Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
360 Polymer Electrolyte Fuel Cells
Irreversible Modes of Chemical Degradation
1. Electrolyte Loss Electrolyte material can be lost through a variety of physico-
chemical mechanisms. For PEFCs, the polymer itself can degrade physically and
chemically, particularly from peroxide radical attack [73]. This results in loss of
mass and conductivity in the electrolyte and possibly catastrophic pinhole forma-
tion. Mechanical stress and cycling have also been linked to accelerated polymer
degradation [74]. Over time, the electrolyte in a PEFC can thin considerably as the
ionomer is lost. This can result in a temporary increase in performance, since the
ohmic losses decrease with a thinner electrolyte. However, over time, the thinning
electrolyte is more susceptible to pinhole formation and excessive crossover, lead-
ing to failure. This mode of damage is especially amplified in extreme conditions
of high temperature and low humidity. In PEFCs, electrolyte degradation can be
monitored by measurement of the fluorine content in effluent liquid and vapor and
has been found to correlate with membrane degradation.
2. Platinum Dissolution and Migration Platinum migration from the cathode of a
PEFC occurs due to the following steps [75];
a. Platinum dissolves into Pt–O in the catalyst layer at high potentials. On the
cathode at high potential, a mobile Pt
2+
species is created according to the
reaction
PtO + 2H
+
↔ Pt
2+
+ H
2
O (6.52)
b. The PtO is mobile in Nafion and can diffuse on the surface of Pt/C, further
exposing underlying Pt in clusters.
c. Under the voltage gradient between the anode and cathode, PtO migrates away
from the catalyst layer through the electrolyte toward the anode.
The result is an irreversible loss with fewer active sites in the cathode for the
reaction, resulting in a gradually decreasing exchange current density (i
0
)atthe
cathode. Under load cycling to high cathode potential, the degradation rate is greatly
accelerated. This is because of the continuous formation and disruption of mobile
oxide film leading to mobile Pt
2+
species. The bottom line is that Pt is susceptible
to dissolution and migration toward the anode under high potential and load cycling
conditions, leading to loss of ECSA.
3. Ionic Impurity Contamination Ionic impurities from metals in the fuel cell system
will readily absorb into the fuel cell electrolyte, since it is an ionic conductor.
When the ionic impurity gets into the membrane, it can greatly reduce the ionic
conductivity and alter water transport in the membrane and catalyst layers, reducing
performance from very minute quantities of impurities [76]. Postmortem testing of
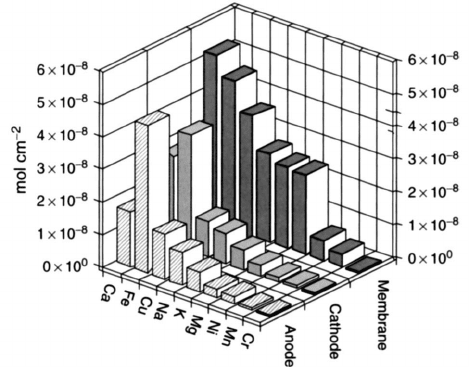
used membrane electrode assemblies (MEAs) has demonstrated the presence of
a surprising array of metallic impurities, such as calcium, iron oxides, copper,
magnesium, and various other metals, as shown in Figure 6.62. As a result, most
fuel cell systems are designed to avoid contact of the reactant flow stream with any
metal connectors or couplings, and special plastics deemed compatible for fuel cell
service have been developed for fuel cell systems.
4. Degradation of Hoses and Gaskets Oxidation of components such as metallic
current collectors, gaskets, and hose components and fittings can become a ma-
jor loss in fuel cells over time [77]. The oxidation and decomposition products
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.5 PEFC Degradation 361
Figure 6.62 Bar chart with cationic impurities found in Nafion 112 electrolyte operated at
0.25 A/cm
2
at 75
◦
C after 9300 h of operation. (Reproduced with permission from Ref. [76].)
from these materials can permanently change the ionomer conductivity and wetta-
bility of components, changing the water management or accelerating membrane
degradation.
Carbon Monoxide Poisoning In the near term, it is likely that a majority of the hydrogen
produced will come from a hydrogen reformation process, in which a carbon-containing
fossil fuel (e.g., natural gas or methanol) is partially oxidized into a mixture of mostly
hydrogen, carbon dioxide, and water [78]. One of the undesired byproducts of the refor-
mation process is a small fraction of carbon monoxide (CO). Although the amount of CO
in the feed stream varies for different reformation processes, even small levels of CO can
adversely affect the performance of low-temperature noble metal catalyst fuel cells, such
as the PEFC. As a result, a large portion of the total fuel cell reformer system must be
devoted to CO removal. Even with bulky and complex CO scrubbing subsystems, nominal
CO levels can still be in the 10–50-ppm range, and transient operation during load changes
or startup can produce sporadic pulses above the 1000-ppm level. The complexity involved
in dynamic control of the reformation subsystem is a barrier in transient automotive and
military fuel cell systems, where it would be especially desirable to utilize a conventional
liquid fuel with onboard reformation to circumvent hydrogen infrastructure limitations.
Although there are alternative theories, the global reaction mechanism of CO
poisoning of hydrogen PEFCs with platinum catalyst has been modeled by Springer and
co-workers [79]:
CO + M ←−−−−−−−−−−−−−−→
k
1 f
k
1R
(M–CO) (6.53)
H
2
+ 2M ←−−−−−−−−−−−−−−→
k
2 f
k
2R
2(M–H) (6.54)
(M–H) −−−−−−−→
k
3 f
H
+
+ e
−
+ M(6.55)
H
2
O + (M–CO) −−−−−−−→
k
4 f
M + CO
2
+ 2H
+
+ 2e
−
(6.56)

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
362 Polymer Electrolyte Fuel Cells
The forward reactions of Eqs. (6.53) and (6.54) dominate the reverse reactions and respec-
tively represent the parallel processes of CO adsorption and H
2
dissociative chemisorption
on an active and available platinum catalyst site, M. Equation (6.53) is the CO poisoning
step. Here, adsorbed CO takes up active catalyst surface sites, rendering them unavailable
for hydrogen dissociative chemisorption of Eq. (6.54) and the desired current-producing
hydrogen electrochemical oxidation reaction of Eq. (6.55). In steady state, surface
coverage of CO sites with gas streams containing as little as 100 ppm CO can reach 95%.
This blockage of available catalyst sites results in reduced fuel cell efficiency (lower cell
voltage for identical current). Equation (6.56) is the electrochemical oxidative desorption
of CO from the catalyst site and is the rate-limiting step in the overall mechanism.
Based on the mechanisms proposed in Eqs. (6.53)–(6.56), an expression relating the
time-dependent fractional surface coverage of the CO (θ
CO
) species can be shown [79]:
ρ
dθ
CO
dt
= k
1 f
y
CO
(
1 − θ
CO
− θ
H
)
− K
1R
θ
CO
− k
4 f
θ
CO
exp
α
CO
Fη
A
RT
(6.57)
where the first term on the right-hand side represents the CO adsorption on the catalyst
surface (poisoning step), the second term represents the desorption of CO (reverse of
poisoning), and the third term represents the electrochemical oxidative desorption rate
equation (6.56). Due to the uncertainty in the various kinetic parameters, the fuel cell
dynamic performance is very difficult to predict without use of arbitrary fitting parameters.
Carbon monoxide poisoning remediation technology is fairly developed, including air
bleeding, alternate catalyst selection, and other methods. In air bleeding, a small percentage
(usually <5%) of air is mixed with the anode flow to promote chemical and electrochemical
oxidation of CO and reduce poison coverage of the anode catalyst [80]. The drawback of
this technique is increased pumping requirements and electrochemical potential losses
associated with fuel/oxidizer mixing in the anode feed. Additionally, the mixing of moist
air and hydrogen poses safety concerns. The use of CO-tolerant catalysts such as Pt–Ru
can greatly reduce the performance loss for operation on moderate amounts of CO in the
fuel feed stream.
In summary, various modes of physical and chemical degradation exist, which limit
life times of operating DEFCs. In particular, membrane moisture variation or chemical
impurities can rapidly degrade performance and must be controlled.
6.6 MULTIDIMENSIONAL EFFECTS
In the study of fuel cells, there is a natural tendency to imagine the profiles of heat, mass,
current, and other parameters as uniform across the active area. There is also a natural
tendency to think that a perfectly homogeneous distribution of these operational parameters
is ideal. In fact, almost nothing is uniform in PEFC stack operation in the channelwise,
through-plane, or plate-to-plate directions. From an engineering design perspective, many
of these gradients can be intentionally exploited for performance optimization. An excellent
example already discussed is that of water management and temperature distribution. In
order to provide a fully moist condition throughout the fuel cell without excessive flooding
and simultaneously reduce the load on the humidifier system, the temperature distribution
can be adjusted through the coolant flow rate to increase steadily from the inlet region to
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.6 Multidimensional Effects 363
the exit region, so that the relative humidity of the flow is approximately uniform and 100%
throughout and the water generated is absorbed as vapor reducing flooding.
The computational or analytical prediction of the current, species, and temperature
distributions in a fuel cell can be generated by extension of the basic concepts of this
text into multidimensional models with various levels of complexity and solved using the
tools of computational fluid dynamics (CFD). Models of various complexity abound in the
literature, and commercial packages now exist from several CFD software developers for
this purpose.
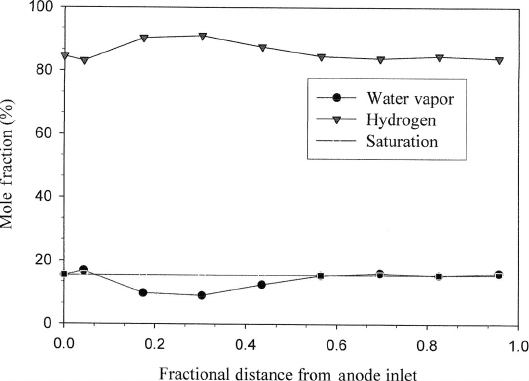
Water and Species Distribution Along the channel path from inlet to exit, one can
anticipate a reduction in the hydrogen and oxygen species concentrations that follow the
consumption by the electrochemical reactions. The mole fractions of the reactant species
may not be decreasing, however, since humidification or dehumidification of the flow can
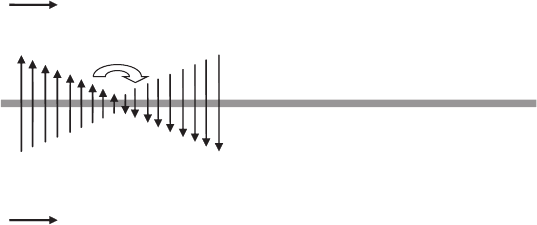
alter the water mole fraction. Consider a wet anode input with a dry cathode input and
cocurrent flow, depicted in Figure 6.63. Conditions of dehumidifying flow can actually
increase the reactant mole fraction, even with consumption, as shown in Figure 6.64. As
the flow enters, the net drag will be heavily positive, favoring flow of water from the
humidified anode inlet toward the dry cathode. The result will be a decreasing water mole
fraction, and increasing hydrogen mole fraction along the anode, despite the fact there is
hydrogen consumption along the channel. At the point where the water vapor concentration
on the anode and cathode are the same, there will be a diffusion flux reversal point.
Temperature Distribution Along the fuel cell channel, the temperature distribution is
directly controlled by the heat transfer boundary conditions. For small fuel cell stacks, with
no active coolant flow, the external boundary conditions control the temperature distribution
and at low current can be considered as uniform in temperature. For larger stacks with active
cooling, the temperature distribution can be engineered to match the desired humidity profile
to control flooding and promote longevity by elimination of dry- and hot-spot locations. In
the in-plane direction, temperature variation exists, with more water accumulation under
generally colder lands, as discussed. The temperature distribution in the stack can be fairly
Moist anode inlet
Dry cathode inlet
Diffusion reversal location
Net water drag
toward cathode
Electrolyte/electrode assembly
-net flux reversal may be different location
Figure 6.63 Schematic of dry cathode–wet anode inlet flow that results in diffusion reversal process
in electrolyte. The arrows represent the direction of diffusion flux, not the net mass flux. (Adapted
from Ref. [11].)
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
364 Polymer Electrolyte Fuel Cells
Figure 6.64 Water vapor and hydrogen mole fraction distributions for a concurrent flowing ser-
pentine isothermal fuel cell with 100% RH anode inlet, 0% RH cathode inlet at 0.7 V opera-
tion. Even though current is being produced and hydrogen consumed, the hydrogen mole fraction
along-the-channel is increased near the inlet due to dehumidification of the anode. (Adapted from
Ref. [11].)
well predicted using known thermal parameters and voltage current behavior to predict heat
generation.
Current Distribution The current distribution in a PEFC can be measured experimentally
with a variety of techniques, as discussed in Chapter 9. For larger fuel cell active areas,
gradients in temperature, reactant concentration, humidity, and liquid water can drastically
alter the local current density and should be considered in any advanced analysis. In order
to predict the current distribution in a cell on a qualitative basis, one has to keep in mind
the major driving forces that control the current at a given voltage:
1. Local relative humidity
2. Local reactant concentration
3. Local liquid water distribution
Since the temperature in the PEFC typically varies by at most ∼15
◦
C, the effect
of temperature distribution on the reaction kinetics will be relatively small compared to
the temperature interaction with the liquid water distribution and relative humidity that
controls membrane ionic conductivity. The local relative humidity in the anode phase
typically controls the local current density of an underhumidified fuel cell because electro-
osmotic drag exacerbates anode dryout, while water generation at the cathode diminishes
any electrolyte dryout in the cathode catalyst layer [11]. That is, if the other parameters
are constant, the local current distribution can be predicted with a knowledge of the anode
in an underhumidified cell [11]. Anode dryout can be the result of the loss of only a few
hundredths of a milligram of water per square centimeter active area in the catalyst layer
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.6 Multidimensional Effects 365
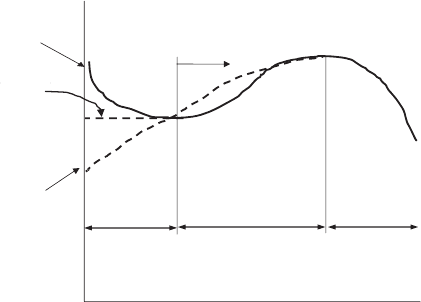
Current
Location
(
x/L
)
Flooding/concentration
losses (may or may not
occur)
Dip due to
anode dry-out
at high i
Moist
anode/high
Dry anode
inlet
Water flux
reversal
to anode
Peak height increased
with current, net water
imbalance
Region II
Region I
Region III
Balanced
water
content
inlet
current inlet
Figure 6.65 Characteristic local current curve for undersaturated inlet conditions. (Adapted from
Ref. [11].)
[10]. Since the catalyst layer is only on the order of 15 µm thick and consists of about 30%
ionomer by weight, a very small amount of local drying can significantly decrease the ionic
conductivity of the catalyst layer.
Figure 6.65 is a sketch of the generic relative current versus location along the flow
channel for different combinations of inlet humidity on the anode and cathode with a
concurrent serpentine design and is key to understanding local performance for underhu-
midifed conditions. The generalized performance curve is qualitatively sinusoidal in shape
(although not necessarily symmetric or with equal amplitude or wavelength, as shown for
convenience). These results were obtained for a uniform coolant temperature boundary
condition, so any effects of a nonuniform temperature are not included. Region I begins at
the inlet of the fuel cell. If the anode is dryer than the cathode and the current is relatively
low, the net flux of water is always neutral or toward the anode, resulting in an increasing
performance through region I. The steepness of the slope of this portion is exacerbated
with increased moisture imbalance between anode and cathode, increased current density,
and other thermodynamic and geometric parameters. In contrast, if the water vapor im-
balance initially favors the anode (dryer cathode), diffusion and electro-osmotic drag are
both initially toward the cathode, resulting in a drying condition at the anode catalyst layer
and electrolyte, reducing performance until a local minimum is reached, which terminates
region I. The minimum in performance is exacerbated by increased current density and
moisture difference between anode and cathode as well as flow rates and other thermody-
namic and geometric parameters. Note that for closely hydrated anode and cathode at low
to moderate current density performance in region I can be homogeneous, as illustrated in
Figure 6.65. Note that the end of region I is shown as the same current for dry anode and dry
cathode conditions, when this is not necessarily the case. In fact, region II for a relatively
dry anode inlet is really a continuation of region I and no discrete boundary between the
two regions is defined.
In region II, the case with relative dry anode feed continues increasing performance to
a maximum (if reached), which ends region II. The decrease from the maximum current is
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
366 Polymer Electrolyte Fuel Cells
a result of decreasing reactant availability or flooding and depends on operating conditions,
and therefore may not be severe, as shown in Figure 6.65. The dry relative cathode case
begins region II at the point of water mass flux reversal from cathode to anode. In this
situation, this reversal may not occur with a thick electrolyte if back diffusion never
overcomes electro-osmotic drag to the cathode. This flux reversal reduces anode-side
dryout performance loss and initiates an increase in performance. For both the dry relative
anode and cathode cases, region II concludes with a maximum in performance. Note that
the location of the maximum is dependent on the fuel cell and operating conditions.
In region III, for convenience, both dry anode and cathode cases are shown to peak at the
same location, although this depends on the individual conditions and is not necessarily the
case. Following the maximum local current, there is a downward trend resulting from local
flooding or gas-phase mass transport losses at the electrode(s). This peak and downward
trend will only occur if a reactant starvation condition (via flooding or high utilization) is
reached.
The generalized performance curve of Figure 6.65 closely follows anode gas channel
water vapor content through region II, indicating anode catalyst layer and electrolyte mois-
ture content plays a key role in controlling the distributed performance of an undersaturated
inlet PEFC. Cathode conditions are generally locally moist at the catalyst layer and control
the location of the maximum performance which initiates region III. The region I trend
depends on the side with greatest hydration and local water activity. The amplitude of the
peaks is increased with increasing current density and water imbalance between anode and
cathode at the inlet. The width of regions I–III depends on current density, flow rate, water
imbalance, and membrane and electrode thicknesses as well.
In general, under high-humidity conditions with neat hydrogen flow, the current profile
follows oxygen distribution. The current distribution is reduced along locations of flooding,
which typically occurs near the cathode exit or in cold spots in the fuel cell under landing
areas. In this case, excessive liquid water buildup restricts oxygen flow to the catalyst layer,
and local current can be greatly reduced. In single-cell and stack situations, it is quite
possible that certain locations of the fuel cell are dry, and others are flooded.
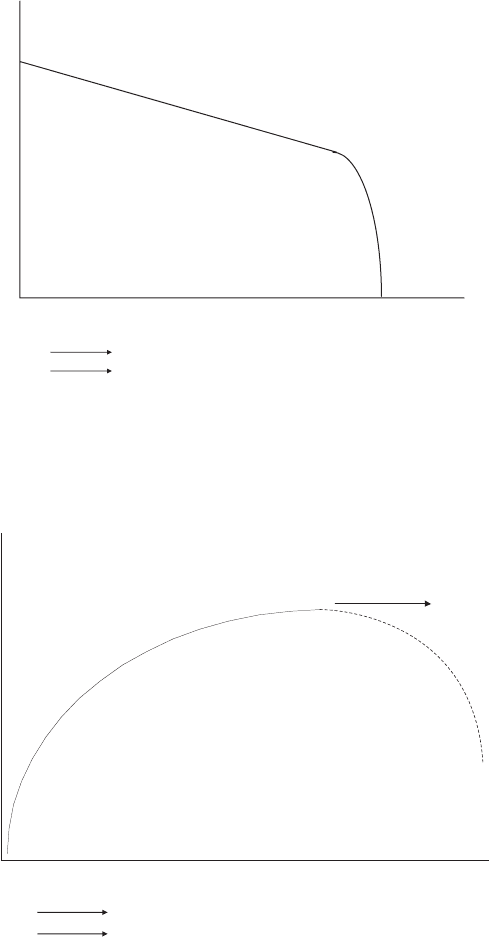
Example 6.7 Qualitative Prediction of Current Profiles in Simple Fuel Cell System For
an isothermal neat hydrogen fuel cell at moderate current density with adequate cathode
flow stoichiometry, sketch the current distribution from inlet to exit for the following cases:
(a) Fully moist inlet anode and cathode, concurrent flow
(b) Dry anode and cathode inlet, concurrent flow
(c) Dry anode and cathode inlet, countercurrent flow
(d) Dry cathode and fully moist anode inlet flow, concurrent flow
SOLUTION
(a) In this case, the flow is fully moist at both the inlets, so that the current should be
dominated by the oxygen mole fraction. The current should decrease from the inlet
to the exit in a decaying fashion, since the oxygen consumption will follow the
same qualitative shape as the current decays. At some point, the effects of flooding
will likely induce severe performance loss, as sketched.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.6 Multidimensional Effects 367
Distance from Inlet
Current Density
Cathode
Anode
(b) In this case, both the anode and cathode are dry, and thus the performance should be
quite low at the inlet and gradually increase as water is produced along the channel
and hydrates the electrolyte. If the flow path is long enough, the performance will
peak when the membrane is fully humidified and can eventually decrease due to
flooding effects.
Distance from Inlet
Current Density
Possible flooding
Cathode
Anode
(c) In this case, both the anode and cathode are dry but the flow is countercurrent.
This is a flow configuration often employed in portable applications, where no
humidifier system is used. In this underhumidified case, the performance should
be dominated by the anode moisture content. Along the anode flow channel, the
moisture content will gradually increase to a peak as moisture is transferred by
diffusion from the cathode, then it should decrease toward the exit as the moisture
is transferred back to the cathode. The current profile will look follow the water
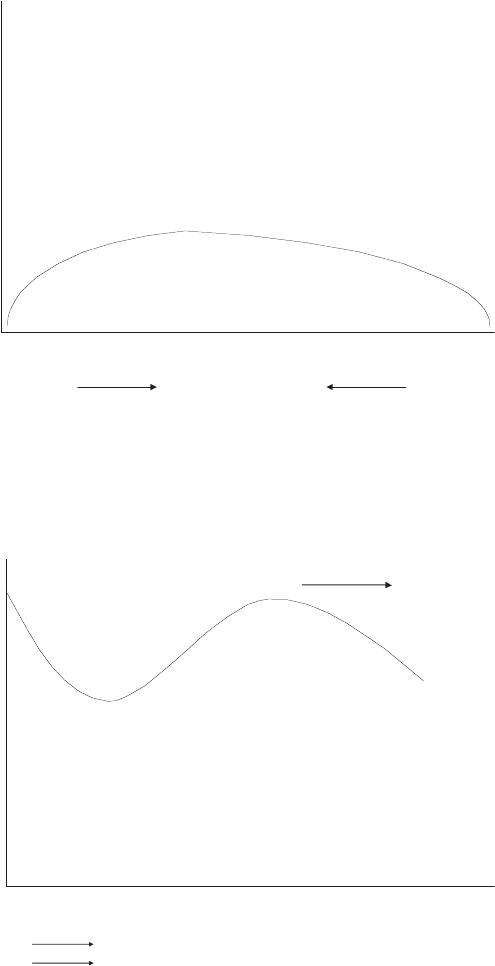
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
368 Polymer Electrolyte Fuel Cells
uptake trend, although the peak current location will depend on the relative flow
rates of the anode and cathode dry flows.
Distance from Inlet
Current Density
Anode
Cathode
(d) In this case, the current distribution will be dominated by anode moisture content,
since the overall flow is underhumidified. As the water is transferred from the
moist anode to the cathode, the current will decrease to a minimum then increase
again after flux reversal. Given a long enough channel length, the performance will
eventually decline due to flooding.
Distance from Inlet
Current Density
Possible flooding
Cathode
Anode
COMMENTS: The sketches drawn should be thought of as qualitative tools only, to help
in understanding the internal distributions of the various controlling parameters.

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.7 Summary 369
6.7 SUMMARY
The goal of this chapter is to present the reader with a summary of the operational issues
and design constraints of the PEFC. Within the PEFC scope is the hydrogen-powered
PEFC and the alternative fuel PEFC, normally called a DAFC, since the vast majority of
the alternative fuels proposed are alcohol based. The DAFCs all have inherently reduced
fuel cell performance compared to the H
2
PEFC because the anode kinetics are more
complex. Additionally, issues of fuel and water crossover and anode catalyst poisoning
from intermediate reactions further reduce the performance relative to the hydrogen fuel
cells. The reduced performance of DAFCs is deemed tolerable in portable applications,
however, where system size and simplicity are valued more than efficiency. Portable DAFC
systems can be designed to eliminate the humidification, cooling, and bulky hydrogen
storage needed for H
2
PEFC systems.
Despite the fact that the PEFC is a water generation reactor, some humidification of
the reactants is usually necessary to enable high performance and longevity. A dry inlet
feed results in poor local performance, hot spots, and internal stresses that can lead to
short lifetimes. There are various active and passive humidification methods used to ac-
complish humidification, including membrane humidification, direct injection, and internal
or external recirculation.
One of the most difficult engineering challenges in the PEFC is achieving a net water
balance without flooding. Like dryout, a flooding condition with liquid water accumulated
in the pores of the various media or in the flow channels can reduce performance and
exacerbate various degradation mechanisms. From a global perspective, we seek to remove
the net water produced from the fuel cell to maintain a steady-state condition in terms
of water storage in the fuel cell. This condition is accomplished via Eq. (6.16), derived
from a control volume balance on the entire fuel cell assumed at steady state and repeated
here:
λ
O
2
y
O
2
,in,dry
− 1
χ
out,c
2
1 − χ
out,c
−
λ
O
2
y
O
2
,in,dry
χ
in,c
2
1 − χ
in,c
+
λ
H
2
y
H
2
,in,dry
− 1
χ
out,a
1 − χ
out,a
−
λ
H
2
y
H
2
,in,dry
χ
in,a
1 − χ
in,a
+
˙
n
slugs,out,a
+
˙
n
slugs,out,c
iA
2F
= 1
whereχ = RH · P
sat
(T )/P.
The condition of a balance can be obtained in practice through tailoring the inlet
humidity, flow rates, exit temperature (through coolant flow manipulation), and pressure
drop though the fuel cell. Since a condition of exact balance is rarely achieved and an
overall balance may not satisfy the desire for maximum performance, the fuel cell is
typically operated in a slightly flooded situation, and a periodic growth and rejection cycle
of liquid droplet slugs from the fuel cell achieves a quasi-steady balance condition.
Even when the fuel cell achieves a global water balance, there can be areas of dry-
out and flooding inside the fuel cell itself. Water transport inside the fuel cell through
the electrolyte is governed by diffusion, electro-osmotic drag, hydraulic permeability (of
gas and liquid phases), and temperature gradient effects. Electro-osmotic drag is always
toward the cathode, but the diffusion, temperature, and hydraulic permeability gradients
can be engineered to achieve a favorable water balance. The liquid permeability direction
