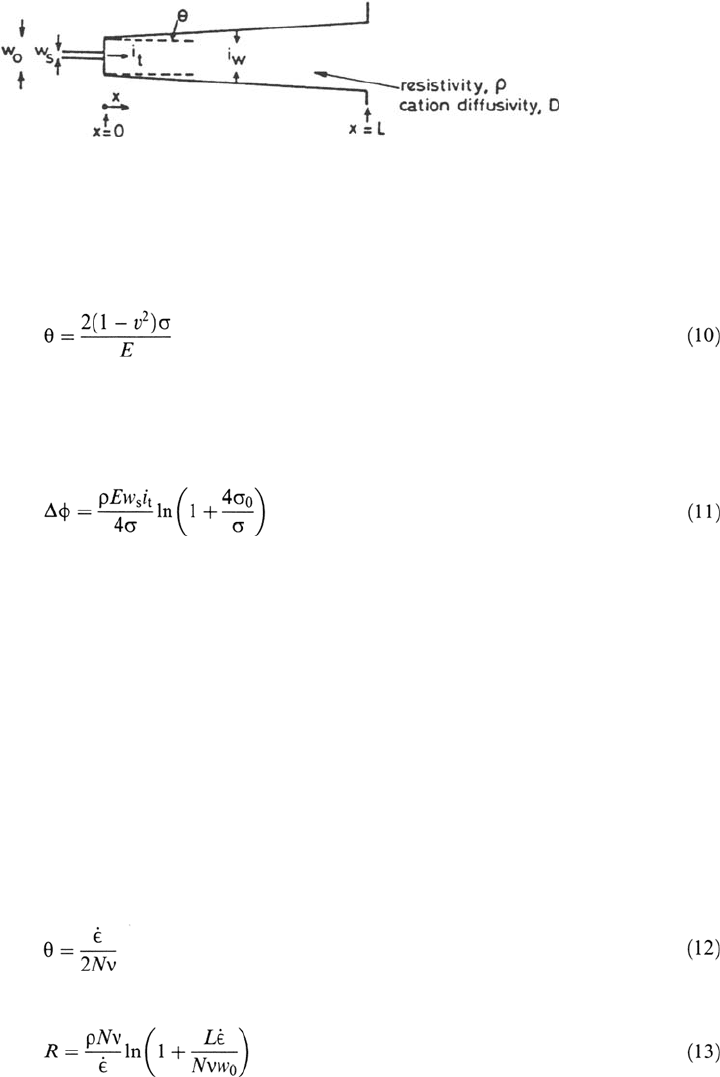
For small-scale yielding, the crack half-angle θ is given by the elastic solution:
424 Newman
Figure 25 Crack geometry for calculation of the potential drop and metal ion concentration
in a crack [33].
where
υ
is Poisson’s ration, about 0.33. The IR potential drop or dissolved metal
cation concentration in a growing crack (with small-scale yielding, passive walls,
and an active path of width w
s
embedded in w
0
) was shown to depend on the stress
and the crack velocity but not on the crack length [115]:
where ρ is the electrolyte resistivity and i
t
is the anodic current density on the
width w
s
of active material at the crack tip [i.e., i
t
≡ i in Eq. (2)]. This provides an
interesting similitude argument for constant-load SCC tests of for practical SCC
failures: no decrease in crack velocity with increasing crack length should be
expected under constant load. The preceding argument is only slightly affected by
the observation that real stress-corrosion cracks are sharper than would be expected
from fracture mechanics [16,34,38,66,116], owing to the existence of uncracked
ligaments that shield the crack tip from some of the applied stress intensity—the
region over which this occurs (about one grain) is small compared with the crack
length as a whole.
One can also analyze the strain-rate dependence of SCC for a slow strain rate
test with general rather than small-scale yielding, assuming that plastic stretching
at the crack sites dominates the specimen extension from shortly after crack initiation
[33,115]. The crack half-angle θ is now given by
and the crack electrolyte resistance by
Values of θ at least 10 times those obtained in static tests are readily achieved in a
slow strain rate test, with a large corresponding decrease in the IR potential drop or
crack tip metal cation concentration for a given value of i
t
. Such arguments show that
concerns about the saturation of metal ions or very high IR potential drops within
cracks are unfounded for slow strain rate tests, even if there is no active path
Copyright © 2002 Marcel Dekker, Inc.
w
s
. Historically, this is an important issue, as these concerns were partly responsible
for the abandonment of the slip-dissolution model by Galvele [20,118].
For a resistive system, the crack-tip anodic current density i
t
can be written
Stress-Corrosion Cracking Mechanisms 425
where E is the potential at the external surface, E
m
is the mixed (corrosion) potential
of the straining crack tip, and R is the electrolyte resistance in the crack. The crack
velocity can be derived on this basis as
As the log term is nearly a constant, this equation explains the commonly observed
relationship [29]
without considering any of the details of the i(t) transient. It would be valuable to
incorporate some of these crack geometry considerations into more rigorous
electrochemical crack models [119].
Type B SCC includes a large number of passive systems in which cracking is
induced by chloride ions. All these systems share an acid crack environment and an
active or semiactive state at the crack tip. For a while it was thought that Al-Li-Cu-
Mg alloys might be an exception to this rule because alkaline crack chemistries are
possible under special conditions (little or no cathode area outside the crack), but
it soon became clear that rapid cracking in this system is associated with acidification
in the crack [120,121].
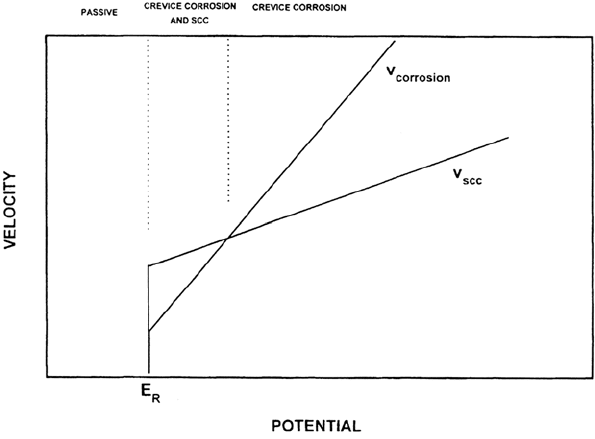
A fundamental requirement in type B SCC is that the crack velocity must be
able to exceed the localized corrosion velocity; otherwise the crack will degenerate
into a blunt fissure [68] (Figs. 26 and 27). This leads us to expect SCC over a
range of potentials between the repassivation potential of the localized corrosion
process (E
R
) and a higher potential (E
u
) at which the localized corrosion velocity
equals the crack velocity at the prevailing stress intensity value. By manipulating
the composition of a susceptible austenitic stainless steel (Ni, P, Mo, Cu, Al), these
potentials can be made to coincide, guaranteeing immunity to SCC [111,112]. An
increase in temperature is then required to increase E
u
sufficiently for SCC to
occur; i.e., the manipulation of alloy composition increases the critical temperature
for SCC initiation from a crevice. All the applicable alloying elements alter both
the SCC velocity and the crevice corrosion kinetics, so a complete analysis would
be quite complicated.
It is evident that type B SCC rarely occurs by the slip-dissolution mechanism.
The metal in the crack is already in an essentially active state, although there may
be a porous oxyhydroxide layer and possibly a dealloyed metallic layer [78,79]. If
SCC does occur by anodic dissolution in such a system, it is of the trivial kind seen
in sensitized stainless steel or underaged Al-Cu alloys [122] in chloride solutions:
the kinetics of an intergranular pitting process are simply enhanced by the opening
of the crack, reducing the ohmic resistance according to Eq. (13).
All type B systems generate hydrogen within cracks, and in some strong alloys,
such as martensitic stainless steels [91], the cracking is obviously due to hydrogen
Copyright © 2002 Marcel Dekker, Inc.
embrittlement. High-strength Al-Zn-Mg-Cu (7000 series) alloys also have a proven
role of hydrogen in SCC [54,55] (Fig. 28). Both the formation and the entry of hydro-
gen are favored by the active state in the crack, but the presence of hydrogen does not
guarantee a role, especially in fcc systems. A further complication is that in some Al
alloys, slow crack growth occurs in the absence of an environment, due to creep-like
processes or solid-metal embrittlement by lead particles in the alloy [54,123].
The mechanism of chloride-SCC of austenitic stainless steels is still debat-
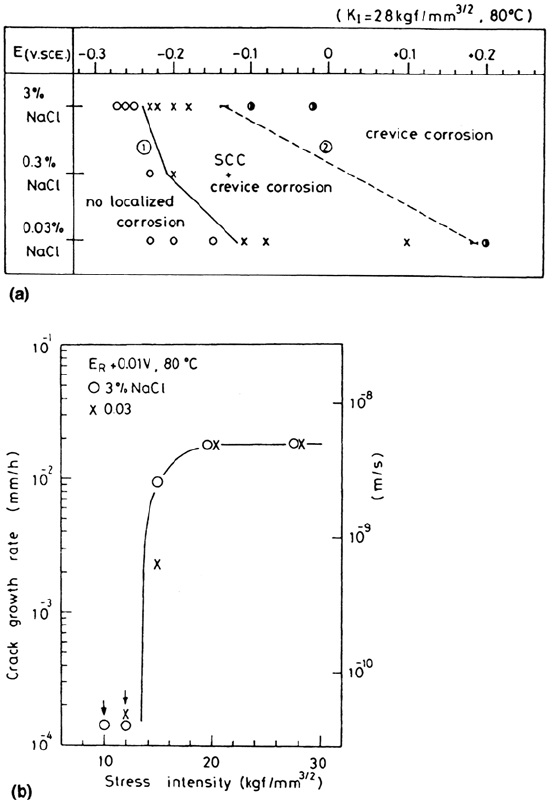
able. We may discount the slip-dissolution model for crack growth at 80°C [68]:
the required anodic current density of some 50mA/cm
2
(a velocity of about 2 ×
10
–8
m/s) is not available in the crevice environment, except during the first
milliseconds of exposure of a fresh surface, and t* [Eq. (3)] could never be this
low. Hydrogen may play a role, especially in unstable austenites at relatively low
temperatures, but it is difficult to rationalize a hydrogen effect in stable austenitic
steels at very high temperatures such as 300°C [56]. The film-induced cleavage
model [40,41] has indirect support: dealloying (Ni enrichment) has been demon-
strated in simulated crack solutions [78] and observed on the sides of cracks [79],
and the dependence of SCC on Ni content [73,74] is very similar to that seen in
Au-Cu alloys as a function of Au content [75] (Fig. 9). However, there is no direct
evidence for film-induced substrate cleavage of the kind found in Cu-Zn, Au-Ag,
and Au-Cu alloys [46–51]. The austenitic steel cracks only from sites of localized
corrosion, so “single-shot” cleavage experiments are much more complicated than
in systems that show uniform dealloying.
The electrochemistry of Cl-SCC in duplex (α-γ) stainless steels was discussed
earlier and displayed in Figure 17. The ferrite phase has a higher E
R
value, due to its
higher Cr content, but dissolves more rapidly (due to its lower Ni content) when both
phases are in the active state [124]. The crack approaches (from above, due to IR
426 Newman
Figure 26 Rationale for occurrence of type B (localized corrosion-related) SCC over a
range of potentials [68,111,112].
Copyright © 2002 Marcel Dekker, Inc.
limitations) a mixed potential where the austenite is a net cathode and the ferrite
a net anode. The ferrite is thus polarized above its normal (isolated) cracking
potential and cracks rapidly, while the austenite is below its isolated cracking
potential and cracks slowly or not at all. Cracks propagate in the ferrite and
tend to be arrested by the austenite. Another situation is possible if the ferrite can
remain passive in the crack while the austenite corrodes; now cracking occurs in
the austenite and is hindered by the ferrite.
Stress-Corrosion Cracking Mechanisms 427
Figure 27 (a) Localized corrosion and SCC regimes from Tamaki et al. [68] using
potentiostatic tests on creviced samples of 316L stainless steel in NaCl solution at
80°C. (b)
υ
-K curves measured in two different concentrations of NaCl just above
the respective repassivation potentials (E
R
), showing that SCC is controlled by local
chemistry. (Courtesy of NACE.)
Copyright © 2002 Marcel Dekker, Inc.
Addition of H
2
S or its oxidation product S
2
O
2–
3
(thiosulfate) greatly enhances
localized corrosion and Cl-SCC of austenitic and duplex stainless steels [91,125,126].
The effect of H
2
S is slight up to a critical concentration around 0.01 M because below
this concentration it is depleted in deep pits and cracks [127]. “Nonpropagating”
cracks found around this H
2
S concentration have run out of H
2
S at the crack tip
(Fig. 29). Enhancement of SCC by H
2
S is often ascribed to a hydrogen effect, by
analogy with low-alloy steels, but for stable austenites such as 310SS this argument
is not tenable. Adsorption of sulfur on the active surface in the pit or crack changes
the dissolution and passivation kinetics of the material, specifically by catalyzing
Fe and Ni dissolution, and possibly by hindering passivation (but the passivation
potential is not necessarily altered because no interaction between Cr and S is
expected in aqueous systems) [128–130]. Austenite, with its lower Cr and Mo content,
will tend to show more sulfur-induced activation than ferrite [130,131]; thus the
kinetic difference between the phases will be reduced when adsorbed sulfur is
presnet provided that both phases are in the active state. This may be one aspect
of the increased susceptibility of duplex steels in Cl
–
/H
2
S systems, but obviously
there is also an effect of S
ads
on the atomistics of SCC.
SCC of austenitic or duplex stainless steels in Cl
–
/H
2
S environments usually
occurs from pits, even at moderate temperatures such as 80°C, whereas crevice or
underdeposit corrosion is normally needed to nucleate Cl
–
-SCC at such temperatures.
This may be understood from Figure 26: pits in Cl
–
solutions at 80°C are normally
growing faster than cracks (so no SCC occurs), whereas in Cl
–
H
2
S solutions the
crack velocity is increased and the pitting velocity can be lower without
repassivation, owing to the stabilization of pit dissolution by adsorbed sulfur and
possibly also the resistive effect of the black corrosion product that forms in the pits.
In Cl
–
H
2
S systems, the effect of Ni content is basically the same as in Cl-SCC,
except that there is easier cracking around 20–30% Ni, and a new form of mainly
428 Newman
Figure 28 Notched hollow tube experiment of Ratke and Gruhl [55], used to show that
SCC of high-strength Al-Zn-Mg alloys could involve internal crack initiation by hydrogen.
(From Ref. 54. Courtesy of NACE.)
Copyright © 2002 Marcel Dekker, Inc.
intergranular cracking appears at high T and [H
2
S]; this latter cracking does not
respect the Ni content [132].
Molybdenum alloying is not particularly beneficial for SCC resistance of
austenitic stainless steels; nickel is the most important beneficial element.
Referring to Figure 26, Mo increases E
R
and E
u
by about the sme amount, through
its effect on the localized corrosion velocity, while Ni (above 10%) decreases the
crack velocity, thus lowering E
u
[68,111,112].
Type C (dealloying-related) SCC has been mentioned in the context of
austenitic stainless steels, but direct analytical evidence of dealloying is lacking in
that case. However, a number of systems definitely show dealloying and can crack in
an oxide-free state or with a loose, precipitated tarnish film, such as Cu-Zn, Cu-Al,
Au-Cu, and Au-Ag. The original SCC system (brass in ammonia) clearly comes into
this category [133], and the ease of dealloying in brass explains why it cracks in so
many different environments, especially in slow strain rate tests [134,135]. In brass
the dealloying is quite superficial (less than 100nm) but correlates with SCC and can
trigger foil fractures in cuprous [Cu(NH
3
)
2
+
] solutions that simulate the crack
environment [46,72,87,136,137] (Figs. 10 and 30). In gold alloys, the correlation can
be demonstrated more easily, as the electrochemistry is much simpler without any
competing anodic reaction on the gold [47–51]. The attempt of Flanagan, Lichter, and
others [80,81] to account for SCC of Au-Cu alloys without dealloying is premature,
as rapid dealloying may well occur in the chloride-rich environment of a crack.
In several systems, including Au and Cu alloys, gross macrodealloying in
chloride solutions has been shown not to favor SCC or microcleavage
[33,47,48,87,135,136]. One view is that this represents a kind of crack blunting (in a
Stress-Corrosion Cracking Mechanisms 429
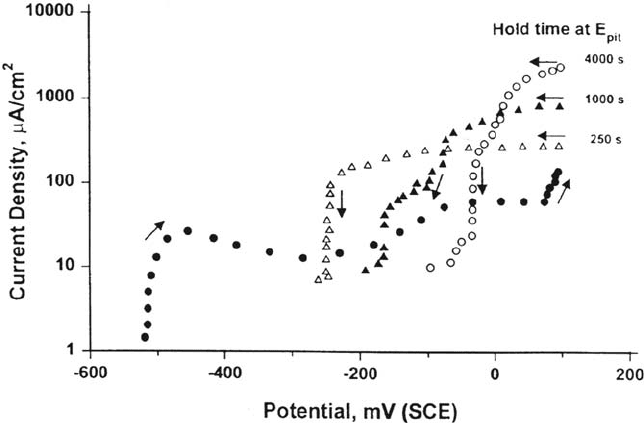
Figure 29 Potentiodynamic pittin g scans for 25Cr duplex stainless steel in 20% NaCl + 0.03
M H
2
S, pH 3.4 (acetate-buffered) at 80°C, with different hold times just above the pitting
potential, showing the paradoxical raising of the repassivation potential (E
R
) with increasing
pit (crack) depth, due to depletion of H
2
S at the dissolving surface. (Courtesy of S. B. Mat.)
Copyright © 2002 Marcel Dekker, Inc.
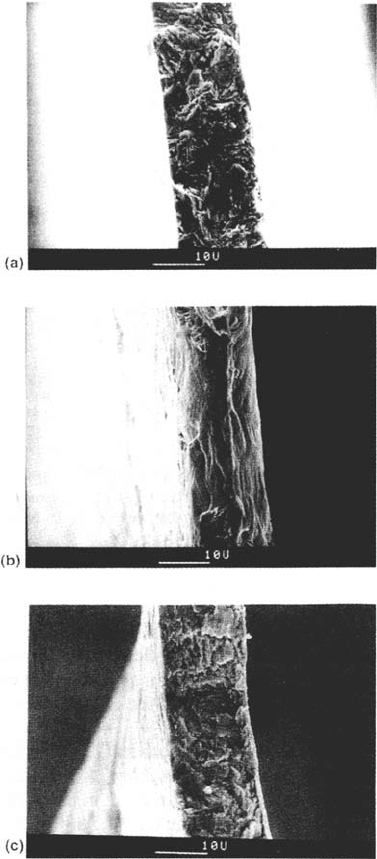
slip-dissolution context) [135], but we have proposed a more detailed interpretation
based on the film-induced cleavage model [47–49,87,88]. Essentially, gross dealloying
is indicative of coarsening of the pores within the dealloyed material (by surface
self-diffusion), to the point that the pores are too coarse to nucleate a brittle crack in
the unattacked substrate. This aging phenomenon has been demonstrated directly in
430 Newman
Figure 30 (a) Single-shot cleavage failure of α-brass rapidly strained in a cuprous ammonia
solution after 100 min immersion. (b) Identical specimen rinsed in deionized water and
dried with cool air before fracturing, showing aging of the dealloyed layer to a harmless
form. (c) Identical specimen quenched in liquid nitrogen without rinsing, then fractured
near 77 K, confirming that cleavage can occur with no liquid environment present [46].
Copyright © 2002 Marcel Dekker, Inc.
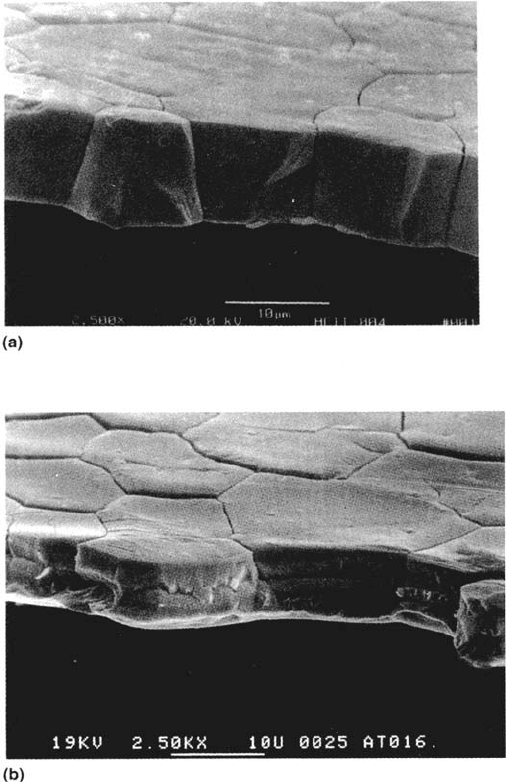
gold alloys (Figs. 31 and 32) and in brass it has been shown that alloyed arsenic
causes a transition from macro (μm) to micro (nm) dealloymg [87,136], favoring SCC
in chloride environments but having no effect in ammonia, where microdealloying
occurs irrespective of the presence of arsenic; ammonia and arsenic are both believed
to hinder surface mobility of Cu, by specific adsorption in cationic or uncharged
form [87,88,136–138]. The dealloyed layers formed on brass in ammoniacal solutions
Stress-Corrosion Cracking Mechanisms 431
Figure 31 (a) Ag-20 at % Au foil specimen, dealloyed to a depth of 3μm in perchloric
acid without stress, then fractured after stepping the potential to 0 V (SCE) for 5 min (brittle
intergranular failure). (b) Dealloyed and stepped to 700 mV for 30 min (ductile failure—the
coarsening of the porosity in the dealloyed layer is more rapid at higher potentials). (c) As
in (a) but with 10 mM NaCl added after stepping the potential to 0 V (ductile failure due
to rapid pore coarsening). (d) As in (c) but with a further addition of 10 mM pyridine (brittle
failure due to counteraction of chloride effect by pyridine) [33,49].
Copyright © 2002 Marcel Dekker, Inc.
