Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
340 Polymer Electrolyte Fuel Cells
(a)(b)
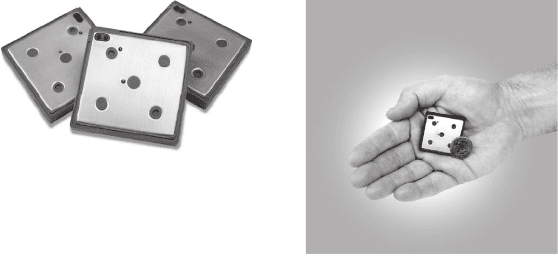
Figure 6.51 9-cm
3
Mobion
R
direct methanol fuel cell chips, which are used by Mechanical
Technology, Inc. (MTI) as the power source for consumer electronics applications. The Mobion
technology is based on a passively fed DMFC, with 100% methanol fuel. A power density of over
50 mW/cm
2
can be achieved. (Image courtesy of MTI.)
such as the coolant and humidification subsystems can be eliminated. In the simplest case,
the system contains only fuel storage and delivery/recirculation systems, the fuel cell,
and microelectronics. By elimination of various subsystems, the portable fuel cell system
profiles can become extremely compact, even though the fuel cell stack (without ancillary
components) could be smaller if operating on pure hydrogen. Figure 6.51 shows 9-cm
3
DMFC chips used to power consumer electronics.
Direct alcohol fuel cells do suffer some drawbacks compared to hydrogen PEFCs:
1. Low operating efficiency
2. Reduced performance, due to more complex kinetics, and flooding issues
3. Increased cost per kilowatt due to much higher catalyst loadings, on the order of
1–8 mg/cm
2
total (anode plus cathode) precious metal loading, compared to 0.2–
1.0 mg/cm
2
total loading for the H
2
PEFC
However, considering the portable market, efficiency and cost are less important than
system size, so these trade-offs are acceptable. The most developed DAFC is the direct
methanol fuel cell (DMFC). Many prototype and nearly commercial DMFC systems exist
for powering small electronics, laptop computers, cell phones, hand-held electronics, and
other devices. Direct alcohol fuel cells are expected to occupy a growing market for portable
power for years into the future.
The search for high-performance alternative fuels to hydrogen has led to many can-
didates. Table 6.4 provides a summary of safety, thermodynamic, and other data collected
on the potential candidates. Some fuels are potentially attractive alternatives due to ease
of handling or improved safety ratings. As with any fuel cell, there is no perfect solution,
and each potential fuel has its own limitations. Alternative fuels to hydrogen are an attempt
to eliminate one or more of the disadvantages of another fuel. One way to think about
alternative fuels is as hydrogen carriers. That is, they are high in hydrogen content but with
a greater hydrogen density than hydrogen gas because they are typically stored in liquid or
solid form. There are many potential fuels that can be used in portable applications where the
desire is for a compact size and low efficiency and higher cost per kilowatt can be absorbed.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
Table 6.4 Properties of Various Methanol Alternatives Being Considered for Portable Fuel Cell Use
Molecular Evaporation Rate Freezing/ Flash
Chemical Weight Density Solubility (relative to butyl Boiling Point Theoretical
Name Composition (g/mol)
c
(g/mL)
c
in Water
c
acetate = 1)
c
Point (
◦
C)
c
(
◦
C)
c
Capacity
Methanol CH
3
OH 32.0312 0.791 Completely Miscible 4.6 −98/64.6 12 5.03 Ah/g
a
Ethanol C
2
H
5
OH 46.0468 0.789 Miscible >= 10 g/
100 mL at 23
◦
C
N/A −114.1/78.3 12 N/A
Dimethoxy-methane
(DMM)
C
3
H
8
O
2
76.0624 0.086 33 g/100 mL 14.4 −105/42 −18 N/A
Trimethoxy-methane
(TMM)
C
4
H
10
O
3
106.078 0.97 N/A N/A −53/101–102 13 N/A
Trioxane C
3
H
6
O
3
90.0468 1.17 > 10 g/100 mL at
20
◦
C
N/A 64/114.5 45 N/A
Formic Acid CH
2
O
2
46.0156 1.21 Completely Miscible 2.1 8.3/100.7 69 N/A
Formaldehyde CH
2
O 30.0156 1.09 Very Soluble 55 g/mL N/A −118/−19.5 60 N/A
Dimethyl Ether (DME) (CH
3
)
2
O 46.0468 Soluble N/A −138.5/−22 −41 N/A
Ethylene Glycol (EG) C
2
H
6
O
2
62.0468 1.1155 Miscible >= 10 g/
100 mL at 17.5
◦
C
0.01 −13/195 111 4.32 Ah/g
a
Dimethyl Oxalate
(DMO)
C
4
O
4
H
6
118.0468 1.148 Limited N/A 52/163.4 75 3.18 Ah/g
a
Oxalic Acid C
2
O
4
H
2
94.0468 1 10 g/100 mL N/A 189/190 N/A 43 Ah/g
a
(Continued)
341
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
Table 6.4 (Continued)
NFPA Hazard Global
Name Rating (H/F/R) Toxicity Reaction Efficiency Losses
Methanol 1/3/0
c
Skin/Eye irritant, Poisinous in large
quantities
CH
4
O + O
2
→ CO
2
+ 2H
2
O Membrane Crossover
(causes mixed potential,
parasltic O
2
consumption)
Ethanol 0/3/0
c
Concentrations below 1000 ppm
usually produce no signs of
intoxication. It is a central nervous
system depressant in humans.
C
2
H
5
OH + 3O
2
→ 2CO
2
+ 3H
2
O Carbon-Carbon bonds
make CO
2
formation
very difficult
Dimethoxymethane
(DMM)
1/3/2
c
Irritates Eyes, Skin, and Airways C
3
H
8
O
2
+ 2O
2
→ 3CO
2
+ 4H
2
O Hydrolyzes in the fuel cell
to produce methanol
Trimethoxymethane
(TMM)
N/A N/A C
4
H
10
O
3
+ 5O
2
→ 4CO
2
+ 5H
2
O Hydrolyzes in the fuel cell
to produce methanol
Trioxane 2/2/0
b
Can evolve formaldehyde when
heated strongly or in contact with
strong acids. Permitted as an
additive in food for human
consumption.
C
3
H
6
O
3
+ 3O
2
→ 3CO
2
+ 3H
2
ON/A
Formic Acid 3/2/0
b
Toxic. Proved very harmful to test
animals.
CH
2
O
2
+ 1/2O
2
→ CO
2
+ H
2
ON/A
Formaldehyde 2/4/0
b
Cancerous CH
2
O + O
2
→ CO
2
+ H
2
ON/A
Dimethyl Ether (DME) 2/4/1
c
Mild anesthetic. Large quantites can
cause bloodthining.
c
(CH
3
)
2
O + 3H
2
O → 2CO
2
+ 12H
+
+ 12e
−
N/A
Ethylene Glycol (EG) 1/1/0
b
Mildly toxic by skin contact. A
suspected carcinogen.
C
2
O
2
H
6
+ 2H
2
O → 2CO
2
+ 2H
2
O Lower Energy Conversion
than Methanol
a
Dimethyl Oxalate
(DMO)
N/A N/A C
4
O
4
H
6
+ 1/2O
2
→ 4CO
2
+ 3H
2
O Lower Energy Conversion
than Methanol
a
Oxalic Acid 3/1/0
b
Oral irritant (poisonous if swallowed) C
2
O
4
H
2
+ 1/2O
2
→ 2CO
2
+ H
2
ON/A
a
E. Peled (A38 - A41)
b
http://www.orcbs.msu.edu/nfpa/nfpa.html
c
http://chemfinder.cambridgesoft.com/
Source: Adapted from Ref. [43].
342
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.4 Direct Alcohol Polymer Electrolyte Cells 343
6.4.1 Direct Methanol Fuel Cell
The global electrochemical reactions for the DMFC are as follows:
Anode oxidation reaction:
CH
3
OH + H
2
O → 6e
−
+ 6H
+
+ CO
2
(6.42)
Cathode reduction reaction:
6H
+
+
3
2
O
2
+ 6e
−
→ 3H
2
O (6.43)
Overall cell reaction:
CH
3
OH +
3
2
O
2
→ 2H
2
O + CO
2
(6.44)
Notice that at the anode some water is consumed in the global oxidation reaction, although
the overall fuel cell is still a net water generator. On the cathode of the DMFC, the main
reactions are the same as for the hydrogen fuel cell. Even though methanol and water are
used as the fuel, charge transfer at the anode catalyst and through the electrolyte is still
the same; hydrogen protons are generated by the methanol oxidation and travel though the
electrolyte.
The DMFC has similar design features as the hydrogen PEFC, except that a liquid
solution of methanol and water is used as the fuel, rather than hydrogen gas. A schematic
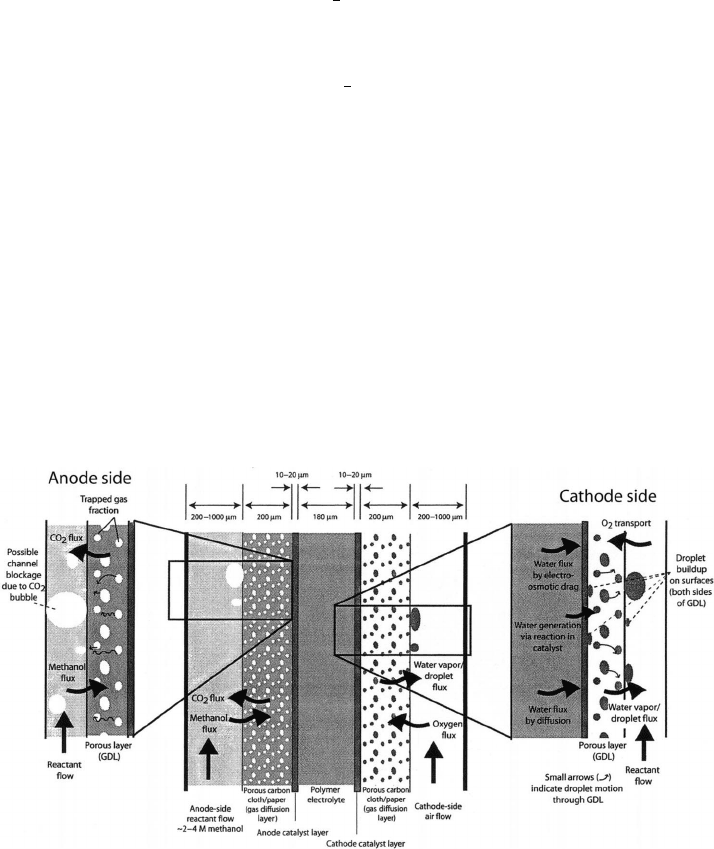
of the various transport phenomena in a DMFC is shown in Figure 6.52.
In the DMFC, a liquid solution of methanol and water is fed to the anode and air
is supplied to the cathode. At the anode side, a countercurrent flux of carbon dioxide
bubbles formed via electrochemical reaction fights through the DM against the liquid-
phase transport of methanol from the flow channel to the catalyst layer. Due to the potential
Figure 6.52 Schematic of direct methanol fuel cell and associated mass transfer processes. (Cour-
tesy of K. Sharp, Penn State University.)
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
344 Polymer Electrolyte Fuel Cells
blockage effect and the need to efficiently transport methanol solution to the anode surface,
the anode-side DM material is typically highly hydrophilic porous carbon cloth or paper.
On the cathode side, the same flooding issues prevalent in the hydrogen PEFC are even
more severe, since the diffusion gradient from the anode to the cathode is always toward
the cathode and the electro osmotic drag coefficient is higher (∼2–5) for liquid contact
with the membrane due to Schroeder’s paradox. To mitigate flooding and achieve a more
favorable net water transport coefficient, a highly hydrophobic microporous layer and DM
are typically used.
For the DMFC, both anode and cathode activation polarizations are significant. How-
ever, reduced performance compared to the H
2
PEFC is tolerable in light of other advantages
of the DMFC, namely:
1. Because the anode flow is mostly liquid (gaseous CO
2
is a product of methanol
oxidation), there is no need for a separate cooling or humidification subsystem.
2. Liquid fuel used in the anode results in lower parasitic pumping requirements
compared to gas flow. In fact, many passive DMFC designs operate without any
external parasitic losses, instead relying on natural forces such as capillary action,
buoyancy, and diffusion to deliver reactants.
3. The highly dense liquid fuel stored at ambient pressure eliminates problems with
fuel storage volume. With highly concentrated methanol as fuel (>10 M), passive
DMFC system power densities can compare favorably to advanced Li ion batteries.
4. No reformer system is needed.
5. Methanol is ubiquitous and transportable, and an infrastructure already exists.
The DMFC solves many problems associated with the hydrogen PEFC system; however,
there are of course limitations to the DMFC. The main technical issues affecting perfor-
mance and design are as follows:
1. Water Management Even though external humidification is not needed in the
DMFC, prevention of cathode flooding is critical to ensure adequate performance.
2. Methanol Crossover There is a high crossover rate of methanol from anode to
cathode because of the high concentration of methanol at the anode side. This
results in a mixed potential at the cathode from crossover methanol oxidation and
greatly reduces the open-circuit voltage of the DMFC from the theoretical value of
∼1.2 V to around 0.7–0.8 V.
3. Poor Anode Kinetics The kinetics are inherently slower because of the more
complex anode oxidation reaction. Tafel kinetics are appropriate at both electrodes,
and compared to the H
2
PEFC, an order-of-magnitude higher precious metal loading
is typically used.
4. Counterflow and Removal of Carbon Dioxide Carbon dioxide is produced at the
anode surface, resulting in countercurrent two-phase flow in the anode DM that can
block access to the catalyst layer.
5. Methanol Safety Methanol is slightly toxic, spreads more easily into the ground
than gasoline, and is highly flammable and miscible in water so that contamination
with reservoirs is very simple.

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.4 Direct Alcohol Polymer Electrolyte Cells 345
Water Management in DMFC In DAFCs, some water is needed at the anode to provide
additional oxygen for the oxidation reaction. As a result, flooding at the cathode can be
more severe because the diffusion gradient will favor flow toward the cathode and electro-
osmotic drug is enhanced. From Eq. (6.42), 1 mol of water is needed per mole of methanol
for the overall oxidation reaction. One can define an anode methanol and an anode water
stoichiometry in this case:
λ
CH
3
OH
=
˙
n
CH
3
OH
iA
6F
λ
H
2
O
=
˙
n
H
2
O
iA
6F
(6.45)
Ideally, some of the water generated in the reaction at the cathode can be directed to
the anode, and concentrated methanol can be used as the feed fuel, reducing the fuel
storage volume requirements. This balance can be achieved passively if the net transport
coefficient can be maintained to the proper level using capillary pressure management or
other techniques. For the DMFC
˙
n
H
2
O
net,a−c
= α
d
iA
F
(6.46)
From Eq. (6.45), if α
d
=−0.17, then operation on neat methanol with only product water
back-fed to the anode can be theoretically achieved. Each DAFC will have a different value
for this coefficient, depending on the number of electrons exchanged in the fuel oxidation.
This condition must be achieved with a capillary or gas-phase pressure difference, since the
electro-osmotic drag and diffusion are always toward the cathode in the DMFC. Practically,
methanol crossover reduces performance, so that some methanol dilution is typical, as
described in the next section.
External humidification is not needed in the DMFC, due to the liquid anode solution,
but prevention of cathode flooding is critical to ensure adequate performance. To combat
flooding and methanol crossover in the DMFC, several approaches have been used, as
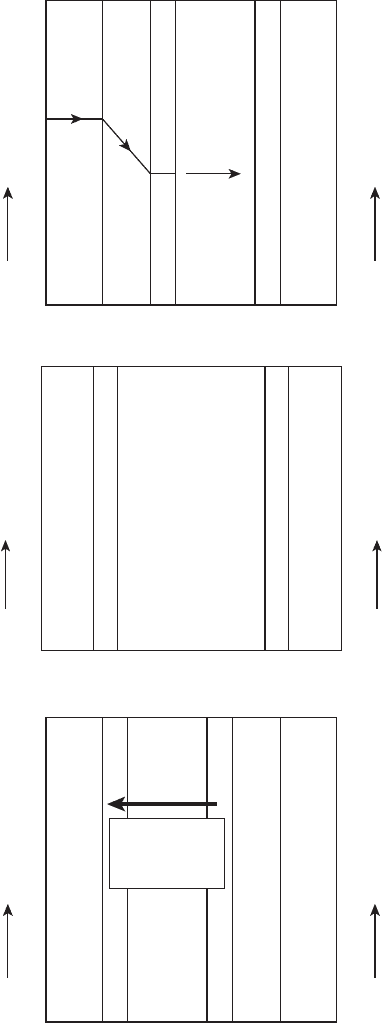
schematically shown in Figure 6.53:
1. Increased Electrolyte Thickness This also limits the methanol crossover from
diffusion through Fick’s law:
˙
n
x-over
= DA
C
a
− C
c
δ
PEM
(6.47)
where δ
PEM
is the electrolyte thickness and D is the diffusion coefficient of methanol
in the electrolyte. As δ
PEM
increases, the diffusion rate of water decreases. However,
this solution reduces performance since the path length of ion travel is increased. In
typical DMFCs, the electrolyte thickness is greater than 100 µm to limit methanol
and water crossover.
2. Capillary Pressure Management Use of a highly hydrophobic microporous layer
on the cathode can be used to maintain a capillary pressure difference between
the liquid-filled hydrophilic pores of the cathode and anode, and pump water (and
crossover methanol) back toward the anode by capillary pressure forces.
3. High Cathode Flow Rate The crossover water and generated water can be removed
by a high flow rate of dry air. This has the drawback of increasing parasitic losses for
the cathode flow. However, many compact fans provide adequately high flow rate.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
346 Polymer Electrolyte Fuel Cells
(a)
DM CL PEM
Diffusion
barrier
DMCL
Air flow
C
CH
3
OH
Methanol
solution
CH
3
OH
Crossover
Sharp
drop
DM CL PEM DMCL
Air flow
C
CH
3
OH
Methanol
solution
(b)
(c)
DM CL
PEM
MPL
DMCL
Air flow
C
CH
3
OH
Methanol
solution
Capillary back-
flow of
water/CH
3
OH
Figure 6.53 Schematic of various water and methanol management approaches used in DMFC: (a)
diffusion barrier approach, (b) thicker membrane approach, and (c) capillary back flow management.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.4 Direct Alcohol Polymer Electrolyte Cells 347
To prevent flooding by airflow, the cathode air flow must be adequate to remove water at
the rate that it arrives and is produced at the cathode surface. The water arrival and production
at the cathode surface by diffusion, electro-osmotic drag, and hydraulic permeability can be
expressed by Eq. (6.46). For the case where the cathode is just flooding, the diffusion to the
cathode will be minimal. Therefore, an estimate of the minimum amount of water that must
be removed by the cathode to avoid flooding without capillary pressure management can
be derived by balancing the water generated and dragged by electro-osmosis to the cathode
with the drying capability of the cathode flow. The maximum amount of water vapor that
can be removed at the exit for an air cathode can be shown as [44]
˙
n
H
2
O,removed
=
(
λ
c
− 1
)
iA
0.84F
P
g,sat
(T )
P
t
− P
g,sat
(T )
(6.48)
where λ
c
is the cathode stoichiometry at operating conditions and P
g,sat
is the saturation
pressure. In this calculation, the assumed oxidizer is air, which results in the factor of 0.84
in the denominator. This does not allow for water removal in the liquid phase. For the case
of flooding just at the exit, P
t
is equal to the total pressure of gas leaving the fuel cell. The
minimum stoichiometry required to prevent liquid water accumulation in the limit of zero
water diffusion through the membrane can be shown as
λ
cathode, min
=
2.94
P
g,sat
/(P
t
− P
g,sat
)
+ 1inair
14
P
g,sat
/(P
t
− P
g,sat
)
+ 1inO
2
(6.49)
The factor of 1 in Eq. (6.49) is a result of the consumption of oxygen in the cathode by the
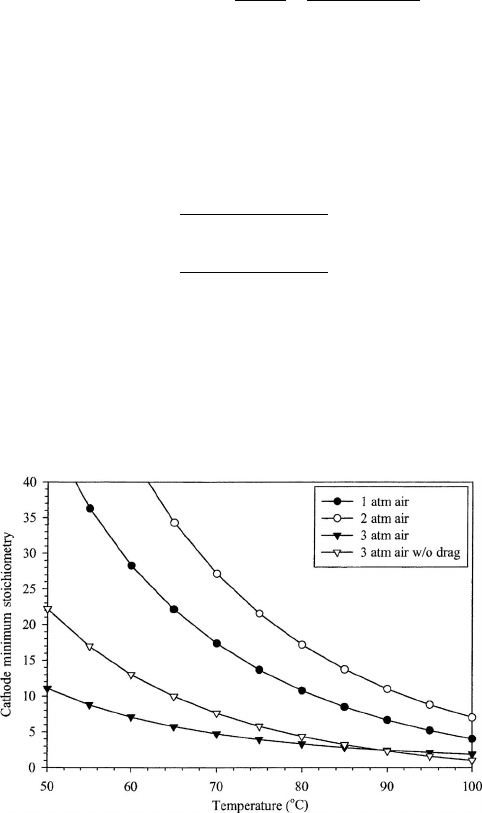
electrochemical oxygen reduction reaction. A plot of this minimum boundary as a function
of pressure over the relevant temperature range is shown in Figure 6.54. This boundary
serves as a baseline for discussion purposes. Depending on the use other methods to control
the net drag coefficient of water, this boundary can shift considerably.
Figure 6.54 Plot of minimum stoichiometry requirements for flooding avoidance as function of
operating temperature and pressure for DMFC. (Adapted from Ref. [44].)

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
348 Polymer Electrolyte Fuel Cells
It can be seen that, without capillary pressure management, the minimum cathode
stoichiometry for a DMFC is determined by flooding avoidance rather than by electro-
chemical requirements. Therefore, cathode stoichiometries in the DMFC are typically
much greater than unity. Also note from Figure 6.54 that the lowest curve is that of air at
3 atm pressure with no electro-osmotic drag. For this curve, Eq. (6.49) was modified to
eliminate the electro-osmotic drag term, which represents a result achievable by capillary
pressure management. This results in a vast reduction in the required cathode flow rate
to avoid flooding and shows the potential of capillary pressure management to improve
performance.
Methanol Crossover Another critical issue in the DMFC is methanol crossover from
the anode to the cathode from diffusion, electro-osmotic drag, and hydraulic permeation.
Methanol crossover from the anode to the cathode results in the following performance-
limiting effects:
1. A mixed potential on the cathode and oxidation of methanol at this location both
poison the cathode catalyst, consume oxygen, and greatly reduce the OCV, even
more so than hydrogen crossover in H
2
PEFC systems. Typical OCVs of DMFCs
are significantly below 0.8 V.
2. The parasitic leakage of fuel through the membrane without current generation
represents an inefficiency. Without special design, or purging on shutdown, the
leakage will continue and consume methanol under idle conditions.
Methanol crossover to the cathode is quickly oxidized to CO
2
via Eq. (6.42). This consumes
O
2
, dilutes the cathode flow, and creates a mixed potential parasitic current (I
p
) reaction at
the cathode:
I
p
= 6F
˙
n
CH
3
OH,cathode
(6.50)
The loss from this equivalent current can be modeled in the same way as hydrogen crossover
shown in Chapter 4.
Methanol crosses from anode to cathode by diffusion, hydraulic permeability, and
electro-osmotic drag:
˙
n
CH
3
OH
=−DA
C
c−a
x
+
iA
F
λ
drag
−
kk
r
µ
A
P
c−a
l
(6.51)
Typically, the hydraulic permeability is either neutral or directed toward the anode by
capillary pressure management. Of the three modes of methanol crossover, diffusion
(estimated as 10
−5.4163-999.778/T
m
2
/s [45]) is dominant under normal conditions, espe-
cially at higher temperatures. Since the driving potential for oxidation is so high at the
cathode, the methanol that crosses over is almost completely oxidized to CO
2
, which
sets up a sustained maximum activity gradient in methanol concentration across the
electrolyte.
The electro-osmotic drag coefficient of methanol is estimated to be 0.16 CH
3
OH/H
+
[46], or 2.5y, where y is the mole fraction of CH
3
OH in solution [47]. The electro-osmotic
drag coefficient is relatively weak compared to water, which is a result of the nonpolar
nature of the molecule.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.4 Direct Alcohol Polymer Electrolyte Cells 349
In order to combat methanol crossover, several approaches have been used:
1. Use of Dilute Methanol Solutions Use of a low-molarity solution (0.5–2 M)of
methanol reduces the concentration of methanol at the anode, thus reducing the
crossover. However, this approach results in unacceptably large fuel storage tanks
and excessive water-pumping requirements.
2. Use of Thicker Electrolyte Similar to the water crossover, a thick electrolyte can
restrict crossover but also limits performance via increased ohmic losses through
the electrolyte.
3. Diffusion Barrier on Anode A diffusion barrier (shown in Fig. 6.53) is an al-
ternative to the use of a thicker electrolyte that places the diffusion restriction at
a location that will not affect ionic transport. With a diffusion barrier, there is a
steep concentration gradient from the DM through barrier, so that the methanol
concentration at the catalyst layer is minimized, reducing crossover [48].
4. Capillary Pressure Management This establishes a hydraulic pressure gradient
favoring liquid flow toward the anode (Figure 6.53). The use of capillary pressure
management and an anode diffusion barrier permits simultaneous methanol and net
water crossover management, so that highly concentrated methanol solutions can
be used as the fuel source.
Of the various methods to restrict methanol crossover, typically a combination of thicker
electrolyte (∼100 µm) with diffusion barriers and capillary pressure management is used.
This approach is the most successful because it simultaneously enables water and methanol
management, with a high-concentration methanol solution at the anode, resulting in a
compact system design.
Anode Kinetics Since the cathode ORR is the same in DMFCs as it is in H
2
PEFCs and the
anode methanol oxidation is a complex series of intermediate reaction steps, Tafel kinetics
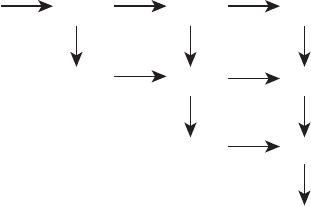
are generally valid for both the anode and the cathode. The theorized intermediate reaction
pathways are given in Figure 6.55 from [49]. There are believed to be two main routes to
methanol oxidation. The preferred pathway is to generate formaldehyde (CH
2
O), followed
by formic acid (CH
2
O
2
) oxidation to carbon dioxide (CO
2
). The nonpreferred route is also
initiated by generation of formaldehyde, which then oxidizes to carbon monoxide (CO),
finally oxidizing to produce carbon dioxide. The overall oxidation pathway (either preferred
CH
3
OH CH
2
OH COH
CH
2
O
CO
COH
HCOOH COOH
CO
2
CH
2
O
Figure 6.55 Proposed methanol electrochemical oxidation pathway at DMFC anode. (Adapted
from Ref. [49].)
