Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
350 Polymer Electrolyte Fuel Cells
or nonpreferred) results in a six-electron, six-proton generation, but certainly not in a single
step. The CO oxidation pathway is not preferred since CO is a known poison of platinum
catalysts. Typically, a different catalyst, platinum ruthenium, is used because it facilitates
oxidative removal of the CO at lower overpotentials than pure platinum [50] and therefore
mitigates poisoning from the nonpreferred pathway.
There have been myriad research efforts aimed at reducing the precious metal loading
for DMFCs and decreasing the anodic activation polarization, with some success. However,
even with advanced catalyst selection, the anodic reaction generally requires a high precious
metal loading of 10 times a hydrogen fuel cell. Around 2–4 mg/cm
2
of catalyst is typically
needed (compared to ∼0.2 mg/cm
2
for the H
2
fuel cell) for the anode and cathode. The
cathode electrode generally has to have additional catalyst loading to withstand the methanol
crossover oxidation. Because of the high metal loading in the catalyst layers, use of a carbon-
supported structure results in a prohibitively thick electrode (and high ionic transport losses)
therefore, an unsupported catalyst electrode (i.e., without carbon supporting particles in the
CL) design is typically used. The bottom line is that the DMFC anode kinetics are poor
compared to hydrogen oxidation and require a higher catalyst loading. This probably
eliminates the DMFC for all but portable applications.
Carbon Dioxide Blockage and Removal At the anode side, a countercurrent flux of
carbon dioxide bubbles formed via electrochemical reaction must travel through the anode,
into the diffusion media, and out through the channel, against the liquid-phase transport of
methanol solution from the flow channel to the catalyst layer (Figure 6.56). Because the
vapor-phase density of CO
2
is so much lower than the liquid fuel solution (∼1000 times!),
the CO
2
bubbles can quickly fill flow channels. At high current densities, the volume fraction
of the carbon dioxide bubbles in the flow channels can reach up to 95% [51], reducing the
area through which liquid-phase reactant can penetrate to the catalyst layer. Blockage of
methanol solution transport to the reactive surface in the DM and flow channels can result
in mass-limited performance. In portable system applications, a hydrophobic microporous
membrane is often used to passively separate and reject the product CO
2
to the atmosphere
from the methanol solution flow that is recycled back to the anode.
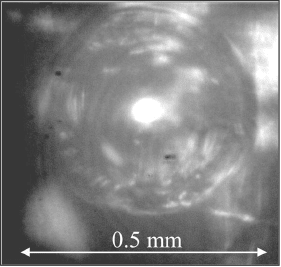
Figure 6.56 Photograph of carbon dioxide bubble produced in anode reaction in anode flow channel
of transparent DMFC. (Adapted from Ref. [52].)

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.4 Direct Alcohol Polymer Electrolyte Cells 351
Table 6.5 Health Effects for Average 70-kg Human of Methanol Exposure
Exposure Amount in Body and Result
Background methanol in body ∼35 mg
Hold liquid methanol in hand for 2 min 170 mg
0.8 L of aspartame-sweetened diet drink 40 mg
Drink 0.2 mL of methanol 170 mg
Drink >25 mL of methanol >20,000 mg, Death
Inhale 40 ppm vapor for 8 h 170 mg
Inhale 15 ppm vapor for 15 min ∼150 mg
Inhale 2.5% vapor for 1 s Very dangerous, possible damage
Source: Based on data from the Methanex Corporation [53].
Methanol Safety Methanol safety is an issue in the design of DMFCs. Methanol itself is
toxic in liquid and vapor form if imbibed orally or inhaled in large quantities and absorbs
through skin, as shown in Table 6.5. It is also highly flammable and spreads more rapidly
into groundwater than gasoline. Its colorless flame is difficult to detect, and the solution
itself is more corrosive than gasoline. To be fair to methanol advocates, this is no different
in most ways to the gasoline used by millions for automotive transportation on a daily basis
or the caustic materials used in the batteries the DMFCs would replace. However, the fact
that methanol vapor and liquid are not benign means that special precautions must be taken
to provide safe power, and the system should be designed with little potential for leakage.
6.4.2 Other DAFCs
Although the design and operation of the hydrogen and direct methanol fuel cells are
the most developed among PEFCs, both the DMFC and the H
2
PEFCs have significant
limitations. Since the power density of a pure hydrogen PEFC is higher, the use of hydrogen
alternatives is meant to overcome hydrogen system drawbacks and eliminate ancillary
system components for system compactness. The particular designs of DAFCs are very
similar to the DMFCs, except that other catalysts besides Pt–Ru may be used to have slightly
better anode kinetics, and a different membrane electrode assembly and DM configuration
may be used to manage water or crossover more effectively. In terms of kinetics and
catalysts for DAFCs, a few general trends are notable:
1. In general, the more complex the molecule (i.e., the more bonds), the higher the
activation polarization since there are more intermediate steps required for complete
oxidation.
2. An intermediate reaction pathway in the carbon-containing fuel oxidation will
include carbon monoxide, which poisons platinum catalysts at the low temperatures
of PEFCs. Thus, a catalyst such as Pt–Ru that has a CO tolerance will generally be
more effective than pure platinum.
3. A molecule with a carbon–carbon (C–C) bond is generally more difficult (higher
polarization) to electrochemically oxidize than one without a C–C bond. Methanol
and some other fuels do not have a C–C bond, while ethanol and other hydrogen
alternatives do [54].
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
352 Polymer Electrolyte Fuel Cells
Current density (A/cm)
Voltage (V)
2
0 200 400 600 800 1000 1200
0.0
0.2
0.4
0.6
0.8
1.0
21 C operation
o
30 C operation
o
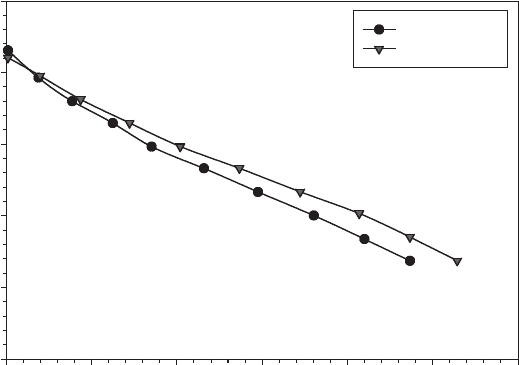
Figure 6.57 Polarization curve of a fuel operating at 21 and 30
◦
C with a 10 M formic acid solution
as fuel. Anode catalyst palladium, with platinum cathode catalyst. (Adapted from Ref. [55].)
Formic Acid and Formaldehyde The use of formic acid (HCOOC) or formaldehyde
(CH
2
O) for a DAFC has the potential to achieve better anode polarization kinetics than
methanol, since formic acid and formaldehyde are intermediate reaction products in the
overall methanol oxidation process and have no C–C bonds (Figure 6.55), so that lower
activation losses should be achievable. The formic acid fuel cell has a particular potential
because it may avoid significant CO generation and poisoning by avoiding the undesired
pathway to CO oxidation proposed for methanol shown in Figure 6.55. A polarization curve
from a formic acid fuel cell is shown in Figure 6.57. Besides the DMFC, the formic acid
fuel cell is the most developed DAFC [e.g., 55–57]. The formaldehyde fuel cell had some
early interest but is no longer being developed.
From a health standpoint, formic acid and formaldehyde are undesirable choices be-
cause these are known toxins with a National Fire Protection Association (NFPA) health
rating of 3. Formaldehyde is also chemically unstable, making it even more difficult to
work with. While both are very soluble in water (formic acid is completely miscible), these
compounds pose considerable health risks and have relatively low energy storage densities,
considering the low electron transference numbers (n) of 4 and 2 for formaldehyde and
formic acid, respectively.
Dimethyl Ether Another potential alternative fuel is dimethyl ether (DME, CH
3
OCH
3
),
presently used as an aerosol and propellant for spray paints and agricultural chemicals and
cosmetics and a potential diesel fuel replacement [58, 59]. The storage and handling of
DME is similar to standard propane; DME can be stored as a liquid at 0.6 MPa in standard
propane tanks, and it throttles to a gas at atmospheric pressure. This is similar to the fuel
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.4 Direct Alcohol Polymer Electrolyte Cells 353
Throttle to low-
pressure gas phase
Liquid butane
under pressure
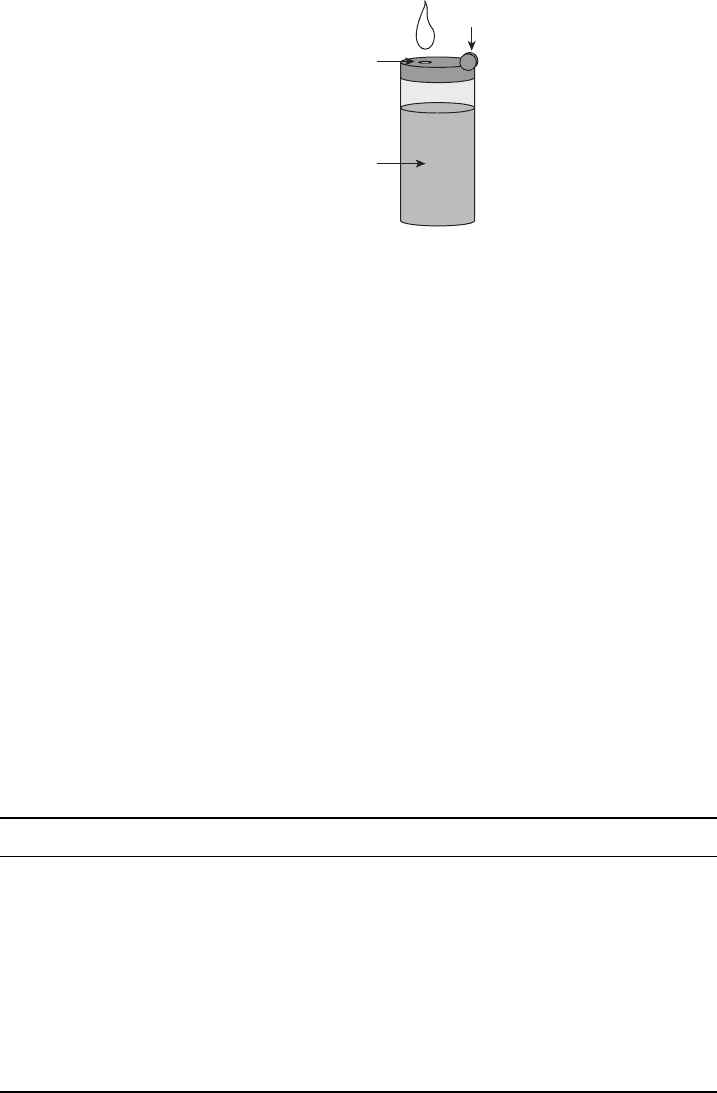
Figure 6.58 Butane and DME are both stored as liquid under pressure but are easily throttled to
gas phase at atmospheric pressure.
in a butane cigarette lighter (Figure 6.58). Table 6.6 presents some basic thermochemical
parameters of DME and other fuels for comparison.
Besides the fuel storage advantage, following are some other specific benefits of the
use of DME for fuel cells [60]:
Ĺ High electron transfer number of 12 for complete oxidation (methanol is 6 and
hydrogen is 2), resulting in reduced theoretical fuel requirement. However, this does
also indicate a more complex molecule requiring a greater number of intermediate
reaction steps and pathways and generally worse kinetics.
Ĺ Lack of C–C bond makes complete direct electro-oxidation possible with minimal
kinetic losses.
Ĺ Reduced net crossover rate due to the reduced dipole moment of DME compared to
methanol.
Ĺ The low toxicity of DME is comparable to that of liquid propane. Compara-
tively, methanol is toxic upon skin contact and ingestion. Dimethyl ether has a
higher autoignition temperature and lower flammability limit than gasoline [61],
however.
Table 6.6 Properties of DME and Comparative Fuels
DME Propane Methane Methanol Diesel Fuel
Chemical Formula CH
3
OCH
3
C
3
H
8
CH
4
CH
3
OH N/A
Boiling Pt,
◦
C −25.1 −42.0 −161.5 64.6 180–370
Liquid density, g/cm
3
@20
◦
C 0.67 0.49 NA 0.79 0.84
Gas specific gravity relative
to air
1.59 1.52 0.55 NA NA
Saturated vapor pressure at
25
◦
C, atm
6.1 9.3 246 NA NA
Ignition temperature,
◦
C 235 470 650 450 250
Explosion limits, % 3.4–17 2.1–9.4 5.0–15 5.5–36 0.6–6.5
Net heating value, kcal/kg 6,900 11,100 12,000 4,800 10,000
Note: Hydrogen lean flammability limit = 4%.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
354 Polymer Electrolyte Fuel Cells
Ĺ Handling properties are similar to those of propane and butane; therefore, existing
liquid propane infrastructure and handling technologies can be used to store and
transport DME.
Ĺ Dimethyl ether will not spread into groundwater as does methanol.
Ĺ The DME lower explosion limit is higher than that of propane, and DME has a
visible flame. In comparison, hydrogen and methanol flames are nearly invisible.
Ĺ Dimethyl ether can be produced with very low greenhouse gas emissions, especially
if produced from biomass.
M
¨
uller and co-workers examined DME fuel cell performance and fuel utilization compared
to the DMFC at high pressure (5 atm) and temperature (130
◦
C) and found performance
similar to DMFC under these operating conditions [62], although the high pressure and
temperature studied are not suited for portable applications. A few groups have published
research on the DME PEFC and have shown substantial performance enhancement for
elevated temperature (100–130
◦
C) and pressure (4.5 atm) [60, 63]. Also, DME has been
applied with good performance results in solid oxide [64] and molten carbonate [65] fuel
cells. The fuel cell performance of the DME is still currently lower than the DMFC due to
the more complex anode kinetics involved.
Example 6.6 Determination of Anode and Cathode Global Reactions for DME Deter-
mine the global anode oxidation and cathode reduction reactions for DME.
SOLUTION The overall redox reaction is
CH
3
OCH
3
+ 3O
2
→ 3H
2
O + 2CO
2
At the anode, we take 1 mol of fuel and solve for the carbon dioxide generated as a product
of oxidation by balancing on the carbon. Then, we add an appropriate amount of water
to the reactants to balance the oxygen on both sides of the reaction. Finally, we add the
appropriate number of protons and electrons on the products to balance the total hydrogen
on the reactant side:
CH
3
OCH
3
+ 3H
2
O → 12H
+
+ 12e
−
+ 2CO
2
For the DME fuel cell, the electron transfer number is 12.
On the cathode side, the reaction is the same as the PEFC:
4H
+
+ 4e
−
+ O
2
→ 2H
2
O
which can be scaled up to match the anode reaction by multiplying by 3. We can check the
accuracy of the result by adding the oxidation and reduction reactions, making sure they
add to the global redox reaction.
COMMENTS: There are of course many intermediate reactions at the anode and cathode.
Despite the high electron transfer number of 12, the elementary charge transfer step(s) at
the anode involve only one or two electrons.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.4 Direct Alcohol Polymer Electrolyte Cells 355
Trioxane Trioxane (C
3
H
6
O
3
) is solid at room temperature (melting point is 64
◦
C), has
lower toxicity than methanol, and is derivable from natural gas. Other advantages of trioxane
are as follows:
1. High electron transference number of 12 and hence high theoretical energy density
2. Lack of C–C bond
3. Lower anode-to-cathode crossover rate than methanol for the same electrolyte and
an ability to control resting crossover loss by keeping in the solid phase until
operation [63]
4. Higher boiling and flash points than methanol
However, there is no significant trioxane fuel infrastructure, and the fuel must be above
55
◦
C to dissolve sufficiently, which means a power-assisted startup is needed. Trioxane
tablets or granules could be easily shipped and provide desired molar concentrations of
fuel. Trioxane use in fuel cells was studied by Narayanan et al. [66], but the power density
was much lower than a comparable DMFC.
Dimethoxymethane and Trimethoxymethane Dimethoxymethane (DMM, C
3
H
8
O
2
) and
trimethoxymethane (TMM, C
4
H
10
O
3
), can be grouped together due to their similar prop-
erties. Both are liquids at room temperature and have very low melting points and similar
molecular composition and structure. While DMM is an irritant, it is not known to pose any
long-term health risks. Initial interest in TMM and DMM arose from several advantages
compared to methanol. Both TMM and DMM do not have any carbon–carbon bonds, both
have higher energy storage densities compared to methanol, and TMM has a higher flash
point and boiling point than methanol. Testing has shown that DMM sustained current
densities three to four times that of TMM but less than a modern DMFC. While DMM
showed significant promise, the main drawback appears to be lack of infrastructure and
performance compared to methanol. Dimethoxymethane does have a potential for use that
is unique considering the boiling point lies at 40
◦
C at ambient pressure. Therefore, an
operating fuel cell could be used to boil liquid DMM solution and create a flow of fuel and
vapor without the use of a pump.
Ethanol Ethanol (ethyl alcohol or grain alcohol, C
2
H
5
OH) is a clear, colorless liquid
with a characteristic odor and is mildly toxic, although it has a NFPA health rating of
0 since it can be consumed and digested safely in moderate quantities. Ethanol has a
number of potential commercial advantages. In some countries such as Brazil, ethanol
already has a well-established infrastructure. Ethanol is a renewable energy source derived
from the fermentation of sugar cane, corn, or other biodegradable sources. Because of
a carbon–carbon bond, the ethanol fuel cell has a performance disadvantage related to
the DMFC but has been studied by several researchers [67, 68]. The main disadvantages
of ethanol use are the high activation polarization related to the C–C bond and the high
relative cost. Besides high activation overpotential, fuel containing the C–C bond often
suffer incomplete electro-oxidation and severe CO poisoning.
Dimethyl Oxalate and Ethylene Glycol Like trioxane, dimethyl oxalate (DMO, C
4
H
6
O
4
)
offers simplified handling as well as limiting crossover current because it is a solid at
room temperature. Dimethyl oxalate also has a high electron transference number of 14
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
356 Polymer Electrolyte Fuel Cells
(which concomitantly means a more complex molecule and generally unfavorable kinetics)
but suffers from limited infrastructure and low demonstrated performance compared to
methanol, due in part to molecular complexity and C–C bonds.
Ethylene glycol (EG, C
2
H
6
O
2
) is ubiquitously used in the automotive industry as
an engine coolant, and hence a distribution infrastructure already exists. Also, EG has a
crossover current density roughly half that of methanol [69]. However, PEFC performance
with EG is still relatively low, with a fuel cell specific energy density about 20–40% less than
that of the same fuel cell utilizing methanol. Additionally, EG has been shown to rapidly
degrade PEFC electrolyte material, which obviously limits its potential PEFC applications.
6.5 PEFC DEGRADATION
Although begining of the life (BOL) performance is important, fuel cell and system durabil-
ity and time-dependent performance are just as critical. The lifetime of a fuel cell is expected
to compete with existing power systems it would replace. The automotive fuel cell must
withstand load cycling and freeze–thaw environmental swings with minimal degradation
(3–5%) over a lifetime of at least 5000 h. A stationary fuel cell must withstand over 40,000
h of steady operation with minimal downtime. The fuel cell environment is especially
conducive to degradation, since a voltage potential difference exists which can promote
undesired reaction, and PEFCs operate at slightly elevated temperature with a corrosive acid
electrolyte. There are many phenomena that can result in gradual degradation and perfor-
mance loss in PEFC systems. These include assorted chemical and mechanical degradation
modes [70]. Many different modes of physicochemical degradation are known to exist that
can generally be grouped into two categories: reversible and irreversible damage.
The state-of-the-art in terms of performance degradation for a PEFC is on the order of
1–10 µV/h. However, this is at steady state in relatively high humidity conditions [71]. The
degradation in PEFCs is exacerbated through many mechanisms. In general degradation is
accelerated by
1. operation at high-temperature or low-humidity conditions,
2. aggressive load cycling from low to high cell voltages, and
3. large temperature or humidity swings in the environment, also including freezing.
6.5.1 Physical Modes of Degradation
Reversible Modes of Physical Degradation Some modes of physical degradation are a
result of reversible phenomena, including the following:
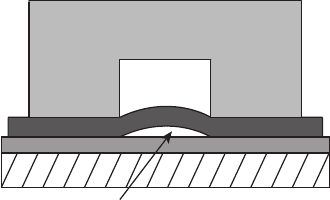
1. Diffusion Media Channel Intrusion Figure 6.59 illustrates the phenomenon of DM
tenting, or sagging into gas flow channels. This phenomenon occurs mostly with
flexible cloth DM. The result is a lack of contact with the catalyst layer under the
channel and excessive pressure drop in the affected channel. In large stacks this can
cause severe flow maldistribution effects and the gap created can serve as a liquid
pooling location under the DM. This is reversible in principle by replacing the DM
in the affected fuel cell.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.5 PEFC Degradation 357
ChannelLand
Catalyst layer
PEM
DM
Gap
Figure 6.59 Diffusion Media tenting in flow channels, resulting in poor electrical contact and
increased gas-phase pressure drop.
2. Flooding or Dryout These losses, already discussed, are reversible through a
variety of methods, including changing flow rate of reactants or coolant and inlet
humidification or coolant flow modification.
3. Reactant Starvation If a location in the catalyst layer of the anode or cathode is
blocked with liquid or the flow rate to a stack cell is reduced due to maldistribution,
poor performance from fuel or oxidizer starvation can result. Prolonged fuel star-
vation may result in voltage reversal and in some cases carbon corrosion, which is
irreversible.
4. Voltage Reversal (Benign) Some fuel cell voltage reversal reactions are benign
and thus reversible as soon as conditions in the cell are returned to normal.
5. Physical Intrusion of Unwanted Particulate Matter Dirt, sand, and other foreign
matter it in the air can be brought into the fuel cell and block flowchannels.
Irreversible Modes of Physical Degradation
1. Diffusion Media Plastic Deformation Under compression from the lands (usually
>1.5 MPa), the DM can be plastically deformed. This may not be critical as long
as the fuel cell stack remains assembled but may prohibit the reuse of the DM upon
stack disassembly.
2. Catalyst Layer Cracking and Delamination Catalyst layers are typically sprayed,
deposited, or spread onto the electrolyte from a viscous mixture. This mixture is then
baked at an elevated temperature, which drives off volatile compounds in the catalyst
mixture used to control mixture viscosity and dispersion. As a result, small fissures,
or “mudcracks” are common in catalyst layers, as shown in Figure 6.32, with widths
much greater than the average pore size in a continuous portion of the catalyst layer.
Over time, and as a result of the electrolyte expansion and contraction with water
content variation, these cracks can grow and lead to delamination or catalyst layer
degradation.
3. Electrolyte Fracture Electrolyte fracture can result from rapid or severe temper-
ature and or humidity cycling, including frozen conditions. Electrolyte fracture
is not always catastrophic but results in increased hydrogen crossover, leading to
failure over time. Freeze–thaw cycling can potentially also result in catalyst layer
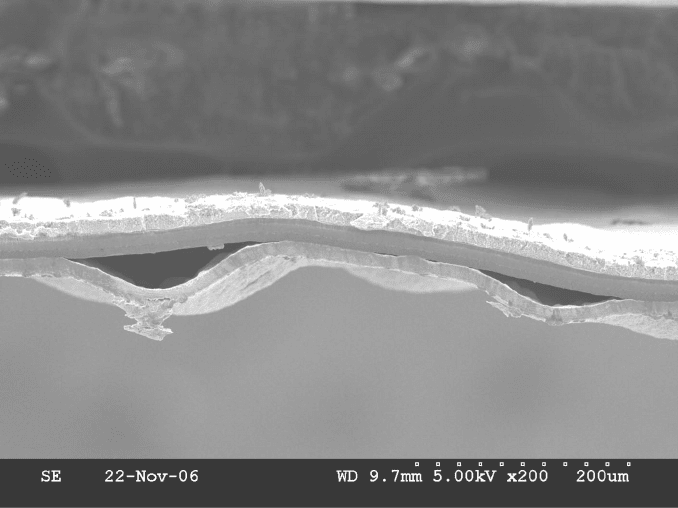
delamination, as shown in Figure 6.60.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
358 Polymer Electrolyte Fuel Cells
Figure 6.60 Scanning electron micrograph of membrane electrode assembly that had catalyst
layer delamination resulting from unrestrained freezing in liquid water. The gaps caused by this
delamination can serve as pooling locations for liquid during subsequent operation.
4. Diffusion Media Hydrophobicity Change As a result of regular operational cycling
or minute impurities introduced from gaskets and other components of the fuel cell
system [72], the wettability of the DM can change over time, altering the water
management and increasing flooding.
5. Morphology Changes or Loss in Catalyst Layer or Other Components For all
fuel cells, the catalyst layer ECSA is a determining factor in overall power density,
and nanosized catalysts and supports are present in a complex three-dimensional
electrode structure designed to simultaneously optimize electron, ion, and mass
transfer. As a result, any morpholological changes can result in reduced perfor-
mance. Commonly observed phenomena include catalyst sintering, dissolution and
migration, catalyst oxidation, supporting material oxidation (e.g., carbon corrosion
for carbon-supported catalysts), and Oswald ripening [71]. These effects result in
loss of ECSA and are irreversible.
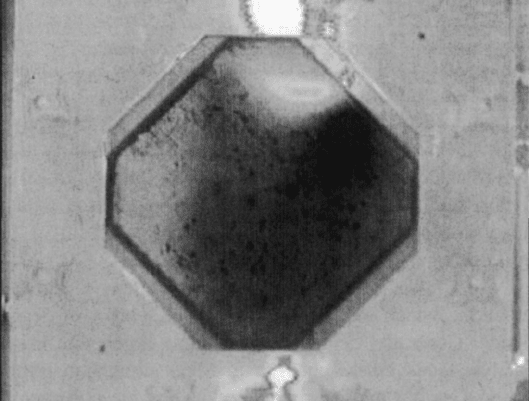
6. Pinhole Formation As a result of internal stresses, localized hot spots and dryout,
or other factors, small pinholes can develop in the electrolyte (see Figure 6.61).
This leads to a gradually increasing hydrogen crossover problem and eventual
failure. Pinholes often occur at locations near the anode inlet and are believed to be
germinated from one of three sources:
(a) Dust or other impurities embedded in the electrolyte during manufacturing. This
is highly unlikely, since quality control at electrolyte manufacturing plants now
prevents this and the problem still exists.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.5 PEFC Degradation 359
Figure 6.61 Photograph of pinhole found in PEFC electrolyte from Ballard fuel cell. (Reproduced
from Ref. [71].)
(b) Mechanical stresses generated by locations of electrolyte dryout and high local
temperature.
(c) Local areas of membrane weakness caused by platinum migration from the cath-
ode and subsequent precipitation inside the main electrolyte. These locations
serve as nucleation sites for further damage growth.
6.5.2 Chemical Modes of Degradation
Reversible Modes of Chemical Degradation
1. Certain Types of Gas-Phase Impurities There are several different species that
preferentially absorb on the catalyst surface and can degrade the electrochemical
activity of that surface. Platinum oxides on the surface of a catalyst can be cleansed
with a quick excursion to low cell voltages (<0.4 V). Another major reversible
poison is carbon monoxide (CO). Carbon monoxide poisoning is described in more
detail later in this section. Other types of potential environmental pollutants can be
detrimental to performance such as dust, aerosols, alcohol vapors, carbon dioxide,
hydrocarbons, various sulfur-containing gases, ammonia, halogenated compounds,
inert gases, and nitrogen oxides. Some of these pollutants cause reversible damage,
and others, especially sulfur-containing compounds such as hydrogen sulfide, can
cause irreversible loss of performance.
2. Coolant Conductivity Increase Since the coolant system is in contact with the
entire stack, the coolant must be highly nonconductive. Over time, ionic impurities
in the coolant stream can degrade the coolant performance, causing shorting within
the stack and reducing performance. Coolant flushing or filtering can be used to
reverse this effect, and the coolant recirculation system must also be designed to
have minimal contact with potential sources of ionic impurities.
