Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

age very rapidly when out of contact with the solution, losing the ability to trigger
cleavage in thin foils (Fig. 30).
Cracking of silver alloys has been reported by Galvele and others in a series
of papers [139–141]. Interesting SCC phenomena were reported in both aqueous
and gaseous environments, including halogen vapor. Clearly, all these systems
involve selective reaction of the silver in the alloy, which in halide solutions or
atmospheres generates a composite of silver halide and nearly pure noble metal
(Au or Pd); this layer should behave mechanically much like a dealloyed layer
formed under conditions of free silver dissolution, so the mere occurrence of SCC
does not help to distinguish between SCC mechanisms. Foil-breaking experiments
[33,46–51] should be performed in some of these environments to test the generality
of the film-induced intergranular fracture mechanism.
Type D SCC involves surface films that are neither oxides nor dealloyed layers.
The cracking of high-strength 4340 steel in dry Cl
2
[102,103] or dissociated N
2
[142]
432 Newman
Figure 31 Continued
Copyright © 2002 Marcel Dekker, Inc.
appears to be a film-induced process; Sieradzki [103] proposed that FeCl
2
acted
as a stiff layer, causing a ductile-to-brittle transition due to the modulus mismatch
with the substrate. Later the lattice parameter was emphasized as a casual factor
[143]. A detailed rationalization of SCC was made for iron exposed to anhydrous
ammonia-methanol, where anodic oxidation of ammonia leads to interstitial
penetration of nitrogen [69,100]. According to the film-induced cleavage model,
such very thin, brittle layers are sufficient to allow cleavage through several μm of
a body-centered cubic (bcc) material, whereas in fcc systems a nanoporous metallic
layer 10–100 nm thick is a specific requirement as it is the only way of epitaxially
coupling a brittle reaction product of sufficient thickness to the fcc substrate. A
special case may be the SCC of pure copper or silver, where a micropitted or
tunneled zone is a possible brittle layer that could nucleate cracking [144,145].
There are several other film-induced SCC processes, such as cracking of Zr
alloys in gaseous iodine [101]. In no case has monolayer adsorption been validated as
the cause of SCC—every known case involves a 3D film. However, liquid metal
embrittlement often occurs, even in fcc systems, without alloy or compound
formation [15]. Possibly there is a regime of very rapid adsorption-induced cracking
that is rarely accessed in aqueous systems as it requires the adsorption process
to be faster than plastic relaxation processes at the crack tip; hence in aqueous
environments this is likely to occur only in very reactive metals (Al, Ti, Mg).
Type E SCC (embrittlement of steels by cathodic hydrogen in the active
state) is quite well understood except for the atomistic action of the hydrogen, which
is still debatable. Briefly, hydrogen atoms are produced by water reduction during
corrosion in neutral solutions, and some of them enter the steel, especially if their
recombination to H
2
is poisoned by adsorption of S, As, P, or Sb. Once in the steel, the
Stress-Corrosion Cracking Mechanisms 433
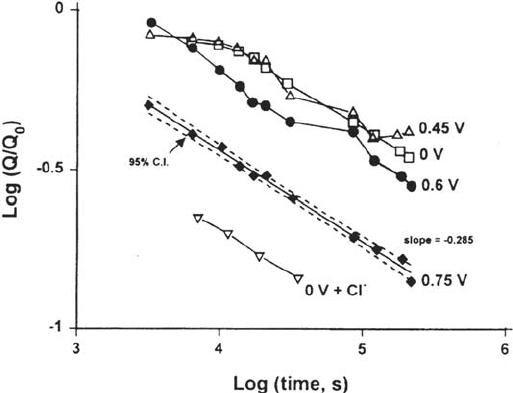
Figure 32 Measurements of double-layer capacitance (proportional to pore surface area;
inversely proportional to pore radius) as a function of time during potentiostatic aging of
dealloyed layers on Au-Ag, showing the effects of potential and chloride ions [48].
Copyright © 2002 Marcel Dekker, Inc.
hydrogen can move with an effective diffusivity (D
eff
) of 10
–8
to 10
–4
cm
2
/s,
depending on the microstructure as well as temperature [21] (e.g., small carbides
in tempered martensite act as traps and greatly reduce (D
eff
). The lattice (interstitial)
hydrogen solubility is elevated ahead of stressed cracks or notches, and these sites
accumulate higher hydrogen concentrations. The same hydrostatic tension that
increases the H solubility in the lattice also enhances hydride phase precipitation in
434 Newman
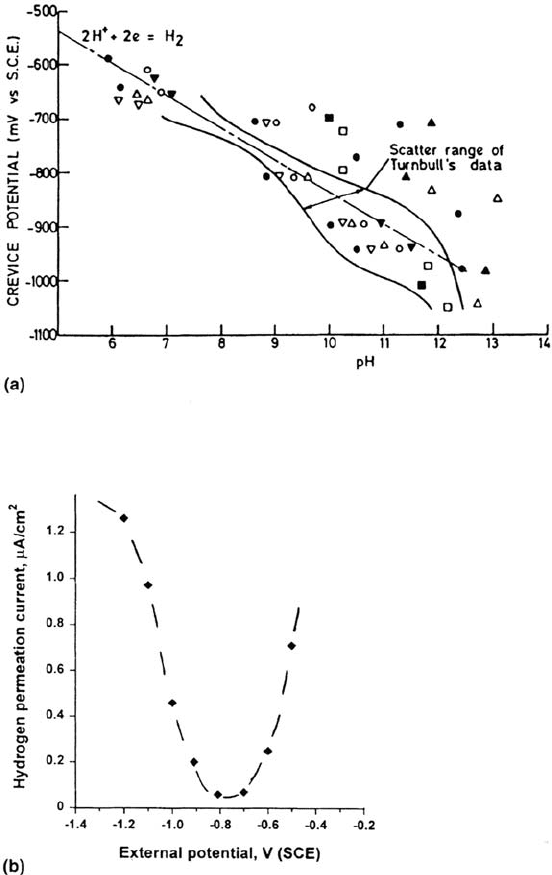
Figure 33 (a) Crevice potential and pH and (b) hydrogen permeation rate, as a
function of potential for carbon steel crevices in NaCl solution. (From Ref. 154.
Courtesy of HMSO.)
Copyright © 2002 Marcel Dekker, Inc.
metals such as Ti and Nb [101]. Hydrogen segregrates to interfaces, including
grain boundaries, and may weaken these in combination with other segregants
such as P and Sb [146]. Once it reaches its site of action, and provided it is in a strong
microstructure, the hydrogen causes local fracture by cleavage [147], intergranular
separation [146], or enhanced microplasticity [148], or some combination of these
processes. In low-strength steels, dynamic loading is required for cracking. If the
input fugacity of hydrogen is high enough (hundreds of atmospheres in media such
as acidic H
2
S), recombination at internal sites such as nonmetallic inclusions may
cause blistering or cracking (hydrogen-induced cracking, or HIC). This is minimized
in pipeline steels by careful microstructural control.
The altered solution chemistry within cracks is an important aspect of SCC
and corrosion fatigue in structural and high-strength steels. Turnbull [149–151]
has shown that crack acidification, as proposed by Brown [152], is not the norm
for steels corroding freely in NaCl solutions or seawater. Only in Cr-containing
steels with very short cracks could any acidification be predicted [153]. Possibly some
of Brown’s historic measurements were flawed by oxidation of Fe
2+
by atmospheric
oxygen; more recent work shows that deep cracks normally become net cathodes
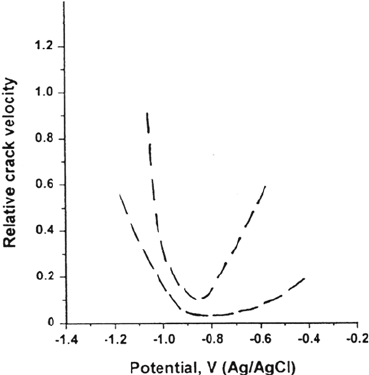
and reach a pH of about 9 [154] (Fig. 33). Nevertheless, with assistance from the
IR-induced isolation of the crack enclave, the potential can remain below that of a
reversible hydrogen electrode, and in sufficiently strong steels embrittlement will
occur above some K
ISCC
value. An excellent correlation exists between hydrogen
uptake on the walls of simulated cracks and SCC of high-strength steels in the
same solution [154] (Fig. 34). Cathodic protection, within limits, can reduce the
hydrogen uptake from cracks by increasing the local pH [149–151]; however, this
is rarely used for high-strength steels that are sensitive to hydrogen, even though
major benefits can be demonstrated in the laboratory. The problem in practice is
that most cathodic protection systems locally reduce the potential to values that
are dangerously low for high-strength steels.
Stress-Corrosion Cracking Mechanisms 435
Figure 34 Band of results showing the effect of potential on the rate of SCC, for a range
of high-strength steels in seawater [152]; compare with Figure 33b.
Copyright © 2002 Marcel Dekker, Inc.
Hydrogen effects often show the same kind of strain rate sensitivity as
slip-dissolution processes. Low-strength steels are immune to SCC under static
loads in salt water, but steels of all strengths can suffer hydrogen-assisted fatigue
crack growth [105,155]. High strength continues to be detrimental, but relatively
less so than under static loading.
STRESS-CORROSION TESTING IN RELATION TO
MECHANISMS OF CRACKING
Because standard methods of SCC testing have been excellently reviewed by
Sedriks [156], we focus on the implications of mechanisms for testing and vice versa.
To rationalize or predict service performance, and to test models of SCC, the
slow strain rate test [26,27,113,157] is convenient as it enables a large number of tests
to be conducted at a large number of potentials within a defined time; the maximum
duration is simply the failure time in air (Fig. 35). The maximum load, elongation to
failure, and percent reduction in area at fracture are measured in the test environment
and normalized to values measured in an inert environment. The disadvantages are
that the mechanical condition is relatively undefined when there are multiple cracks,
the crack velocity cannot be measured continuously, and failures occur in materials
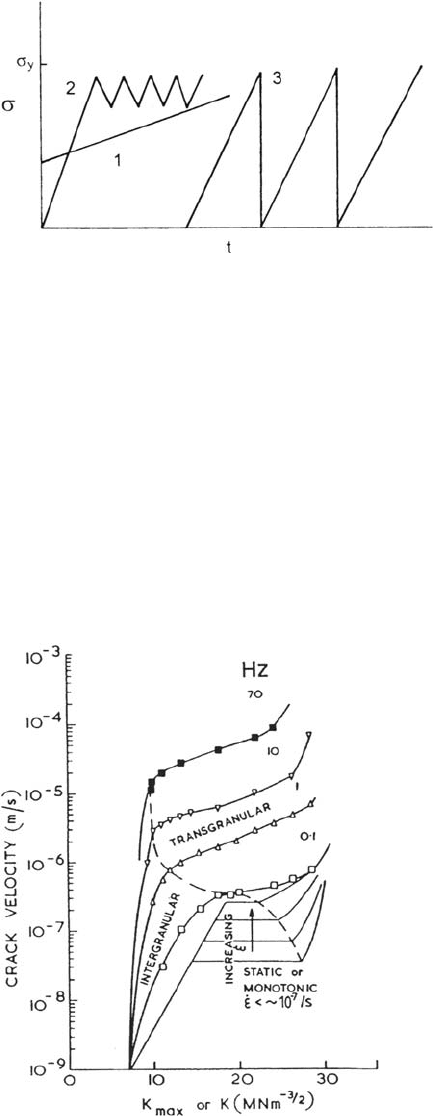
that would never fail by SCC in service. An elastic slow strain rate or ultraslow cyclic
test [67] is a useful compromise that maintains the dynamic loading without gross
plastic straining (Fig. 36), but these tests are difficult to carry out in large numbers. It
has been suggested that slow strain rate and cyclic-loading tests are part of a
continuum [28–30,113,158] and that in some systems SCC and corrosion fatigue are
also a continuum with a single mechanism (Fig. 37); certainly the fractography of
slow strain rate SCC and low-frequency fatigue crack growth can be very similar,
e.g., in low-strength steels. The most notorious cases in which the slow strain rate test
overestimates the susceptibility of a material are the hydrogen-induced cracking of
low-strength steels under cathodic protection [159], the SCC of commercial-purity
titanium in salt water [160], and the hydrogen-induced cracking of duplex stainless
steels [161]. In every case the K
ISCC
value, if it exists, is extremely high, at least
50 MPa m
1/2
. Sometimes this hydrogen-induced cracking, which would not occur in
practice, overshadows a “genuine”-SCC phenomenon that occurs at a lower velocity
but has a much lower K
ISCC
value, e.g., SCC of carbon steel in CO-CO
2
-H
2
O
solutions [162,163]. One approach to this kind of problem (without changing the test)
is to study the distribution of secondary cracks on the failed tensile specimen.
Cathodic hydrogen embrittlement is confined to the necked region [159,164], but the
genuine SCC is distributed as secondary cracks along the whole length of the tensile
specimen [162] (Figs. 38 and 39).
Having classified CO
2
-induced cracking as possibly an artifact, we note that
this is a favored mechanism for a transgranular cracking phenomenon seen in
high-pressure gas transmission pipelines. Clearly, the hydrogen uptake in CO
2
or
NaHCO
3
solution must be high compared with cathodic protection in salt water,
where similar steels do not crack, or else the coexistence of localized corrosion
and hydrogen entry helps to maintain the crack tip strain rate. Dynamic service
stresses are also an important factor.
436 Newman
Copyright © 2002 Marcel Dekker, Inc.
Having reviewed some of the flaws of slow strain rate testing, we must stress
that this is an outstanding useful test. It is always desirable to have the slow strain
rate information, even if it is not used for direct prediction of service performance.
Neither the pipe cracking in boiling water reactors [59] nor the SCC of high-pressure
gas transmission lines [27,67] (both type A systems) could have been predicted by
static-load tests on smooth specimens.
Loading rate, or dK
I
/dt, affects SCC initiation and growth in precracked
specimens [113] (Fig. 37) and a good appreciation of the cracking mechanism is
Stress-Corrosion Cracking Mechanisms 437
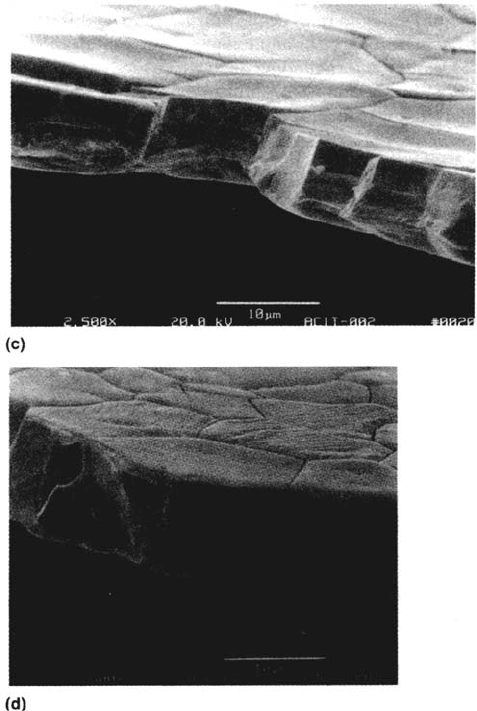
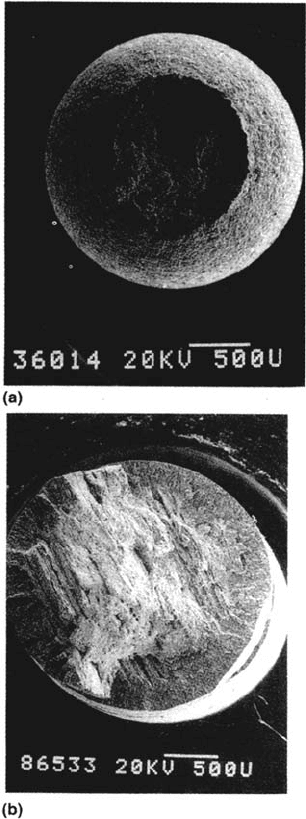
Figure 35 Typical fractures of C-Mn steel obtained in slow strain rate tests: (a) in air;
(b) in anhydrous ammonia-methanol. (Courtesy of I. M. Hannah and W. Zheng.)
Copyright © 2002 Marcel Dekker, Inc.
essential to avoid surprises in service, as not all variants of K
I
(t) can be tested in
the laboratory. Attempts to rationalize all such data with the slip-dissolution model
(for high-temperature aqueous environments) have been more in the nature of a
sophisticated fitting exercise than a first-principles approach [29], and there is a
great divergence of views on the cracking mechanisms, especially in pressurized
water reactor (PWR) pressure vessel steels, where everything from slip dissolution
[28–30] to hydrogen embrittlement [165] to film-induced cleavage [42] to surface
mobility [166] has been suggested.
438 Newman
Figure 36 Various types of elastic slow strain rate test.
Figure 37 The effect of crack tip strain rate, via loading rate, on crack growth in a
high-strength aluminum alloy [54,158]. (Courtesy of NACE.)
Copyright © 2002 Marcel Dekker, Inc.
In some systems, SCC is initiated at a very large number of sites, and the
crack tip strain rate is greatly reduced at each individual site, leading to near
arrest of the cracks (another reason for crack arrest might be discontinuous
segregation or precipitation; recall Fig. 13). Coalescence of two or more cracks is
then required to produce a dominant crack that causes final failure [27,167]
(Fig. 40). Statistical physicists are very interested in similar problems [168],
ranging from mud cracking to fracture of random composite media. Multiple crack
interactions are a major growth area in fracture mechanics. Such phenomena are
peculiar to smooth specimens and provide a strong argument for carrying out several
kinds of laboratory test.
A major area of development in SCC testing is the role of crevices in crack
initiation. We have discussed the essential role of localized corrosion in Cl-SCC of
austenitic stainless steels [68,111,112], and topical problems with duplex stainless
steels necessitate extension of the same approach to these more resistant materials.
The incorporation of crevices into slow strain rate specimens is not at all trivial, as
we discovered during 2 years’ work by Suleiman at UMIST [169]. Tamaki et al. [68]
showed that a fine notch or precrack could be used to establish a reproducible
condition in a fracture mechanics specimen, but this too is difficult work, especially
if one attempts to carry out the work potentiostatically. Tamaki et al. found that,
for 316L stainless steel at 80°C, SCC sometimes occurred only within a few tens of
mV above the repassivation potential of the crevice (E
R
) (Figs. 25 and 26). Without
accurate prior knowledge of E
R
, or in a complex service environment, such work can
be very time consuming, so we have proposed a galvanostatic variant in which a
low anodic current is applied that automatically establishes a steady potential just
above the lowest possible value of E
R
before starting the tensile machine (see Fig. 7c).
This galvanostatic technique is also applicable to the rapid screening test used
by Shinohara and colleagues [112], in which two sheets of austenitic steel are spot
Stress-Corrosion Cracking Mechanisms 439
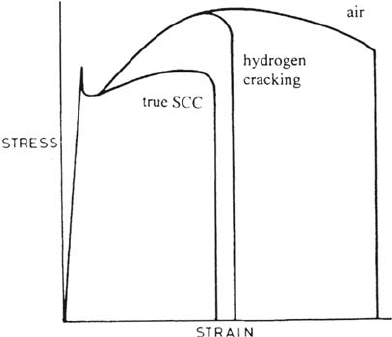
Figure 38 Stress-strain curves for “real” SCC (cracks initiated soon after yield) and
cathodic hydrogen embrittlement (where cracking is confined to the necked region), for
low-strength steels.
Copyright © 2002 Marcel Dekker, Inc.
welded together and polarized in a chloride solution. All these procedures are
recommended for further development except perhaps the use of the creviced slow
strain rate test.
NOTES ON OTHER PROPOSED SCC MECHANISMS
Magnin’s model of SCC and corrosion fatigue [16,17] proposes that the motion of
crack-tip dislocations is impeded by an obstacle ahead of the crack tip, which in
440 Newman
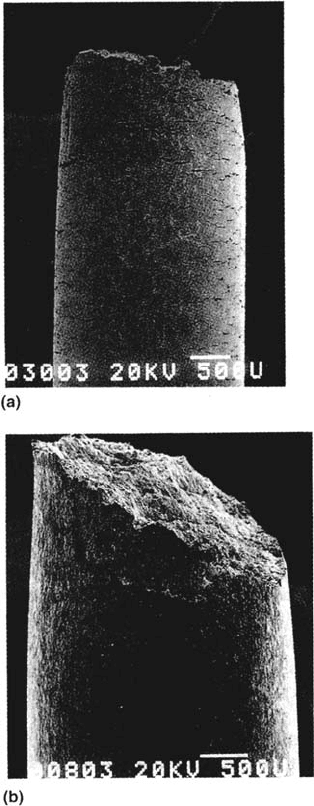
Figure 39 Fractography corresponding to Figure 38 [162], for C-Mn steel in CO-CO
2
-H
2
O
solution: (a) genuine SCC; (b) cathodic hydrogen embrittlement. (Courtesy of I. M. Hannah.)
Copyright © 2002 Marcel Dekker, Inc.
the purest case might be a Lomer-Cottrell lock. Dissolution down a slip band toward
the obstacle leads to the achievement of K
Ic
at the obstacle and the propagation
of a cleavage crack on the slip plane back toward the crack tip; in addition (or
alternatively), this decohesion of the microfacet can be facilitated by hydrogen
adsorption on the fracture plane (Fig. 41). Such a model is consistent with certain
observations in corrosion fatigue cracking, and with fractographic studies of
Dickson and others [66,170] on transgranular SCC surfaces, but is not yet adapted
to explain the multitude of special environmental factors in SCC. Application of
this model to alloy 600 in hydrogenated high-temperature water [17] might be
considered arbitrary in comparison with Scott and Le Calvar’s [60] highly focused
investigations of intergranular oxidation. Magnin’s model is elegant and plausible
but can be tested only by fractography, which is bound to be inconclusive.
The model of environmental cracking proposed by Lynch [19] will be
considered only briefly as it accounts for none of the special environmental effects
in SCC. This does not mean that the model is wrong, only that it is untestable
except by microscopic means. The concept of enhanced local plasticity leading to
plastic microfracture is an important aspect of hydrogen embrittlement [148] and
has interesting implications in liquid metal embrittlement. Most authorities reject
Lynch’s adsorption-based approach to hydrogen effects preferring to appeal to
effects of internal hydrogen on plasticity, yet Lynch has a powerful argument
based on the similarity between liquid metal–induced and hydrogen-induced
fractures in certain systems. There is no doubt that plastic microfracture will form
part of the complete SCC spectrum, should this ever be elucidated, but we must
reject the notion that all SCC can be explained by such mechanisms.
“Universal” models of environmental fracture rarely survive for long, and SCC
specialists such as Parkins [27,171] have devoted much effort to promoting a
spectrum of mechanisms from mainly chemical (intergranular slip-dissolution) to
mainly mechanical (hydrogen-induced SCC of high-strength steel). Nevertheless,
Stress-Corrosion Cracking Mechanisms 441
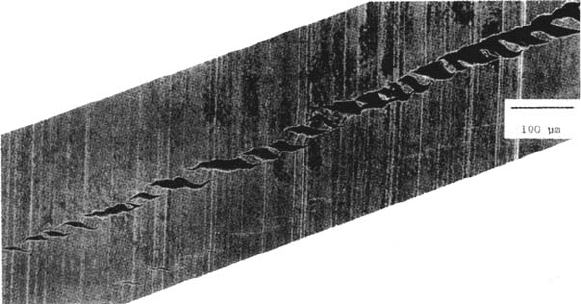
Figure 40 Crack coalescence of {110} microcracks leading to a dominant slip-band
crack for a Cu monocrystal dynamically strained in NaNO
2
solution [65].
Copyright © 2002 Marcel Dekker, Inc.
