Marshall L. Stoller, Maxwell V. Meng-Urinary Stone Disease
Подождите немного. Документ загружается.


432 Ong and Jarrett
71. Dunnick NR, Long JA, Javadpour N. Perirenal extravasation of urographic contrast medium
demonstrated by computed tomography. J Comp Asst Tomog 1980; 4(4): 538–539.
72. Gold IW, Sternbach GL. Spontaneous extravasation following intravenous pyelography, Ann
Emerg Med 1982; 11:9: 485–486.
73. Hafiz A, Rodko EA, Extravasation of contrast during excretory pyelography. J Assoc Can Radiol;
1970: 21:46–50.
74. Blandino A, Gaeta M, Minutoli F, et al. MR urography of the ureter. AJR Am J Roentgenol 2002;
179: 1307–1314.
75. Hosomi M, Oka T, Miyake O, Matsumiya K, Takaha M, Pak S. Spontaneous rupture of a
pyelocaliceal diverticulum. Urol Radiol 1989; 11: 136–138.
76. Sandhu DPS, Iacovou JW, Fletcher MS, Kaisary AV, Philip NH, Arkell DG. A comparison of
intramuscular ketorolac and pethidine in the alleviation of renal colic. Br J Urol 1994; 74: 690–
693.
77. Oosterlink W, Philp NH, Charig C, Gilles G, Hetherington JW, Lloyd J. A double-blind single dose
comparison of intramuscular ketorolac tromethamine and penthidine in the treatment of renal
colic. J Clin Pharmacol 1990; 30: 336–341.
78. Bihl G, Meyers A. Recurrent renal stone disease-advances in pathogenesis and clinical manage-
ment. Lancet 2001; 358(9282): 651–656.
79. Lingeman JE, Lifshitz DA, Evan AP. Surgical management of urinary lithaisis. In: Campbell’s
Urology, 8th Ed., (Walsh, PC, ed.). Elsevier Health Sciences, UK, 2002; pp. 3361–3365.
80. Pearle MS, Pierce HL, Miller GL, et al. Optimal method of urgent decompression of the collecting
system for obstruction and infection due to ureteral calculi. J Urol 1998; 160(4): 1260–1264.
81. Joshi HB, Obadeyi OO, Rao PN. A comparative analysis of nephrostomy, JJ stent and urgent in
situ extracorporeal shock wave lithotripsy for obstructing ureteric stones. Br J Urol Int 1999;
84(3): 264–269.
82. Mokhmalji H, Braun PM, Martinez et al. Percutaneous nephrostomy versus ureteral stents for
diversion of hydronephrosis caused by stones: a prospective, randomized clinical trial. J Urol
2001; 165(4): 1088–1092.
83. Lee WJ, Mond DJ, Patel M, Pillari GP. Emergency percutaneous nephrostomy: technical success
based on level of operator experience. J Vasc Interv Radiol 1994; 5(2): 327–330.

Chapter 23 / Anatomical Considerations 433
433
From: Current Clinical Urology, Urinary Stone Disease:
A Practical Guide to Medical and Surgical Management
Edited by: M. L. Stoller and M. V. Meng © Humana Press Inc., Totowa, NJ
23
Anatomical Considerations
in Urinary Stone Disease
Louis Eichel, MD and Ralph V. Clayman, MD
CONTENTS
URETHRAL ANATOMY
BLADDER ANATOMY
URETERAL ANATOMY
RENAL ANATOMY
IMPACT OF ANATOMY ON SELECTION OF STONE THERAPY
SUMMARY
REFERENCES
Key Words: Kidney; ureter; calyx; anatomy.
URETHRAL ANATOMY
In the urinary tract, all roads lead to the urethra. An understanding of its anatomy,
in both the male and female, is important for the successful and safe removal of bladder
calculi and for the safe passage of both cystoscopes and ureteroscopes.
The male urethra spans approx 20 cm from the distal meatus to the bladder neck. The
urethral meatus can vary greatly in its diameter but in general is the tightest portion of
the entire urethra. Occasionally it is necessary to manually dilate the urethral meatus
or perform a meatotomy to pass an instrument or to extract a distally lodged stone.
For practical purposes the male urethra is divided into three sections: the pendulous,
membranous, and prostatic urethra. The pendulous urethra encompasses the distal
penile and more proximal bulbar portions of the urethra, which lie within the corpus
spongiosum. The urethra will normally accommodate instruments up to 28 French. As
the penile urethra runs directly into the bulbar urethra, its course curves anteriorly and
enters the membranous urethra. The membranous urethra is intimately surrounded by
the external (voluntary) sphincter. This point of anterior deflection and muscular

434 Eichel and Clayman
based occlusion is a potential site of trauma where false passages can be made poste-
rior; understandably it is the most common point of stricture formation. It is important
for the endoscopist to realize that if a false passage has been made with a catheter or
rigid endoscope, that the flexible endoscope may be most helpful as it can be manipu-
lated such that its tip will hug the anterior portion of the urethra and facilitate passage
of a guide wire or the endoscope itself into the true urethral lumen. When stone passage
is inhibited by short strictures along the course of the urethra, direct vision urethro-
tomy with retrograde manipulation of the stone back into the bladder can usually be
performed followed by cystolitholapaxy. More significant strictures that cannot be
easily dilated may require transvesical extraction of a bladder stone followed by ure-
throplasty (1,2).
The prostatic urethra extends from the male external sphincter in the membranous
urethra to the bladder neck (internal sphincter). Here, the urethral lumen is usually at its
widest. Under certain pathologic circumstances that cause obstruction of the bladder
neck such as prostatic hyperplasia and bladder neck contracture or bladder neck hyper-
trophy, there can be difficulty passing stones out of the bladder or passing an endoscope
into the bladder. Obstruction at this level may require transurethral incision of the pros-
tate or bladder neck.
In females, the urethra is typically 4 cm in length. The female urethra can usually be
dilated to 32 Fr to accommodate endoscopes of varying size and for the removal of
bladder stones up to 9 mm in diameter. In addition, stones can form inside urethral
diverticuli. These are best treated with diverticulectomy and primary repair.
BLADDER ANATOMY
The normal functioning bladder is able to store urine at low pressure and then, in a
coordinated fashion with the urethral sphincters, empty to completion. The majority of
the bladder wall is composed of large bundles of interconnecting smooth muscle fibers
that form a powerful meshwork capable of expansion and contraction depending on the
stage of the voiding cycle. These fibers react primarily to cholinergic input. In contrast,
the smooth muscle configuration of the trigone and bladder neck is more organized and
performs several key functions. The trigonal muscle provides a thick supportive backing
for the ureters, which enter the bladder base posterolaterally and travel medially for 1.5–
2.5 cm to open into the bladder lumen at the trigone. As the ureter passes through the
bladder wall it is compressed and narrowed, which can prevent passage of a stone
antegrade or a ureteroscope retrograde. Difficulties with ureteral access can be encoun-
tered following ureteroneocystotomy (discussed later in this chapter).
The bladder neck is intimately related to the trigone and the two as a unit act to funnel
urine into the urethra during the active phase of micturition. Difficulty with bladder
emptying caused by a neurogenic problem, an obstructive process, or poor detrussor
function may lead to urinary stasis and increase the risk of infection. Stasis and infection
are the two main risk factors for bladder stone formation.
Several other factors can lead to bladder stones. In particular, bladder diverticuli can
be a source of urinary stasis and thereby promote stone formation. Foreign bodies such
as iatrogenically placed sutures, indwelling Foley catheters, and indwelling ureteral
stents can each act as a nidus for vesicolithiasis. Last, in patients who have undergone
bladder augmentation or continent urinary diversion, mucous formation and stasis can
create a lithogenic environment (3).

Chapter 23 / Anatomical Considerations 435
URETERAL ANATOMY
The ureter originates from the renal pelvis and transports urine to the bladder in
peristaltic waves. The length of the ureter varies from 22 to 30 cm (4). Its course runs
from the ureteropelvic junction medially where it travels along the medial border of the
psoas muscle just lateral to the transverse processes of the lumbar vertebrae. In the supine
position, this is an uphill climb as the ureter travels over the sacral promontory. At
the L3-L4 level the ureter is crossed anteriorly by the gonadal vessels. As the ureter
passes over the pelvic brim it crosses directly over the bifurcation of the common iliac
artery and then proceeds downhill hugging the contour of the pelvis just medial to the
internal iliac artery. Once the artery branches, the ureter courses between the inferior
vesical artery inferomedially and the superior vesical artery superolaterally. Just before
its oblique insertion into the bladder base (the ureterovesical junction), the ureter is
coursed anteriorly in males by the vas deferens and in females by the round ligament and
then slightly more caudal, laterally by the obliterated umbilical artery (i.e., the medial
umbilical ligament).
Stones tend to lodge at four common points of narrowing along the course of the
ureter: the ureteropelvic junction, the pelvic brim as the ureter courses over the iliac
vessels, the ureterovesical junction, and the ureteral orifice. Among these, the ure-
terovesical junction is often the narrowest, with a diameter of 2 mm. The combination
of these areas of narrowing and the relatively serpiginous course that the ureter takes
from the kidney to the bladder, can make passage of guide wires or endoscopes within
the ureteral lumen quite challenging. For example, it is sometimes necessary to balloon
dilate the intramural portion of the ureter or the ureterovesical junction in order to allow
passage of a ureteroscope. In addition, when passing a ureteroscope up the ureter over
a guide wire in a supine patient, one must visualize the three dimensional course of the
ureter coursing laterally and downward through the bladder wall and then taking a sharp
turn upward (anteriorly) and medially as the instrument needs to be guided anterior out
of the pelvis and over the iliac vessels. The pulsations of the common iliac artery are
clearly transmitted to the ureter at this point. On occasion, it may be difficult to pass a
semi rigid ureteroscope over the pelvic brim, especially in the male patient. This is a
potential cause of ureteral and/or endoscope damage and can be avoided by changing to
a flexible endoscope. Alternatively, this angulation can be modified in the male by
manually pushing the suspensory ligament of the phallus posterior thereby helping with
the alignment of the urethra toward the ureteral orifice.
The upper ureter can, on occasion, J hook making the passage of a guide wire difficult.
This is more common in elderly patients with ptotic kidneys. A variety of endourological
methods for overcoming this problem have been described; however, the simplest
approach is to place the patient in a steep head down position and proceed to manually
push the kidney upward by pressing upward along the upper quadrant of the abdomen
subcostally (Mertz maneuver) (5).
After summiting the sacral promontory it is a slightly downhill course along the mid
and upper ureter toward the renal pelvis, which usually sits at the level of the second
lumbar vertebral body. However, to traverse the ureteropelvic junction, the endoscope
usually needs to be guided even more posterior.
The anterior-posterior course of the ureter as described above is reversed if the patient
is in the prone position during flexible ureteroscopy. In this position, it is most helpful
to first pass a ureteral access sheath to facilitate passage of the flexible ureteroscope.

436 Eichel and Clayman
RENAL ANATOMY
Kidney Position and External Relationships
The kidneys lie in the retroperitoneum on top of the quadratus lumborum and psoas
muscles. Each kidney has a thin walled fibrous capsule that is intimately adherent to the
parenchyma, which in turn is surrounded by perirenal fat. The perirenal fat is contained
by Gerota’s fascia, which in turn is surrounded by another layer of fat (i.e., the pararenal
fat).
Posteriorly, the superior pole of each kidney rests against the diaphragm and the tips of
the 11th and 12th ribs. Deep to this, the underlying pleura attaches to the 11th rib, which
must be considered when a superior pole percutaneous approach is planned, especially
on the left where the kidney lies higher in the retroperitoneum. The adrenal glands rest
on top of the kidneys medially against the cava on the right and aorta on the left.
The anterior surface of the right kidney is associated with the liver superiorly, the
curve of the duodenum over the midportion and the ascending colon inferior and medi-
ally. On the right side, the colon often covers the lower half of the kidney medially. The
anterior surface of the upper pole of the left kidney is covered by the spleen superiorly
and just the tail of the pancreas medially as well as by, the splenic flexure of the colon;
the anteromedial surface of the entire left kidney is covered by the descending colon. A
retrorenal colon can be seen on either side in 1–10% of percutaneous cases depending
on patient positioning; it is more common when the patient is in the prone position.
However, this condition is usually limited to patients with a markedly redundant colon
or patients with a horseshoe kidney (6). Usually the retrorenal colon covers only the
lateral most portion or upper pole of the kidney.
For anatomic purposes, the kidney can be divided into anterior and posterior seg-
ments. The plane of division for these segments rests 30–50° posterior to the frontal
plane of division for the body as a whole owing to the rotation of the renal axis anteriorly
by the psoas major muscle (Fig. 1) (7). The psoas muscle also defines the axis of the
kidneys in the longitudinal plane so that the upper pole is medial and posterior whereas
the lower pole is more lateral and anterior (6). As such, the distance from skin to collect-
ing system is shortest at the upper pole and greatest at the lower pole of the kidney.
Internal Architecture of the Kidney and Collecting System
The glomeruli, proximal tubules, and distal convoluted tubules rest within the renal
cortex, which is the outer most layer of the renal parenchyma. The loops of Henle and
collecting ducts rest within the renal pyramids, which together comprise the medulla of
the renal parenchyma and rest within the center of the kidney. The renal pyramids are
separated by incursions of cortical tissue called the columns of Bertin. The collecting
ducts within each pyramid cone down to drain into a papilla, which is bordered by the
fornix of a minor calyx, the area surrounding the papilla.
Each kidney typically contains 4–14 minor calyces (mean 8) (6,7). The minor calyces
comprising the middle pole of the kidney tend to drain single papilla (simple calyx)
whereas the polar calyces tend to drain two or three papillae (compound calyx). These
papillae may actually be fused to one another in varying degrees. A minor calyx may
drain directly via an infundibulum into the renal pelvis or several minor calyces, as in
the polar areas, may drain into a major calyx, which in turn drains via a single major
infundibulum into the renal pelvis (Fig. 2).
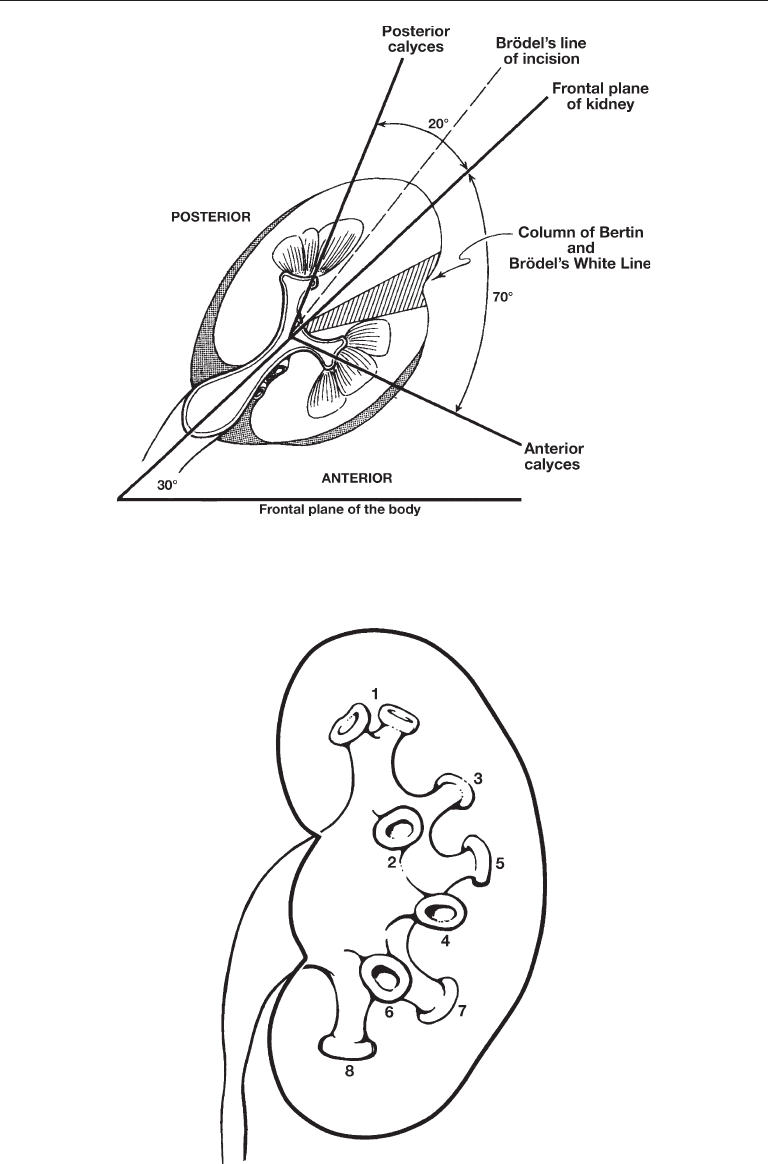
Chapter 23 / Anatomical Considerations 437
Fig. 2. “Classic” left kidney: compound polar calyces (1); anterior calyces (2, 4, and 6) and
posterior calyces (3, 5, and 7). Used with permission from Reference 7.
Fig. 1. Left kidney viewed from above showing anterior calyces projecting 70° and posterior
calyces 20° from the frontal plane of the kidney, as in a classic Brödel-type kidney. Used with
permission from Reference 7.

438 Eichel and Clayman
Sampaio et al. analyzed 140 endocasts of the pelviocaliceal systems of cadaver kid-
neys and derived from this a descriptive classification system based on polar anatomy
(6). Group A kidneys have pelviocaliceal systems comprised of an upper and a lower
pole system. The middle pole minor calyces drain into one of these two major divisions.
Group B kidneys have pelviocaliceal systems in which the midpole calyceal drainage is
independent of the upper and lower pole collecting systems.
The traditional teaching regarding the correlation between the actual anatomy of the
pelviocaliceal and the appearance of the collecting system on intravenous pyelogra-
phy is that on a standard AP view, the anterior calyces appear to be seen in cross section
laterally and the posterior calices appear to be more medial and are seen from a cuplike
end on point of view. Sampaio et al. studied 40 cadaver kidneys in which retrograde
pyelography was correlated with the actual anatomy of the pelviocaliceal system
derived from endocasts (6). In actuality, this relationship (where the anterior calices
are seen laterally and the posterior calices are seen medially) was only present in
27.8% of the kidneys studied. The posterior calices were more lateral in 19.3% of
cases. The largest proportion of kidneys (52.9%) had a mixed pattern. These findings
underscore the importance of obtaining high quality imaging studies before planning
an operative approach. In the case of IVP or retrograde pyelography this should include
lateral and oblique views.
Assessment of the Lower Pole Infundibulopelvic Angle,
Infundibular Length, Infundibular Width, and Calyceal Pelvic Height
It is widely accepted among endourologists that lower pole calculi have a poor clear-
ance rate compared to stones in other locations following shockwave lithotripsy (SWL;
in our study, lower pole anatomy had no impact on clearance after ureteroscopic litho-
tripsy). The dependent position of the lower pole is thought to play a major role. Other
important anatomical relationships to understand with regard to this phenomenon are the
lower pole infundibulopelvic angle (LIP angle), infundibular length (IL), infundibular
width (IW), and calyceal pelvic height (CPH). Several studies exist in the literature that
either support or refute the importance of these parameters with regard to stone clearance
rates and treatment success rates for lower pole stones. When reviewing studies that use
the LIP angle as a potential indicator of the odds of treatment success, it is important to
remember that the angle can be measured several ways. Figure 3A demonstrates this
point. The measurement is calculated using the angle between the central axis of the
lower pole infundibulum and one of the following: the ureteropelvic axis, the vertical
ureteral axis, or the renal pelvic axis (8–10).
The IL is calculated as the distance from the most distal point at the bottom of the calyx
containing the stone to the midpoint of the lower lip of the renal pelvis (8) (Fig. 3B).
Another similar measurement used to predict odds of stone clearance is
the CPH. This is defined as the distance between the lower lip of the renal pelvis the
lowermost point of the stone containing calyx (11,12) (Fig. 3C). The IW is measured at
the narrowest point along the lower pole infundibular axis (Fig. 3D).
Several studies support the theory that lower pole infundibulopelvic anatomy affects
stone clearance rates. In his original article on this subject, Sampaio et al. studied endo-
casts from the collecting systems of 146 cadaveric kidneys. He found three factors that
may play a role in lower pole stone clearance: the infundibulopelvic angle (clearance of
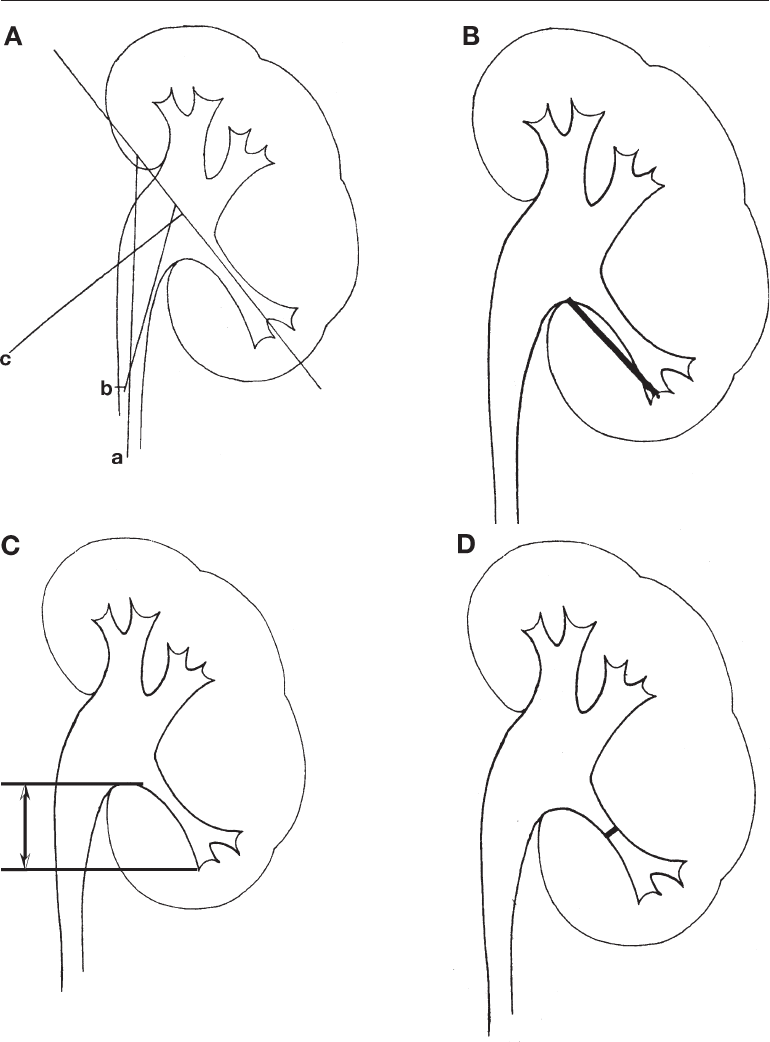
Chapter 23 / Anatomical Considerations 439
Fig. 3. (A) Different method of determining main axis for measuring lower pole infundibulo-
pelvic angle using vertical ureteral axis (a), ureteropelivic axis (b), and renal pelivic axis (c).
(B) Infundibular length measured as distance from the most distal point at the bottom of calyx
containing stone to mid-point of lower lip of the renal pelvis. (C) The calyceal pelvic height
is measured as the distance between the lower lip of the renal pelvis and the lowermost point
of the stone containing calyx. (D) Infundibular width is measured at the narrowest point along
the lower pole infundibular axis.

440 Eichel and Clayman
74% when the angle is >90° and 26% when the angle is <90°), the infundibular diameter
(60% clearance when it is >4 mm and 40% clearance when it is <4 mm), and the inferior
pole calyceal distribution (57% clearance when the calyces were multiple and simple vs
43% when the calyces were single and of a compound nature) (10). In a follow up
prospective study by Sampaio, 74 patients received SWL for lower pole stones. The
stone clearance rates for patients with an LIP angle greater than vs less than 90° was 75%
vs 23% respectively (13). Another retrospective study by Elbahnasy et al. reported 21
SWL patients and 13 ureteroscopy patients who were treated for lower pole stones. In
the SWL group, the infundibulopelvic angle in the stone free vs residual group was
significantly larger (75° vs 51°). There was no difference, however, in the ureteroscopy
group (56° vs 49°). Furthermore, in the SWL group, the IL was significantly shorter and
the IW greater in the success vs nonsuccess (32 mm vs 38 mm and 9 mm vs 6 mm
respectively). Again, there were no significant differences in the ureteroscopy group.
These findings were supported in a later study comparing treatment of solitary lower
pole stones with SWL, PCNL, and ureteroscopy where SWL was sensitive to lower pole
anatomy but PCNL and ureteroscopy were not (14). In this study, they also found that
having two or more favorable or unfavorable parameters had a much greater effect than
any single parameter alone in terms of clearance. Based on both of these studies, the
authors concluded that patients with lower pole stones 17 mm or less who have favorable
anatomy (LIP angle >70°, IL <3 cm, and IW >5 mm) are excellent candidates for SWL
whereas patients with less favorable anatomy (LIP angle <40°, IL >3 cm, and IW <5mm)
would be better served by ureteroscopy or PCNL. In further support of these findings,
Keeley et al. retrospectively analyzed the records of 116 patients with lower pole stones
between 11 and 20 mm with regard to LIP angle and also found a superior stone free rate
among patients with a more obtuse angle (15). Infundibular diameter and calyceal con-
figuration were not found to be significant factors. In the largest study to date, Poulakis
et al. reviewed lower pole calculi results in 680 patients (701 renal units) treated on a
Piezolith 2500. They, like Elbahnasy, found the critical LIP angle to be 45º. A CPH
greater than 15 mm was also found to be a significant variable. In this series all patients
with residual fragments had inversion therapy, and the average follow up was 26 mo
(16). Interestingly, in another recent study, an infundibular length to diameter ratio of
less than 7 and an infundibular diameter of >4mm with a single minor calyx affected in
multivariate analysis appeared to be predictive of clearance (85% in 63 patients) (17).
Looking at ureteroscopic treatment for lower pole stones, however, although
Elbahnasy et al. did not find the LIP anatomy to be a significant factor, Kumar, Joshi,
and Keeley did find a negative impact of an acute LIP angle on stone free rate (18). Thus,
some degree of controversy continues regarding this matter.
Even more controversy exists regarding the significance of lower pole anatomy itself.
Moody et al. analyzed the 26 patients who underwent SWL from the Lower Pole Study
Group with regard to LIP angle, IW, and IL as measured according to Elbahnasy. No
significant differences in these parameters were seen with regard to stone free rate. In
fact, the only significant factor measured was stone size (mean 9.6 vs 15.3 mm for stone
free vs not stone free) (19). However, these few patients were treated at 18 different
institutions using 5 different machines. In a retrospective study of 108 patients with
lower pole stones, Madbouly et al. also were unable to find any correlation between
lower pole anatomy and stone clearance following SWL (9). In this study the only
significant factor that affected stone clearance was a history of pyelonephritis. However,
again in this study, patients were treated with three different lithotriptors, some patients

Chapter 23 / Anatomical Considerations 441
had multiple lower pole stones, uric acid calculi were included, and over half of the
patients underwent multiple SWL treatments. The diversity of the patient population
could well have contributed to the authors’ inability to identify any significant param-
eters. More recently, an article by Sorenson and Chandhoke reviewed 190 patients
treated on a Doli U/50 with lower pole, single, calcified stones all 2 cm. These authors
found no impact of lower pole anatomy on stone clearance rates. Of note, in this study,
all patients had postoperative inversion therapy.
As such, the impact of lower pole anatomy as measured on the intravenous urogram
remains controversial. Perhaps, all of these patients should be treated by post-SWL
inversion therapy as this maneuver in two separate studies singularly increased the stone
free rate from 3% to 40% in one study and from 23% to 88% in another study (20,21).
In the final analysis, in this day and age, the point of lower pole anatomy may well be
on its way to becoming moot owing to the demise of the IVP. Indeed, the ability to
measure these parameters is becoming increasingly more difficult given that most renal
calculi are being diagnosed now by means of CT scan. The CT scan of the kidneys, in
its current state, precludes the ability to measure infundibular length, width, and angle.
In the future, there may be a resurgence of interest in this topic when 3D reconstruction
of the CT scan becomes more commonplace enabling a more reliable and reproducible
measurement of these various parameters.
Intrarenal Vascular Anatomy
The pioneering work of Brödel (22) and Graves (23) defined the distinct anatomical
segments of the kidney with their individual arterial branches. Although wide variation
exists, the renal arteries, in general, originate from the lateral margin of the aorta just
below the level of the superior mesenteric artery. They course posterior to the renal vein
and branch on the appropriate side of the renal pelvis into an anterior and posterior
division. The posterior branch is the first main branch of the renal artery in 50% of cases
and supplies the posterior middle segment of the kidney. The anterior segment typically
divides into four branches (apical, upper, middle, and lower). Kaye in 1982 described
this relationship as a hand (i.e., the main renal artery) grasping a glass (i.e., the renal
pelvis) with the thumb branching early and coursing posteriorly (i.e., the posterior seg-
mental artery) and the 4 fingers spreading out over the anterior surface of the glass (i.e.,
the apical, upper, middle, and lower segmental arteries) (24). The segmental branches,
in turn, split to form interlobar arteries that wrap around the superior and inferior poles
of the infundibula to form the arcuate arteries. The arcuate arteries, in turn, branch and
run between the renal pyramids and columns of Bertin. Small branches of the arcuate
arteries perforate the renal cortex to supply blood peripherally. Each individual arterial
branch is functionally an end artery and injury to a branch can lead to loss of segmental
function owing to infarction (Figs. 4A and B).
In an effort to characterize the renal vascular anatomy more specifically in the context
of percutaneous renal surgery, Sampaio and coworkers performed three-dimensional
endocasts of renal collecting systems, arteries, and veins in fresh cadavers (25–27). They
also studied the extent of vascular injuries sustained from percutaneous punctures of the
renal collecting system at various locations (28). They discovered that there is a high
likelihood of a significant vascular injury if the collecting system is punctured through
an infundibulum or if the renal pelvis is accessed directly because the larger vessels
surround these structures. Significant vascular injuries were discovered in 67%, 23%,
and 13% of upper pole, middle, and lower pole infundibular punctures respectively.
