Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
270 Transport in Fuel Cell Systems
where x is the axial distance along the channel and T
m,i
is the inlet mean fluid temperature.
So for a constant surface heat flux condition, the temperature varies linearly in the channel.
For a constant surface temperature condition, we can similarly derive from an integration
of Eq. (5.119):
T
s
− T
m
(x)
T
s
− T
m,i
= exp
−
Px
˙
mc
p
h
(5.121)
So for a constant surface temperature condition, the temperature varies exponentially in
the channel, asymptotically approaching the constant surface temperature value. Equations
(5.120) and (5.121) can be used with Eq. (5.117) to determine the heat flux when the
average convection heat transfer coefficient is known.
The heat transfer literature is replete with correlations that relate the particular fluid
and other parameters to the heat transfer coefficient. The most basic relationships most
relevant to fuel cell study will be given here, but the student or engineer interested in a
more thorough understanding should consult a heat transfer source for a more complete
treatment.
For free convection in gases, h is very low, ∼2–20 W/m
2
·K. For forced convection,
h can be many orders of magnitude higher, controlled by the flow rate and fluid being
convected. The Nusselt number Nu is an important dimensionless parameter related to the
thermal conductivity and length scale of heat transfer to the convection coefficient:
Nu =
hL
k
t, f
(5.122)
where L is the length scale relevant to the particular geometry (e.g., k
t, f
for a tube L is
the diameter, for a rectangular channel L is the hydraulic diameter) and k
t, f
is the thermal
conductivity of the fluid. There are a multitude of Nusselt number correlations for heat and
mass transfer in different situations.
Similar to the momentum boundary layer entrance length in a closed channel,
there is a thermal boundary layer development region. For laminar fully developed flow
(Re < ∼3000), an exact solution can be found for different boundary conditions. For fully
developed and laminar heat transfer in a rectangular channel with equal depth and width,
the heat transfer coefficient is constant and can be determined as follows
For constant surface heat flux (laminar)
Nu =
hL
k
t
= 3.61 (5.123)
For constant surface temperature (laminar)
Nu =
hL
k
t
= 2.98 (5.124)
Since the true boundary condition in a fuel cell is not exactly isothermal or constant heat
flux, an intermediate value is typically chosen for calculation for bulk analysis. For other
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.6 Heat Generation and Transport 271
Table 5.16 Nusselt Number for Various Rectangular Geometries
Boundary Condition
Uniform Heat Flux, Uniform Surface Temperature,
Cross Section (b × a) b/a Nu = hL/k Nu = hL/k
Circular NA, any diameter 3.11 2.47
Triangular NA, equilateral triangle 4.36 3.66
Rectangular 1 3.61 2.98
Rectangular 1.43 3.73 3.08
Rectangular 2 4.12 3.39
Rectangular 3 4.79 3.96
Rectangular 4 5.33 4.44
Rectangular 8 6.49 5.6
Rectangular ∞ 8.23 7.54
Source: Adapted from [51].
common fuel cell channel geometries,
11
some other Nusselt number values are given in
Table 5.16.
The heat transfer in the entry region is enhanced for the same fundamental reasons as
the pressure drop is increased in the momentum boundary larger entry region. However,
in fuel cells, the entry length heat transfer effect is typically small since the channels are
generally very long, and a small minority of the total heat generated is transferred to the
reacting gas flow streams anyway, so this effect can be neglected at an introductory level
of analysis. The thermal entry length for laminar flow can be shown as
L
e,thermal
d
h
≈ 0.06 Re · Pr (5.125)
For turbulent flow
L
e,thermal
d
h
≈ 4.4
(
Re · Pr
)
1/6
(5.126)
The unitless Prandtl number Pr is the ratio of thermal to viscous dissipation forces, so that
multiplying by the Reynolds number cancels out the momentum forces, leaving the thermal
dissipation:
Pr =
ρα
µ
(5.127)
where the thermal dissipation of the fluid, α,is
α =
k
t
ρc
p
⇒ Pr =
k
t
c
p
µ
(5.128)
11
In this text, we will only discuss basic correlations of greatest relevance to fuel cells. For more detailed
information, the reader is referred to advanced textbooks on convective heat transfer.

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
272 Transport in Fuel Cell Systems
For fully developed turbulent flow (Re > 10,000) in a channel, the Dittus–Boelter equation
is recommended to find the heat transfer coefficient [50]: For Re > 10,000, 0.7 < Pr <
160, L/dh > 10,
Nu =
hd
h
k
t, f
= 0.023 Re
0.8
Pr
n
(5.129)
where n is 0.4 for flow being heated by the channel and 0.3 for hot flow being cooled by
the channel.
In a fuel cell, we often have a mixed case, where a portion of the channel near the
catalyst layer is heating the flow and a portion on the back wall of the channel is cooling
the flow. In this case, we must remember that the h values obtained are simply averaged
correlations based on experimental data and generally measured for larger channels then
used in fuel cells. The use of the heat transfer coefficient h is a simplified way of calculating
the heat transfer compared to more fundamental calculation approaches. Thus, the solutions
provided by these Nusselt number correlations already have inherent error and should
not be treated as exact solutions. In the case of mixed conditions or odd geometries
not exactly within the bounds of those prescribed by the particular correlation: (1) a
more appropriate correlation may be developed and available in the literature or (2) some
alternative approximation can be made. For example, in the laminar-to-turbulent transition
region (3000 < Re < 10,000), a linear interpolation between the proper laminar Nusselt
number and the Dittus–Boelter Nusselt number can be used.
Example 5.17 Estimated Temperature Gradient inside a PEFC Consider a typical
PEFC operating at 0.6 V generating around 0.6 W/cm
2
waste heat flux. Determine the
expected temperature gradient from the 400-µm-thick cloth DM to the cathode catalyst
layer, assuming 50% of the waste heat is removed from the cathode side.
SOLUTION From Table 5.14, we see the thermal conductivity for the cloth is about
0.22 W/m·K. Through the DM, heat transfer should be dominated by conduction, so for
one-dimensional conduction between the catalyst layer and the edge of the DM
q
heat,x
=−k
x
dT
dx
⇒ q
heat,x
x
k
x
= T
Plugging in the numbers, we find
(0.3W/cm
2
)
400 × 10
−4
cm
0.0022 (W/cm · K)
= 5.45 K
So at a typical current density of 1 A/cm
2
, we can expect >5 K temperature difference
between the electrode and the edge of the DM for this DM. At higher current densities, the
temperature difference can even reach >10
◦
C, which has a significant effect on the water
balance and evaporator mass transfer.
COMMENTS: The fraction of heat removed from the cathode side was arbitrarily chosen
as 50% in this example to simplify the analysis. However, this need not be the case and
depends on the geometry, materials, and operating conditions. Obviously, the DM thickness

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.6 Heat Generation and Transport 273
also plays an important role. In the PEFC, the DM behave like insulation on the catalyst
layers, limiting heat transfer to the lands with relatively low thermal conductivity values.
Mass Transfer Analogy It is obvious by comparison that there is a direct analogy between
heat and mass transfer: Each has a developing region, each can be transported by direct
molecular collision (conduction or diffusion), and each can be transported by fluid motion
(convection). Indeed, Fourier’s law of heat conduction is mathematically identical to Fick’s
law of diffusion.
q
th,i
=−k
∂T
∂x
i
n
i
=−D
∂C
∂x
i
As discussed, the Prandtl number is the ratio analogous to the Nusselt number and is the
dimensionless mass concentration gradient.
The Schmidt number is the ratio of momentum and mass diffusivities:
Sc =
v
D
AB
(5.130)
Therefore, appropriate correlations for the mass transport in enclosed channels can be
obtained by taking the relationships shown for heat transfer and replacing Nu with Sh and
Pr with Sc and the temperature with the concentration.
Radiation Heat Transfer Radiation is heat transfer resulting from the emission of pho-
tons or electromagnetic waves from matter undergoing molecular transitions. Unlike the
transmission of heat by conduction or convection, radiation does not rely on intermolecular
collisions and therefore can be transferred in a vacuum. This quality is particularly handy
considering all life on Earth relies on radiation from the sun. Radiation is a complex subject
that will only be briefly discussed here. For more detailed analysis, the reader is referred to
undergraduate-level [50] and graduate-level [52] references. Radiation is only a significant
mode of heat transfer for high-temperature fuel cells, such as molten carbonate and solid
oxide fuel cells. For an enclosed body, the net radiation exchange between the surface of
the body at absolute temperature T
s
and the surroundings at absolute temperature T
surr
is
q
= εσ
T
4
s
− T
4
surr
(5.131)
where ε is the surface emissivity, which is the fraction of radiative energy emitted (0 <ε<1)
and is different for every surface. For a perfect emitter, this value can be assumed to be 1.
The σ is the Stefan–Boltzmann constant (σ = 5.67 × 10
−8
W/m
2
· K
4
). This expression
assumes that the body of exchange has the same emissivity as the surface. Here, T
surr
is the surrounding temperature the body is exchanging radiation with, which is different
from the ambient temperature. For example, consider a SOFC outer shell at 1000
◦
C and
inside surface of the surrounding at 700
◦
C. As a first approximation, the radiation exchange
between the surfaces can be estimated as
12
q
rad
= σ
T
4
s
− T
4
surr
= 5.67 × 10
−8
1273
4
− 973
4
= 98 kW/m
2
12
This is only a basic estimation. For a better approximation, the spectral emission, reflection, and absorption
surface properties of the materials involved need to be incorporated into the analysis.
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
274 Transport in Fuel Cell Systems
This relationship assumes a perfect emitter in a fully enclosed surroundings. This value can
be compared to a typical natural convection current from the surface, with h ∼ 5W/m
2
· K,
q
conv
= h
(
T
s
− T
∞
)
= 5
(
1273 − 973
)
= 1.6kW/m
2
So radiation can be quite important, but only when there is a high temperature difference
between the surface and surroundings, as is the case for high-temperature fuel cells such as
the molten carbonate and solid oxide fuel cells.
Heat Dissipation from Stacks In many high-temperature systems, the high rate of heat
transfer and operating temperature and the need to maintain a safe external environment re-
quire thermal insulation around the unit. For high-temperature systems, it is often desirable
to operate the power system as a combined heat and power (CHP) source. This practice is
common for stationary power systems, not just fuel cells. In a CHP system, some of the
waste heat from reaction is used for some other intentional service, such as providing heated
water or powering a steam turbine. High-temperature fuel cell systems are well suited for
CHP use due to their quality recoverable waste heat. In 1998, Siemens Westinghouse began
operation of a 100-kWe SOFC unit in Westervoort, The Netherlands. The system was also
designed as a CHP unit, to deliver 65 kWh to the residential district as heated water. This
early demonstration unit was able to achieve a direct electrical conversion efficiency of
46% at 109 kWe. When the additional recovery of heat is included in the overall efficiency,
the CHP system achieved a remarkable 73% average energy conversion efficiency. Many
more advanced systems now are in operation that improve on this early prototype.
In many high-power fuel cells, the heat dissipation is high enough that some cooling
is needed. Many fuel cell systems have a small range of operational design temperature for
a variety of reasons. In some liquid electrolyte fuel cells (e.g., an alkaline fuel cell), the
electrolyte itself can be circulated through a heat exchanger and act as the coolant. In PEFC
systems with a solid electrolyte, the material and need for humidification of the electrolyte
limit operating temperature to below 100
◦
C. To avoid a large thermal gradient and possible
overheating in PEFC stacks, coolant channels are typically integrated with the overall stack
design, as illustrated in Figure 5.43. The coolant used is typically water (which can freeze)
or some other coolant such as ethylene glycol with resistance to freeze.
The heat transfer from the bipolar stack plate to the coolant flow can be simply estimated
by consideration of an energy balance on the fluid:
˙
Q =
˙
m
coolant
c
coolant
(T
out
− T
in
) (5.132)
This simple relationship, derived from a conservation of energy around the coolant flow in
the steady state, can be used to estimate temperature rise in the coolant for a given condition
or adjust the coolant flow rate to achieve a desired temperature gradient across the bipolar
plate. Because the thermal mass of the bipolar plates is so much larger than the gas in
the flow channels, the gas temperature will follow that of the bipolar plate at the inlet and
through to the exit.
Example 5.18 Determination of Coolant Flow Rate Required Consider a 100-plate, 10-
kWe PEFC stack operating at 48% thermal efficiency, as determined from a stack voltage
measurement. Each individual fuel cell is to be cooled by a flowing liquid water coolant
channel. In order to balance the water generated in the fuel cell and prevent flooding, it is
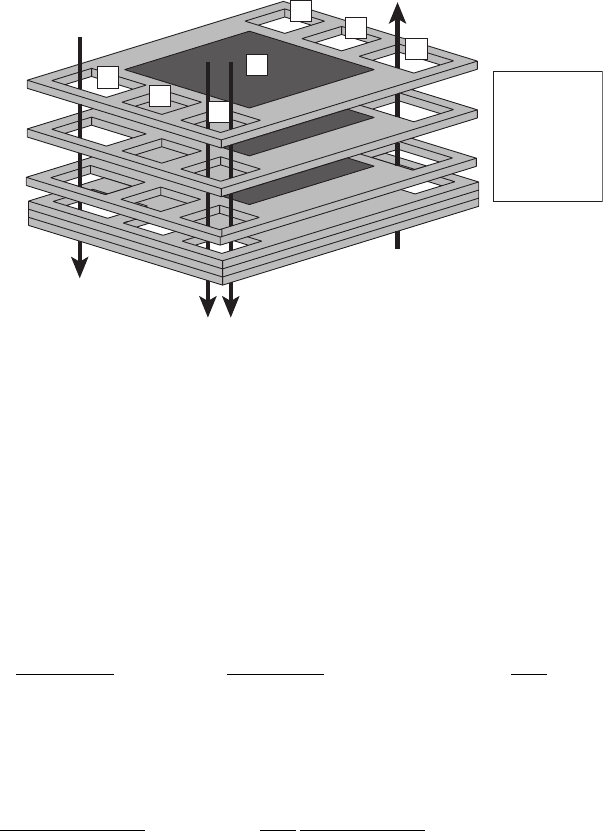
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.6 Heat Generation and Transport 275
Flow
chimney
Air in
Air out
Coolant in
Coolant out
H
2
in
H
2
out
Active area
A:
B:
C:
D:
E:
F:
G:
D
G
E
C
B
A
F
Figure 5.43 Stack plates manifolded with coolant flow channels.
desired for the coolant temperature to increase 10
◦
C from the inlet to the exit of the fuel
cell. Solve for an expression relating the coolant mass flow rate to the desired temperature
increase. From measurement of the gas channel temperature you can assume 80% of the
waste heat is absorbed by the bipolar plates and flows into the coolant and the remaining
20% is removed by the gas flow. The specific heat of liquid water is 4.18 J/g · K.
SOLUTION At 48% thermal efficiency, the stack is generating waste heat:
η
th
=
˙
Q
e
˙
Q
waste
+
˙
Q
e
⇒
˙
Q
waste
=
˙
Q
e
(
1 − η
th
)
η
th
⇒
˙
Q
waste
= (10 kWe)
0.52
0.48
= 10.83 kWh
With 80% of this waste heat into the coolant and a 10
◦
C temperature increase, from Eq.
(5.132),
0.8
˙
Q
waste
c
coolant
(
T
out
− T
in
)
=
˙
m
coolant
=
8.67
4.18
(
kWh
)
(
J/g · K
)
(10 K)
(1000 W/kW) = 207 g/s
If the flow rate were increased, the temperature rise of the coolant would be decreased. As
the power output of the fuel cell increases, the coolant flow rate can be increased to match
the rising waste heat output.
COMMENTS: Much of this example has been simplified to emphasize the use of Eq.
(5.132). In a full-size stack, there would be temperature gradients from plate to plate in the
stack resulting from the external heat loss and differences in the individual performances
of the fuel cells in the stack. Although the overall thermal efficiency is measured at 48%,
individual fuel cells can be operating with very different voltages. In some cases, individual
fuel cells may degrade significantly and generate excess heat.

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
276 Transport in Fuel Cell Systems
5.7 SUMMARY
In this chapter the transport of uncharged and charged species (ions and electrons) was dis-
cussed. For charged and uncharged species, transport by diffusion (concentration gradient
driven) and convection (velocity field driven) occurs. The addition of a voltage gradient can
cause motion of charged species by migration. The governing transport equation combining
these modes is the Nernst–Planek equation:
˙
n
j,i
=−D
j,i
∂C
j
∂x
i
+ C
j
v
i
−
z
j
F
R
u
T
D
j,i
C
j
∂φ
∂x
i
For most fuel cells, the ohmic region is dominated by the electrolyte conductivity. For a
PEFC, the conductivity is a strong function of water content, temperature, and polymer
molecular weight. For the SOFC, the electrolyte conductivity is mostly a function of
temperature and material additives and is not normally ionically conductive at all until an
elevated light-off temperature is reached. For liquid electrolytes, the ionic conductivity is
a function of many parameters, including electrolyte concentration, temperature, ion type,
and ion charge. Electron transport in a fuel cell is typically not limiting, and only contact
resistance between components contributes to any significant voltage loss.
In Section 5.3, gas-phase transport, which occurs by both diffusion and convection,
was discussed. The characteristic transport times of diffusive transport were shown as
τ =
l
2
D
The characteristic diffusion time for gases is orders of magnitude faster than for liquids
or in polymers, which can lead to instabilities and transient variations in low-temperature
PEFCs.
Several different methods for the determination of gas-phase diffusion coefficients
were discussed, the simplest being derived from molecular theory:
D
j
∝
T
3/2
P · MW
1/2
σ
2
While this relationship is commonly used and shows proper qualitative trends, it is not com-
pletely accurate for polar molecules such as water vapor. Methods for improved estimation
of binary diffusion coefficient and diffusion coefficients in mixtures including nonpolar and
polar molecules are presented and should be used when quantitative precision is required.
Using purely gas-phase diffusion, an expression for the gas-phase diffusion limiting
current density was derived:
i
l
=−nFD
eff
C
∞
δ
=−nFDφ
1.5
y
i
P/R
u
T
δ
This expression can be used to see the qualitative relationships between the variables, but
due to film resistances, especially in multiphase PEFCs, the actual limiting current density
is much less than that predicted through this relationship. The relationship can be extended
to include the effects of film resistances and different layers (e.g., catalyst layer resistance)
as well. Film resistances from an electrolyte layer or liquid on the catalyst layer can be
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.7 Summary 277
accounted for using Henry’s law
y
i,liquid/membrane side
=
y
i,gas side
P
gas side
H(T )
Henry’s constant H is a strong function of temperature for liquids. At a phase boundary, the
concentration of the reactant is decreased in the liquid or solid phase according to Henry’s
law and then must diffuse to the catalyst. Any small film resistance on the catalyst of liquid
effectively floods the catalyst location, so that almost no reactant can reach the catalyst. For
ionomer coverage of the catalyst in a PEFC, some reactant penetration is desired. Although
a high reactant diffusivity in the catalyst layer electrolyte is beneficial to increase the mass
transfer limiting current density, it is deleterious for reactant crossover discussed in Chapter
4, so that a proper engineering balance of ionomer content in the electrolyte is used.
In very small pores, Knudsen diffusion, dominated by molecule–pore surface collisions,
can play a role or even dominate. The Knudsen number (Kn) is a dimensionless parameter
that can be used to determine the relative role of Knudsen flow:
Kn =
l
d
=
k
B
T
√
2πσ
2
ii
Pd
Ĺ When Kn > 10, Knudsen flow dominates.
Ĺ When Kn < 0.01, bulk diffusion flow dominates.
Ĺ For 0.01 < Kn < 10, both Knudsen and bulk diffusion are important, and transitional
flow exists.
For Knudsen flow, the effective diffusion coefficient can be determined from the kinetic
theory of rigid spheres [18]:
D
Kn
=
d
3
2k
B
T
MW
i
1/2
(5.133)
The pore size diameters where Knudsen flow dominates are below 0.05 µm, which is below
the normal pore size seen in fuel cells. However, Knudsen flow can play a role within the
catalyst layers of many fuel cells, in parallel with normal diffusion processes.
The pressure drop along flow channels in a fuel cell is governed by frictional pressure
drop, reactant consumption, product uptake, and two-phase slugs or blockages in low-
temperature PEFCs. For low-stoichiometry flows, the consumption can dominate pressure
drop along the flow field. Some fuel cell designs even feature a dead-end anode, where the
consumption is used to draw the hydrogen into the fuel cell. In terms of transport, the devel-
oping boundary layer enhances transport of mass and heat and increases frictional losses.
Many fuel cell designs have a significant portion of the flow field within the developing
region, although fully developed flow is normally assumed in analysis.
Multiphase flow in gas channels is characterized by different regimes. In PEFC flow
channels, slug, annular, and mist flow can occur depending on the flow velocity. In porous
media, the description of multiphase transport is more complex. The momentum equation
reduces to Darcy’s law:
Q =
−kk
r
A
µ
P
L
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
278 Transport in Fuel Cell Systems
where the permeability k is a function of the saturation and intrinsic permeability of the
porous media, which can be related to the Carman–Kozeny relationship:
k =
r
2
φ
3
18τ (1 −φ)
2
and k
r
, the relative permeability for a phase, is typically shown as
k
r,nw
= A(s
nw
)
n
and k
r,w
= B(1 − s
nw
)
m
for the wetting (w) and nonwetting (nw) phases. Although m and n vary, typical values are
around 2.0–3.0 for fuel cell porous media in PEFCs.
The driving force in Darcy flow is the capillary force, which can be written as
P
c
= P
nw
− P
w
=
2γ cos θ
r
This relationship shows the critical role that pore size and surface tension have in controlling
the capillary flow through porous media. The smaller the pore size, the higher the capillary
pressure. This fact is responsible for driving liquid from small-pore-size areas to areas with
larger pore sizes. However, the capillary pressure is not solely a function of pore size. It
is also a function of the level of saturation in the media. In order to link the saturation,
capillary pressure, and liquid flow in porous media, a Leverett function has been written
such that
P
C
= γ cos θ
φ
k
1/2
J (s
l
)
where the Leverett function J(s
l
) is an empirical best curve fit of data measured for a
variety of soils. Although this general approach has been adopted in fuel cell studies, the
particular form of the Leverett function is not appropriate for fuel cell media and can lead to
large errors in calculations. Newer, more appropriate functions according to PTFE content,
compression, and temperature have now been derived.
The modes of heat generation include Peltier heating, Joule heating, and activation
heating, a result of entropy generation or reduction, ohmic losses, and activation or con-
centration losses, respectively. In most larger stacks, some active cooling is needed to
remove waste heat and prevent excessive temperature gradients. In low-temperature fuel
cells, this can be achieved using a recirculating liquid coolant. In higher temperature SOFC
systems, the gas-phase reactant flow can be used to achieve a more uniform internal tem-
perature profile. In systems with recirculating liquid coolant, heat can be removed from the
coolant bath.
APPLICATION STUDY: COGENERATION FOR
FUEL CELLS
In a cogeneration power plant, the effective overall thermodynamic efficiency of the plant
is increased by utilizing a portion of the waste heat generated by the power generation

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
Problems 279
process, that is:
η
eff
=
work + heat utilized
maximum energy output
Such systems based on conventional power generation technology have been used for
stationary power or larger grid power systems, not only fuel cells. For instance, the waste
heat generated by a gas turbine can be used to heat the water or provide steam heat for a
building, eliminating the need for a separate heating system by using some of the thermal
energy dissipated as waste heat for a useful purpose. For this assignment, do some research
and answer the following:
1. Which types of fuel cell systems are well-suited for cogeneration? Explain why.
2. Go online and determine what are the current cogeneration concepts and or working
fuel cell systems. There are many of them. Write a brief summary of the three of
the concepts or systems you find to be the most interesting.
PROBLEMS
Calculation/Short Answer Problems
5.1 Discuss the physical meaning of the terms in the
Nernst-Plank Equation.
5.2 When would Ohm’s law be invalid for an electrolyte
solution?
5.3 Make a plot of water viscosity versus temperature in the
range of 300–400
◦
C. By what percentage does the viscosity
change over this range? Make a plot of the conductivity of
Nafion with respect to temperature for fully hydrated con-
ditions. Do you expect the trends in the two plots match?
5.4 Discuss the physical reason why the conductivity of the
Nafion PEFC electrolyte changes with water content and
EW. If you were to design an electrolyte for low humidity
conditions, would you choose a high or low value of EW
electrolyte? What would be the drawbacks of this choice?
5.5 Why is the Grotthuss mechanism of ion transport more
rapid than the vehicular mode?
5.6 Calculate the expected voltage gain (from ohmic po-
larization recovery only) would you expect from increasing
the average fuel cell relative humidity from 40 to 60% in
a Nafion 112 at 80
◦
C, 1 A/cm
2
. What would the practical
disadvantages of this change be?
5.7 A new electrolyte is developed that needs only 20%
relative humidity at 90
◦
C to achieve a maximum conduc-
tivity of 8 S/cm. What is the RH required for an equivalent
Nafion system to achieve the same level of conductivity?
5.8 Calculate the expected voltage gain (from ohmic po-
larization recovery only) would you expect from increasing
the average fuel cell temperature of an SOFC from 700 to
900
◦
C at 1 A/cm
2
. What would the practical disadvantages
of this change be? Use the ionic and electrical conductivity
of 8% mole fraction yttria given in the text.
5.9 List some ways an SOFC system could rapidly achieve
light off temperature in practice.
5.10 Discuss the physical reasons why the temperature,
ion concentration, viscosity, ion radius and charge number
has an effect on the ionic conductivity of liquid electrolyte
solutions.
5.11 At high water content, the electrolyte in a solid poly-
mer electrolyte membrane such as Nafion behaves similarly
to a dilute electrolyte solution. Do you expect the conduc-
tivity of the Nafion electrolyte to always increase with water
content, or would there be a physical limit?
5.12 Calculate the thickness of a bipolar plate (σ
e
=10,000
S/cm) that will match the voltage drop caused by proton flux
through a Nafion electrolyte at 80
◦
C, 100% RH.
5.13 Develop an equation like Eq. (5.33) for water vapor in
air, and water vapor in hydrogen. Onto what side of a fuel
cell will the moisture more readily go into the gas phase?
5.14 Make a plot of the diffusion coefficient of oxygen into
water vapor versus porosity using the Bruggeman relation-
ship. At what level of porosity is the flow 90% restricted
compared to pure gas-phase flow?
