Myron G. Best. Igneous and metamorphic 2003 Blackwell Science
Подождите немного. Документ загружается.

feldspar at higher grades. Excess Al over that needed
for micas and feldspars stabilizes additional aluminous
minerals, including: at low grades, chlorite and garnet;
at medium grades, garnet, staurolite, and either kyanite
or andalusite; and at high grades, cordierite and
sillimanite.
Chlorite Zone. The lowest grade of metamorphism of
shale protoliths (briefly outlined in Section 14.1.1) cre-
ates a mineral assemblage in aphanitic slates of white
mica, chlorite, and quartz together with possible stilp-
nomelane, Fe–Ti oxides, and pyrite. Tourmaline, zir-
con, and monazite are common accessory minerals.
Calcite and/or ankerite occur in more calcareous pro-
toliths, pyrophyllite in highly aluminous ones, and
paragonite or albite where protoliths are relatively rich
in Na and Al. Graphite and/or other carbonaceous
material is common (Wada and Tomita, 1994). K-feldspar
is stabilized in low-Al protoliths transitional to felds-
pathic and lithic sandstones.
In the following paragraphs, pelitic mineral assem-
blages are tracked from this chlorite-zone assemblage
along an intermediate-P metamorphic field gradient
through the greenschist, amphibolite, and granulite
facies.
Biotite Zone. With increasing T from the chlorite
zone, at least three reactions can cause the appearance
of biotite, commonly as small porphyroblasts. The
first two reactions are discontinuous and the third is
continuous (Figure 18.14a, b). The first discontinuous
reaction occurs in low-Al rocks lacking white mica,
Metamorphism at Convergent Plate Margins: P–T–t Paths, Facies, and Zones
577
Orthopyroxene
Plagioclase
K-feldspar
Quartz
Fe–Ti oxides
Ankerite-
Calcite
Epidote
FACIES Greenschist Amphibolite Granulite
Melt
Albite
Oligoclase Andesine
ZONE
APPROX.
Chlorite
T (°C) 300
Phengite
425 500 550 600 650 750 780 800
Biotite Garnet Staurolite Kyanite Sillimanite Sil Kfs Crd Grt Kfs Opx
MINERAL
Muscovite
Chloritoid
Chlorite
Mg-rich
Stilpnomelane
Biotite
Garnet Mn-rich
Staurolite
Kyanite Metastable
Sillimanite
Cordierite
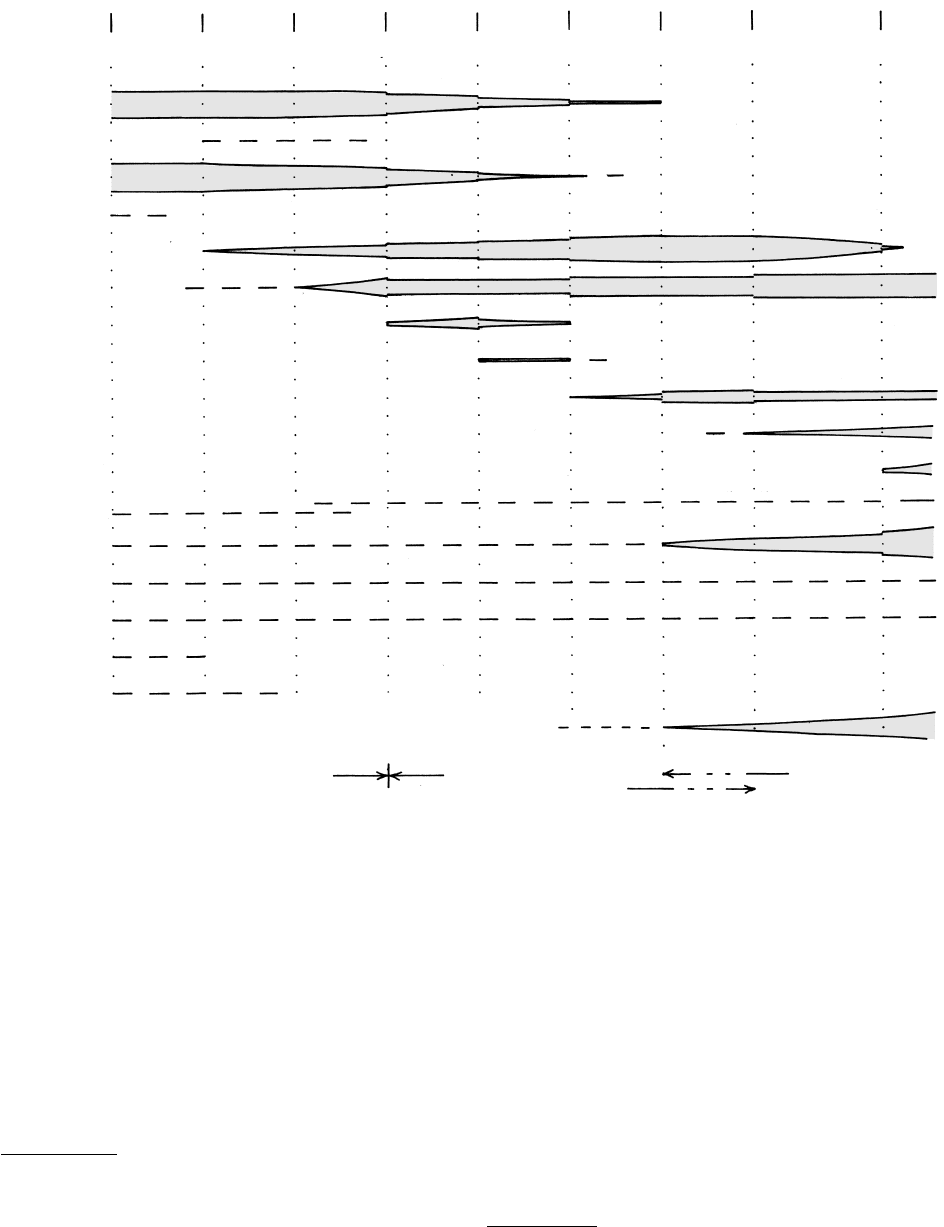
18.13 Generalized mineral compatibilites in typical Barrovian zones in pelitic rocks metamorphosed under intermediate-P conditions. Width
of bands for more prominent minerals, such as micas, garnet, and so on, shows schematically their increased modal amount as produced
by discontinuous and continuous reactions, or their consumption by these reactions. Dashed lines indicate possible mineral stabilities in
nonspecific modal proportions, such as for quartz. Temperatures are approximate. Note possible coexistence of two sodic plagioclases
in the garnet zone. Some petrologists place the transition between the amphibolite and granulite facies at the low-T boundary of the
sillimanite K-feldspar zone, whereas others place it at the high-T boundary.
To K-feldspar
Phengite chlorite →
biotite muscovite quartz H
2
O
Chlorite muscovite → garnet Mg-richer chlortite
biotite H
2
O
Prl
(a) CHLORITE ZONE (b) BIOTITE ZONE (c) GARNET ZONE
Phe Kfs
A
K
M
Bt
Chl
M
AA
Prl Phe Kfs A
K
Chl
Chl
Chl
Cld
Grt
F
Prl
F
Bt
F
F
Bt
Chl
M
A
Cld
Grt
F
Bt
0 0.1mm 0 0.1mm 0 1mm
Muscovite
quartz H
2
O
Muscovite
Muscovite
Biotite
Chlorite
Muscovite
QuartzBiotite
Chlorite
Quartz
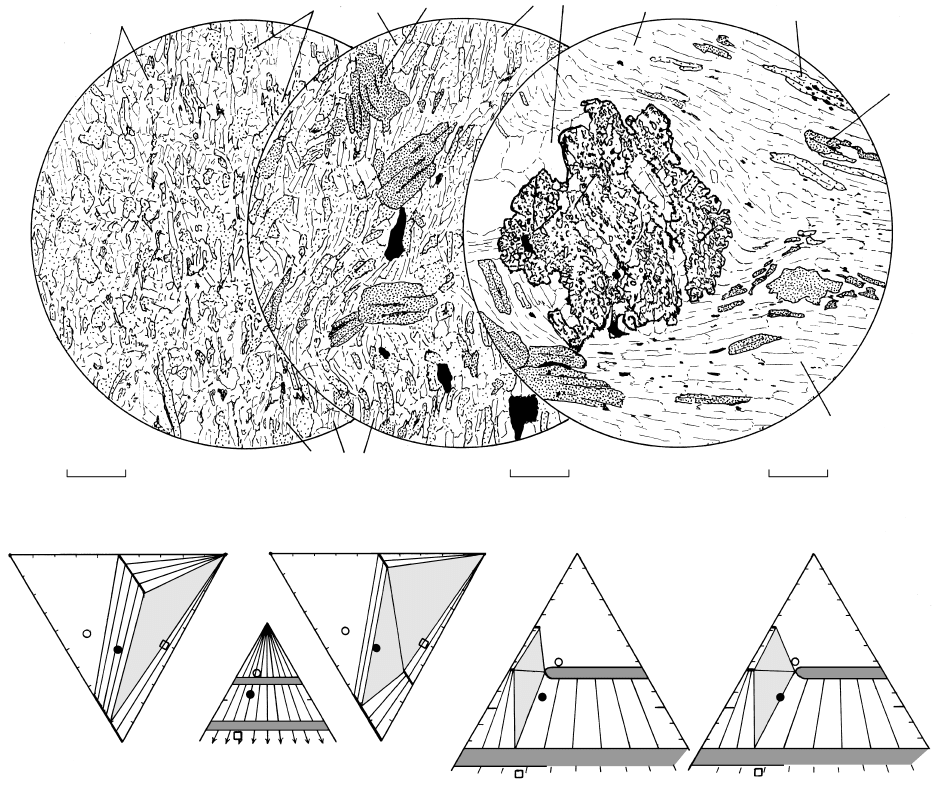
18.14 Prograde regional metamorphism of pelitic rocks as occurs in typical Barrovian zones. T and grade increase from left to right. The top
panel across the pages consists of drawings of thin sections from the lowest grade chlorite zone on the left to the highest grade granulite
facies on the right. Note changes in scales of drawings. Thin sections were provided through the courtesy of William D. Carlson and
Douglas Smith from the University of Texas at Austin collections. Beneath each drawing are one or two composition diagrams showing
stable mineral assemblages for the corresponding zone or facies. The filled circle represents average low-Al shale, open circle Al-rich
shale, and open square Al-poor lithic/feldspathic sandstone and granitoid protoliths from Blatt and Tracy (1996) and Spear (1993). Be-
neath the composition diagrams is the typical reaction producing the characteristic mineral assemblage of the zone or facies. Only in (d)
is a reaction texture preserved to suggest the nature of reactant and product phases. (a) Chlorite zone. Slate from the type area of George
Barrow’s study in the Dalradian Supergroup collected along River North Esk about 1.3 km north of Dalbog in northeast Scotland con-
sists of quartz chlorite phengite graphite(?). Al-rich protoliths can contain pyrophyllite and granitoid protoliths K-feldspar
chlorite and possible phengite. (b) Biotite zone. Weakly crinkled porphyroblastic slate collected along River North Esk about 350 m
northwest of the chlorite-zone sample in (a). Porphyroblasts of biotite and coarse flakes of graphite lie in matrix of chlorite muscovite
quartz. In the smaller AFM diagram projected from muscovite, biotite and chlorite are complete solid solutions between F and M
components. (c) Garnet zone. Locality of schist unknown. High-relief garnet poikiloblast contains abundant inclusions, partly of quartz,
forming a curved pattern. Weakly crenulated schistose matrix of muscovite quartz biotite. The two AFM diagrams represent, on
left, compatibility of biotite and chlorite at a T below the garnet isograd (garnet not stable) in average low-Al pelite. Right-hand diagram
represents the equilibria at the isograd as the three-phase compatibility triangle garnet chlorite biotite expands toward more
magnesian compositions, eclipsing average low-Al pelite. Chloritoid will appear in Al–Fe-rich pelites that are common in New England.
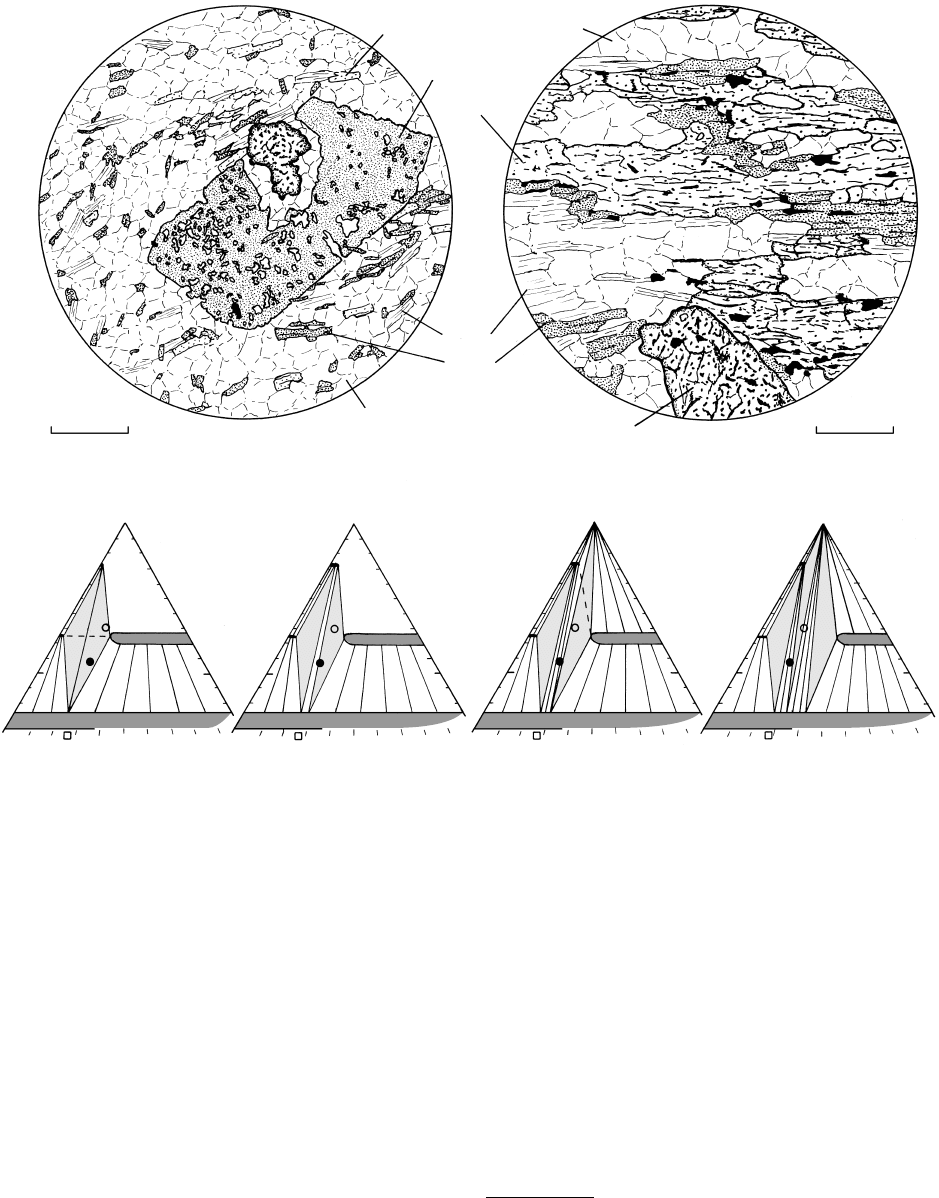
(d) Staurolite zone schist. Locality unknown. Euhedral staurolite poikiloblast that contains abundant inclusions of quartz, defining sieve
texture, partially surrounds anhedral garnet; this may be a reaction texture in which garnet is consumed with production of staurolite
and quartz. Matrix consists of quartz chlorite muscovite biotite. (e) Kyanite zone. Schist from Simplon Road near border of
Switzerland in the Italian Alps. Anhedral garnet porphyroblast with kyanite, quartz, and lesser biotite, muscovite, and graphite. (f) Silli-
manite zone. Schist from near Orange, Massachusetts, made of the assemblage sillimanite biotite garnet quartz muscovite
graphite(?). All grains except quartz are relatively euhedral. Note that most pelitic rocks (circles) contain sillimanite garnet biotite
in contrast to the more variable assemblages in pelites of the staurolite and especially the kyanite zone. (g) Second sillimanite zone, or
sillimanite alkali feldspar zone. Gneiss from the Adirondack Mountains along Route 8 east of Graphite, New York. Rock is composed
of aligned flakes of biotite within an aggregate of quartz alkali feldspar garnet sillimanite Fe–Ti oxides. (h) Granulite-facies
pelitic rock, Tanil Nadu, Maduri block, India. Thin section provided courtesy of Ayati Ghosh. Rock is composed of quartz perthite
cordierite garnet sillimanite spinel Fe–Ti oxides (see, for example, Mukhopadhyay and Holdaway, 1994). Note distinctive
pleochroic haloes around minute zircon inclusions in cordierite.
namely, chlorite K-feldspar → biotite H
2
O, and
the second in calcareous pelites, ankerite muscovite
→ biotite calcite. The third reaction is the one most
commonly accounting for the biotite isograd in average
pelitic rocks. It is a continuous reaction destabilizing
Fe
2
–Mg-rich phengitic white micas in favor of more
typical muscovite
18.1 phengite chlorite →
biotite muscovite quartz H
2
O
(It should be noted here that these and other reactions
listed below are generalized because of the highly vari-
able chemical compositions of the solid solutions. Ex-
act stoichiometrically balanced reactions are not easily
written.) In the AKF diagrams (Figure 18.14a, b), at in-
creasing T, the continuous shrinking of the two-phase
stability field of phengite chlorite allows an average
pelite to be encompassed by an expanding three-phase
field of biotite phengitic muscovite chlorite. In the
AFM diagram (Figure 18.14b), pelites richest in Al do
not record the stable appearance of biotite as do rocks
that contain less Al. Hence, the bulk chemical com-
position of the rock controls stabilization of biotite
and, hence, manifestation of the biotite isograd. It
should also be noted that, at the low temperatures of
the incoming of biotite, there may be manifestations of
the sluggish kinetics of mineral reactions. For example,
Dempster and Tanner (1997) argue that the biotite
isograd in the Central Pyrenees was controlled by
deformation, allowing its appearance at a lower T than
would otherwise have occurred.
Garnet Zone. Typically very small garnets can locally
appear in chlorite zone pelites that have unusually high
concentrations of Mn or of Ca and Fe
3
in strongly
oxidized rocks. Stabilized garnets are essentially spes-
sartine and andradite, respectively. More Mn is parti-
tioned into garnet than other mafic phases. However,
the appearance of Fe
2
-rich almandine porphyroblasts
Metamorphism at Convergent Plate Margins: P–T–t Paths, Facies, and Zones
579
Grt
St
FM
Chl
Bt
Garnet chlorite muscovite → staurolite biotite quartz H
2
O Staurolite chlorite muscovite quartz → kyanite biotite H
2
O
A
(d) STAUROLITE ZONE
Grt
St
FM
Chl
Ky
Bt
A
Grt
St
FM
Chl
Bt
A
Grt
St
FM
Chl
Ky
Bt
A
(e) KYANITE ZONE
01mm 0 0.5mm
Quartz
Garnet
Muscovite
Biotite
Kyanite
Staurolite
Chlorite
Quartz
Muscovite H
2
O
quartz
Muscovite H
2
O
quartz
18.14 (Continued).
of sufficient size to be readily visible in thin section
and even hand sample delineates the lower bound
of the garnet zone in the Barrovian sequence. These
garnets, nonetheless, commonly contain appreciable
concentrations of the spessartine end-member in their
cores (to as much as 30 mole % according to Spear,
1993, p. 354), as well as much of the CaO in the rock.
The exact reaction governing the first appearance of
this critical index mineral is somewhat uncertain but
one possibility is the continuous reaction
18.2 chlorite muscovite → garnet
Mg-richer chlorite biotite H
2
O
The two AFM diagrams in Figure 18.14c depict this re-
action and show that as chlorites become more Mg-rich
with increasing T garnet is stabilized in more alumi-
nous pelites. Chloritoid is stable in highly aluminous
rocks in the garnet zone.
Within the garnet zone, plagioclases become more
calcic, not only in pelites but in other compositional
groups as well. The stabilized oligoclase–andesine con-
trasts with the albite epidote that coexists at lower
temperatures. In some terranes, albite and oligoclase
stably coexist (Figure 18.13) because of a so-called
peristerite gap—a sort of solvus—in the otherwise con-
tinuous solid solution series in plagioclases.
Staurolite Zone. Although many protoliths will con-
tain biotite, chlorite, and muscovite upon metamor-
phism, only ones with sufficient concentrations of Al
580 Igneous and Metamorphic Petrology
Bt
Staurolite muscovite quartz →
sillimanite biotite garnet H
2
O
Muscovite quartz →
K-feldspar sillimanite + H
2
O
Sillimanite biotite quartz →
cordierite K-feldspar H
2
O
Sillimanite biotite quartz →
cordierite garnet K-feldspar H
2
O
Bt Bt
M
Cordierite with
pleochroic
halo around
zircon
Muscovite
Quartz
Sillimanite
Sillimanite
Cordierite
Perthite
Garnet
Garnet
Garnet
Cordierite
Garnet
Cordierite
F
FF
M
M
Sillimanite Sillimanite Sillimanite
Garnet
Biotite
Quartz
0 0.5mm 0 0.25mm 0 0.5mm
AAA
Muscovite quartz
H
2
O
K-feldspar quartz
H
2
O
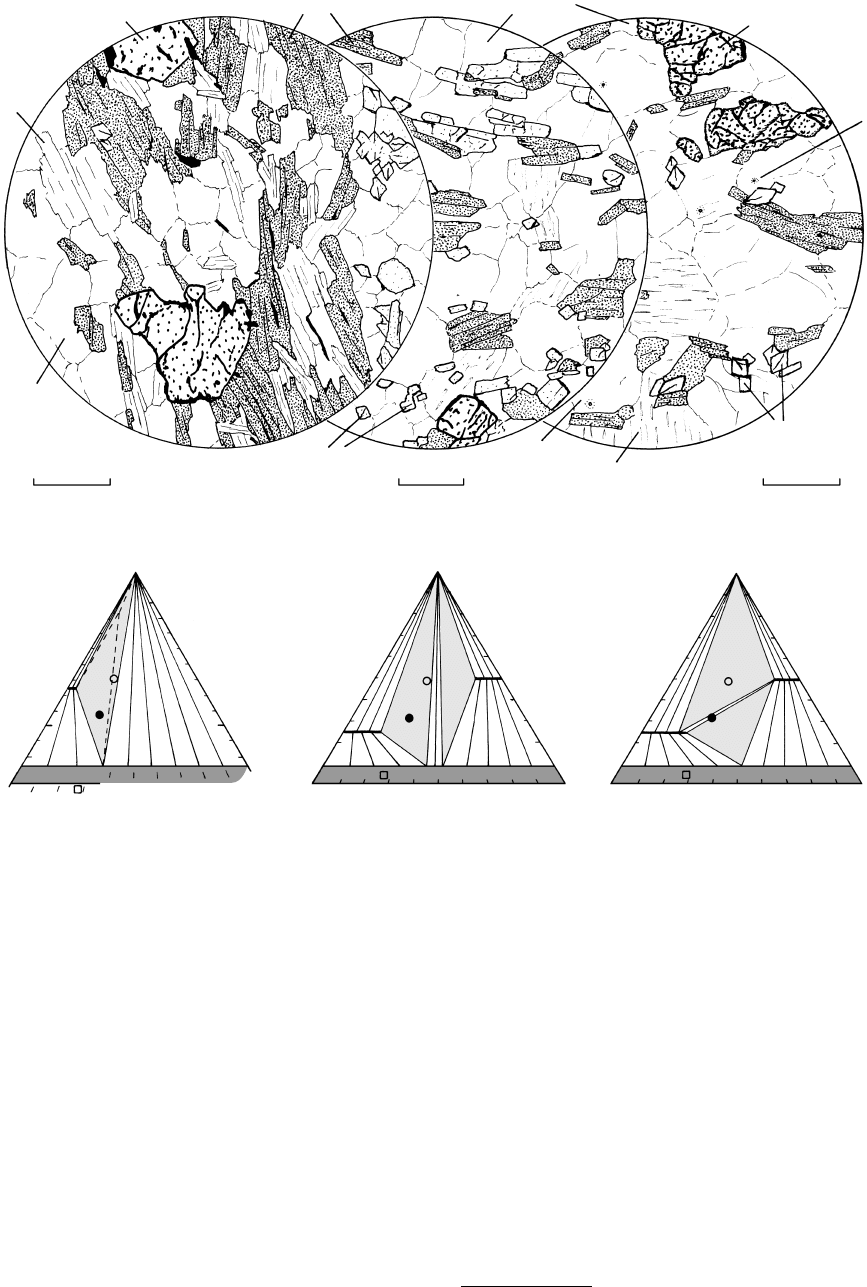
(f) SILLIMANITE ZONE (g) SILLIMANITE K-FELDSPAR ZONE (h) GRANULITE FACIES
18.14 (Continued).

and Fe
2
will contain garnet and staurolite under
suitable conditions. In highly aluminous rocks, stauro-
lite can form by the reaction chloritoid quartz →
staurolite garnet H
2
O. A possible corresponding
reaction texture seen in some staurolite-bearing rocks
consists of garnet poikiloblasts that enclose chloritoid;
the armoring garnet prevented further reaction with
neighboring quartz. A more common discontinuous
reaction, resulting in a tie-line switch in the AFM dia-
gram, is suggested by staurolite poikiloblasts that are
crowded with inclusions of quartz (Figure 18.14d)
18.3 garnet chlorite muscovite →
staurolite biotite quartz H
2
O
The staurolite isograd mapped on the ground is not
merely the first appearance of staurolite, as this mineral
can be stabilized in mafic aluminous rocks just below
the isograd. Rather, the isograd is the first appearance
of the stable pair staurolite biotite.
Though incompatible as a pair in the staurolite
zone, chlorite and garnet individually form stable
three-phase assemblages with staurolite biotite in
relatively magnesian and Fe-rich rocks, respectively.
Chlorite coexistent with staurolite biotite is more
magnesian than the chlorite coexistent with garnet
in the garnet zones. This contraction in chlorite sta-
bility with increasing T is caused by the continuous
reaction
18.4 chlorite muscovite →
staurolite biotite quartz H
2
O
The contraction of chlorite stabilities toward the Mg
end-member and the accompanying expansion of the
two-phase field of staurolite biotite can be seen in
the AFM diagrams for the staurolite zone (Figure
18.14d).
Kyanite Zone. The first appearance of kyanite with
increasing grade involves a similar reconfiguration of
tie-lines in the AFM diagram as for the appearance of
staurolite. In this change in topology, the staurolite
chlorite compatibility is broken by another discon-
tinuous tie-line switching reaction (Figure 18.14e)
18.5 staurolite chlorite muscovite quartz
→ kyanite biotite H
2
O
Hence, the kyanite isograd marks the first appearance
of stably coexistent kyanite biotite in the same way
that the staurolite isograd marks the first appearance of
stably coexistent staurolite biotite. Continued pro-
duction of this Al
2
SiO
5
polymorph through the kyanite
zone is accomplished by the continuous reaction
18.6 chlorite muscovite quartz →
kyanite biotite H
2
O
In an AFM diagram, this reaction is manifest by ex-
pansion toward M of the bundle of kyanite–biotite
tie-lines as the three-phase triangle kyanite biotite
chlorite also shifts continuously toward M (Figure
18.14e). Consequently, the range of stable chlorite solid
solutions is reduced to only the most Mg-rich com-
positions. Many pelitic rocks in the kyanite zone will,
therefore, consist of kyanite biotite staurolite
muscovite quartz plagioclase. Apparently stable
assemblages in some terranes consist of staurolite
biotite garnet kyanite ( muscovite quartz
plagioclase). This four-phase assemblage should not be
allowed in the three-component AFM diagram. A pos-
sible explanation depends on unusually large concen-
trations of components not ordinarily accounted for in
the diagram (Section 15.3), such as Ti stabilizing bio-
tite, Ca and Mn stabilizing garnet, or Zn stabilizing
staurolite. Some staurolites have as much as 2.7 wt.%
ZnO.
Sillimanite Zone. The highest grade zone seen by Bar-
row in Scotland was marked by the appearance of the
high-T Al
2
SiO
5
polymorph, sillimanite. In most rocks,
kyanite grains are not pseudomorphed by sillimanite.
Rather, fine needles and bundles of sillimanite called
fibrolite commonly nucleate within or on muscovite or
especially biotite (Figure 16.2). With increasing T into
the zone more robust prisms of sillimanite develop
(Figure 18.14f).
In the Barrovian terrane of Scotland, the prevail-
ing P (about 6 kbar) during metamorphism allowed
the almost coincidental destruction of staurolite at
the sillimanite isograd according to the discontinuous
reaction
18.7 staurolite muscovite quartz →
sillimanite biotite garnet H
2
O
In Figure 18.14f, tie-lines linking staurolite to garnet,
biotite, and sillimanite all vanish as a single new
three-phase triangle representing coexistent sillimanite
biotite garnet replaces the three lower grade,
three-phase compatibilities. Continued contraction of
chlorite stability (Guidotti et al., 1991) with increasing
T by the continuous reaction 18.6 finally eliminates
even the most magnesian chlorites (Figure 18.13).
Sillimanite + Alkali Feldspar Zone. As temperatures
rise in many Barrovian terranes to above 750°C, mus-
covite solid solutions react with quartz to form a
potassic alkali feldspar plus Al
2
SiO
5
(reaction 14.1 and
Figure 14.31). At lower pressures, andalusite is stable,
whereas higher pressures produce kyanite. However, in
the majority of pelitic sequences, the typical Al
2
SiO
5
polymorph is sillimanite and its first appearance with
alkali feldspar in the field occurs above the upper, or
second, sillimanite isograd.
Metamorphism at Convergent Plate Margins: P–T–t Paths, Facies, and Zones
581
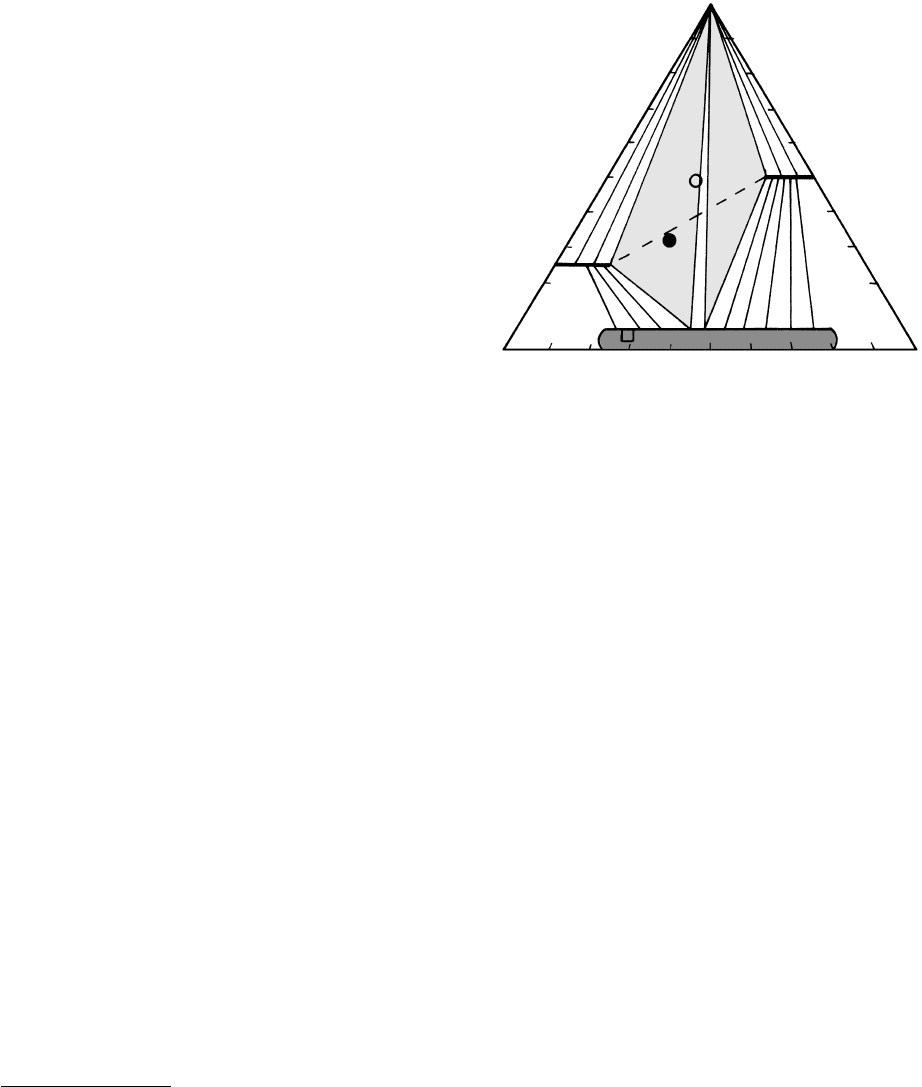
Three additional consequences of muscovite break-
down are petrologically significant.
1. Many petrologists (Miyashiro, 1994; Blatt and
Tracy, 1996) regard the compatibility of sillimanite
alkali feldspar as characteristic of the granulite
facies that is dominated by nonhydrous minerals.
2. With the demise of muscovite, the remaining phyl-
losilicate is only biotite. (Chlorite disappeared in the
lower part of the sillimanite zone.) Consequently,
pelitic rocks tend to be less schistose and more
gneissic.
3. Contributing to the gneissic aspect is the fact that
in many terranes this breakdown of muscovite
more or less coincides with the beginning of partial
melting, possibly prompted by the released water.
Many, if not most, migmatites (Section 15.2.3) are
believed to have formed by in situ dehydration
partial melting. (Production of partial melts was
discussed in Section 11.6 where it should be noted
that the temperatures of melting are relatively high
because of the high pressures of the experiments
and possible overstepping required.)
Partial melting has sometimes been viewed as the
culmination of metamorphism or as ultrametamor-
phism, but it can be debated whether either is appro-
priate. This phenomenon does, nonetheless, represent
a turning point in metamorphism because of the
affinity of the melt phase for any water in the rock—it
is rather effectively dehydrated. Thereafter, any further
reactions at increasing T are dominated by nonhydrous
reactant and product phases.
Graphically, the demise of muscovite and stabilization
of alkali feldspar creates a fundamental change in the
AFM diagram because the point of projection is now
from feldspar in the AFMK tetrahedron (Figure 15.27a).
More magnesian pelites in the sillimanite alkali
feldspar zone may contain cordierite—the most Mg-
rich of the common aluminous mafic minerals (Section
16.5.2). Mineral compatibilities involving cordierite,
shown in Figure 18.14g, suggest it appears by the con-
tinuous reaction
18.8 sillimanite biotite quartz →
cordierite K-feldspar H
2
O
Higher Grade Pelites. At still higher temperatures, the
stability of cordierite may expand toward more Fe-rich
compositions while garnet becomes more Mg-rich,
eliminating the compatibility of sillimanite biotite
according to the continuous reaction
18.9 sillimanite biotite quartz →
cordierite garnet K-feldspar H
2
O
This new equilibria involving the rare compatibility of
garnet cordierite, shown in Figure 18.14h, occurs in
some high-grade migmatites where dehydration partial
melting allowed the liberated water to dissolve in the
melt. According to Yardley (1989), the appearance of
cordierite garnet K-feldspar defines the beginning
of the granulite facies.
Yet higher temperatures in the granulite facies can
cause breakdown of biotite to produce orthopyroxene
K-feldspar that can coexist with combinations of
garnet, cordierite, sillimanite, and quartz (Figure 18.15;
see also the charnockite in Figure 15.20). Extreme
metamorphic temperatures of about 1000°C at 8–10
kbar are recorded in very unusual granulite-facies
rocks of the Precambrian Enderby Land in Antarctica
and the Archean Narryer complex of Western
Australia (Section 19.2.4) that contain the exotic
volatile-free Mg–Fe–Al silicates sapphirine, spinel, and
osumilite in addition to the above phases (Spear, 1993).
18.3.2 P T t Paths and Chronology of Barrovian
Metamorphism
One of the most thoroughly documented determina-
tions of P–T paths in a Barrovian terrane was made by
St-Onge (1987) in pelitic rocks of the early Proterozoic
Wopmay Orogen, Northwest Territories, Canada (Fig-
ure 18.16). GASP and garnet–biotite thermobarometry
(Section 16.11.2) was applied to six rock samples that
contained the basic assemblage garnet biotite pla-
gioclase quartz sillimanite kyanite. Zoned poik-
iloblastic garnets 5–7 mm in diameter are crowded
with inclusions of the same minerals as occur in the
matrix, justifying use of these thermobarometers to
monitor the progress of metamorphism. Six to 17 de-
terminations of P and T were made for each of the six
samples by microprobe analyses of garnet and neigh-
boring inclusions from poikiloblast core outwards,
582 Igneous and Metamorphic Petrology
FM
Orthopyroxene
A
Sillimanite
Crd
Grt
K-feldspar
quartz
H
2
O
18.15 AFM diagram depicting pelitic equilibria with orthopyroxene
in the high-T part of the granulite facies. Under some condi-
tions cordierite garnet (dashed line) is a possible compat-
ibility rather than sillimanite orthopyroxene. Open square
is charnockite in Figure 15.20.
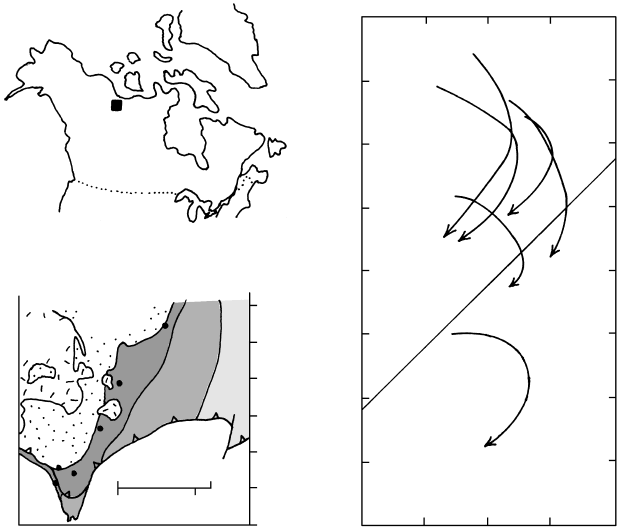
resulting in six separate P–T paths recording garnet
growth during declining P of about 2 kbar but T in-
crease of 25–75°C. The garnet-forming reaction was
probably staurolite muscovite quartz → garnet
biotite Al
2
SiO
5
H
2
O. The six measured paths are
not nested, as predicted for samples at different crustal
depths in tectonothermal models of thickening crust
experiencing thermal relaxation (Figures 14.36 and
18.1c). This is explained by St-Onge in terms of large
amplitude folding that resulted in variable uplift rates
and an irregular crustal T distribution. Nonetheless,
the overall pattern of paths is consistent with the posi-
tion of the samples in the tilted 30-km-thick section in
the Wopmay Orogen.
The time of the Grampian metamorphism in the
Caledonide Orogen of Scotland that created the classic
Barrovian and Buchan zones (Figure 14.30) lasted from
about 480 to 465 Ma during collision between an island
arc and the continental margin of Laurentia (Oliver et
al., 2000). Laurentia (Figure 19.27) lay to the north of
the inferred oceanic trench now marked by blueschist-
facies rocks and ophiolite (Figure 14.30). Constraining
data include K–Ar, Rb–Sr, and Sm–Nd ages on the
metamorphic sole of the ophiolite (Section 18.5),
on metamorphic minerals in pelitic zones, and on
syn- and post-tectonic granitoids. A similar time of
metamorphism has been documented in the northern
Appalachian Mountains in eastern USA that were
connected with the Caledonide Orogen in northwest-
ern Europe before the opening of the Atlantic Ocean.
Dating shows the peak metamorphism in the Barrovian
and Buchan terranes was contemporaneous and within
about 8 My of the time of their exhumation, as indi-
cated by the age of erosional debris that contains meta-
morphic index minerals. These chronologic data show
that collisional orogeny, metamorphism, and exhuma-
tion can all occur within only several million years.
18.3.3 Buchan Metamorphism
Some regional metamorphic terranes preserve mineral
assemblages in pelitic rocks that equilibrated at dis-
tinctly lower P than typical Barrovian assemblages.
Metamorphic zones in the Buchan area of Scotland
north-east of Aberdeen (Figure 14.30) have long served
as the type terrane for such rocks, hence they are called
Buchan zones. Other well known Buchan zones occur
in New England, USA (Blatt and Tracy, 1996) and in
the Proterozoic of Australia (e.g. Johnson and Vernon,
1995; Warren and Ellis, 1996). There is a clear sim-
ilarity with pelitic mineral assemblages in contact
Metamorphism at Convergent Plate Margins: P–T–t Paths, Facies, and Zones
583
P (kbar)
km
10
8
6
4
2
0
10
20
30
020km
020miles
700500 600
T (°C)
6
4
5
3
2
1
Kyanite
Sillimanite
Archean
Chl
Bt
(b)
(a) (c)
1
2
3
4
5
6
Batho-
lith
Ksp Sil
C
a
n
a
d
a
18.16 Barrovian pelitic rocks and their P–T paths, Wopmay Orogen, Northwest Territories, Canada. Redrawn from St-Onge (1987). (a) Index
map showing sample location. (b) Down-plunge cross-section constructed from the geologic map of the structurally tilted metamorphic
terrane that is 30 km thick. Four metamorphic zones are shown; the sampled muscovite Al
2
SiO
5
( garnet biotite plagioclase
quartz) zone is dark shaded. Syntectonic 1.9 Ga batholith is composed of tonalite and quartz diorite. Teeth are on the hanging-wall of a
late-metamorphic fault. (c) P–T paths for six samples whose locations are indicated in (b). For clarity, as many as 17 data points defining
each of the other curves are omitted. See also Figures 16.35 and 18.19.
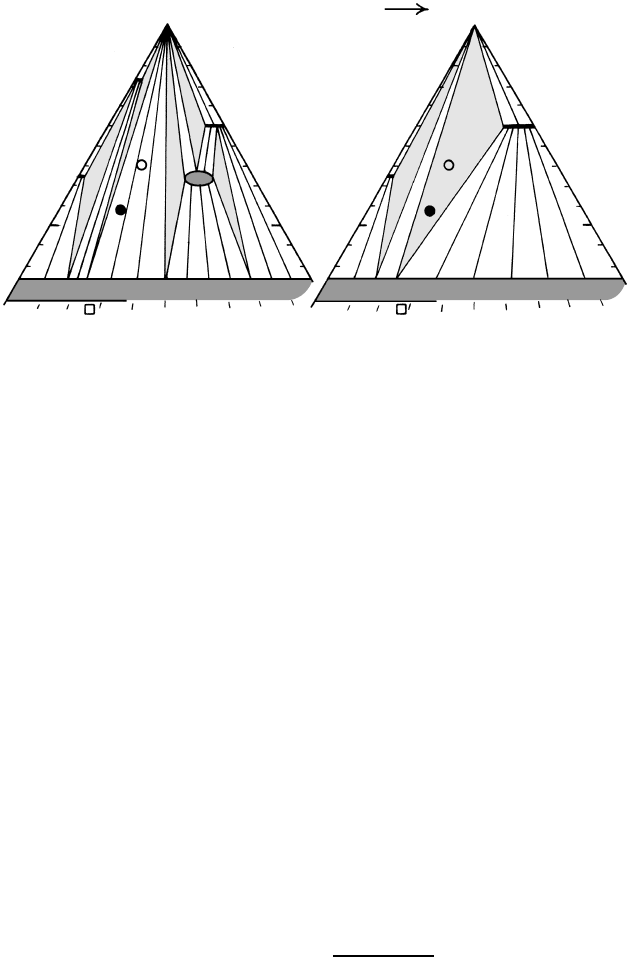
metamorphic aureoles (Figure 14.25) developed around
magmatic intrusions in the shallow (10 km) crust.
The occurrence of abundant synmetamorphic intru-
sions in Buchan terranes lends support to the notion
that the heat for metamorphism was magmatic; thus,
the terranes are often considered to be “regional con-
tact aureoles” possessing tectonite fabric instead of the
hornfelsic fabric of typical aureoles.
The lower pressures of equilibration of Buchan alu-
minosilicates lead to significant contrasts with higher P
Barrovian assemblages (Figure 18.17; see also Figures
14.33 and 14.34a and Yardley, 1989). These contrasts
include:
1. Andalusite and, at higher grades, sillimanite are
stabilized rather than kyanite. Muscovite break-
down can occur in the stability field of andalusite,
producing andalusite K-feldspar compatibility.
2. Garnet and staurolite are less common or even absent
but cordierite is widespread and forms at much
lower temperatures than in some rare Barrovian rocks.
3. Migmatites are less common and developed only at
highest temperatures above the upper sillimanite
isograd where sillimanite K-feldspar are compatible.
The Buchan biotite zone has coexisting muscovite
chlorite quartz as in Barrovian pelites. However,
the lower pressures are manifest in relatively less Al in
chlorites and less phengite end-member in muscovite.
A more striking contrast is found at the upper limit of
the biotite zone where, instead of the garnet isograd in
Barrovian terranes, the next index mineral to appear is
cordierite, manifest as ill-defined “spots” in slaty rocks.
The reaction producing this isograd is
18.10 chlorite muscovite →
cordierite biotite quartz H
2
O
This reaction changes the basic topology of the AFM
diagram in Figure 18.17 and leads to stabilization of
the assemblage andalusite cordierite biotite as this
three-phase triangle sweeps into the AFM diagram
from the AM side, eclipsing bulk pelite compositions.
Changing biotite Fe/Mg ratio in the assemblage ac-
counts for most of the sweep. Like the garnet isograd
in Barrovian terranes, the position of the Buchan
cordierite isograd is composition dependent.
The andalusite isograd can be produced by two
possible reactions in rocks that are not enriched in Fe.
If chlorite has not been consumed by the cordierite-
producing reaction 18.10 above, then andalusite
appears as a result of
18.11 chlorite muscovite quartz →
andalusite cordierite biotite H
2
O
On the other hand, if no chlorite is available, then an-
dalusite is produced at slightly higher temperatures by
18.12 cordierite muscovite quartz →
andalusite biotite H
2
O
P T t Paths. In contrast to Barrovian terranes, Buchan
terranes reveal no clear evidence of crustal thickening.
In fact, Wickham and Oxburgh (1985) describe
Buchan-style metamorphism in the Pyrenees along the
border of France and Spain that they believe occurred
in a continental rift setting where crust was thinned
and probably underplated by mafic magma. H and O
isotopic ratios indicate equilibration with advecting
seawater to depths of 12 km during the high-T, low-P
metamorphism. They suggest a modern analog might
be the Salton Sea area just north of the actively
opening Gulf of California near the international bor-
der of California and Mexico (Figure 13.29 and Special
Interest Box 18.1 and Figure 18.18).
The counter- (anti-) clockwise P–T path of Buchan
metamorphism (Figure 18.19) is inconsistent with the
crustal thickening that typifies Barrovian terranes in
584 Igneous and Metamorphic Petrology
Biotite Biotite
M
CordieriteCordierite
AndalusiteAndalusite
Staurolite
FM
Chlorite Garnet
F
Garnet
AA
Increasing T
Muscovite quartz
H
2
O
18.17 AFM diagrams depicting pelitic mineral assemblages during prograde Buchan metamorphism. With increasing T, staurolite and chlorite
(ellipse) are consumed and the compatibility field of stable andalusite biotite cordierite sweeps toward more Fe-rich compositions,
encompassing most pelites (circles) and creating a cordierite isograd. Redrawn from Spear (1993).
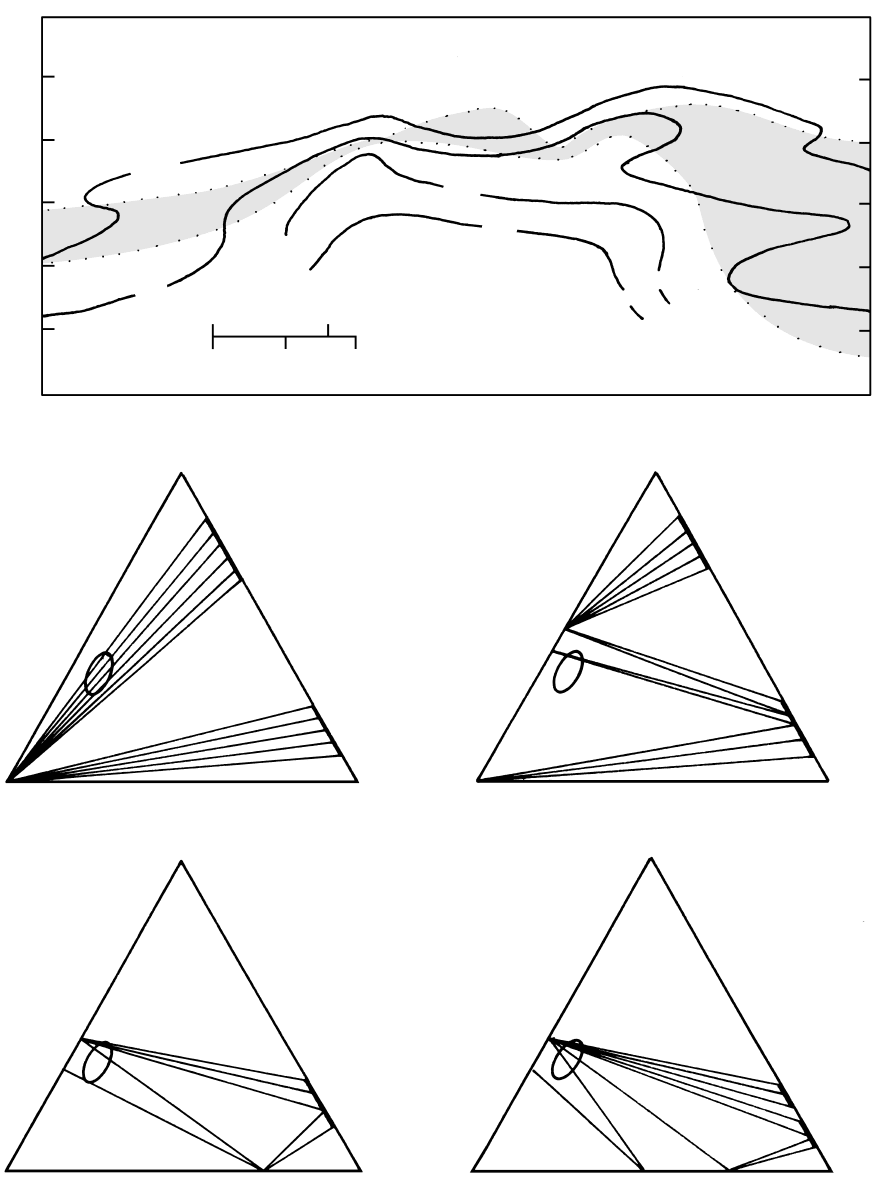
Metamorphism at Convergent Plate Margins: P–T–t Paths, Facies, and Zones
585
Depth (km)
0
1
2
3
CF
Prehnite
Epidote
300°C 320°C
Biotite
Chlorite
Actinolite
A
CF
Prehnite
Epidote
Chlorite
ActinoliteClinopyroxene
A
CF
150°C 230°C
Chlorite
Illite and
white mica
Illite and
white mica
Calcite
A
CF
Epidote
Wairakite
Chlorite
Calcite
A
01km
02miles
1
0
0
°
C
2
0
0
3
0
0
3
5
0
18.18 Cross-section and mineral assemblages in the Cerro Prieta geothermal field, Mexico. Top of diagram shows isotherms and distribution
of calcite-cemented impervious caprock (shaded) overlying geothermal reservoir; constraints are provided by numerous well cores
and cuttings. Mineral assemblages are plotted in terms of A Al
2
O
3
Fe
2
O
3
, C CaO, F FeO MgO MnO. Ellipse represents
composition of carbonate-cemented quartzo-feldspathic sandstone protolith. See Special Interest Box 18.1. Redrawn from Schiffman
et al. (1984).
586 Igneous and Metamorphic Petrology
P (kbar)
Depth (km)
10
8
6
4
2
0
35
30
25
20
15
10
5
0
400 500 600 700 800 900
T (°C)
B
U
C
H
A
N
C
O
N
T
A
C
T
And
Sil
Ky
Chl
Grt
G
r
t
C
h
l
S
t
B
t
S
t
C
h
l
B
t
K
y
G
r
t
S
i
l
B
t
C
h
l
C
r
d
A
n
d
B
t
G
r
a
n
i
t
e
s
o
l
i
d
u
s
B
A
R
R
O
V
I
A
N
H
2
O
-
s
a
t
u
r
a
t
e
d
M
s
Q
t
z
K
f
s
S
i
l
B
t
S
i
l
G
r
t
C
r
d
St
Crd
Crd
18.19 Petrogenetic grid for intermediate grade pelitic rocks
comparing Barrovian and Buchan P–T paths and mineral
assemblages. Depending on rock composition and formative
reaction, cordierite is stable at higher temperatures and lower
pressures than dotted line labeled Crd. Reactions and assem-
blages in intermediate-P Barrovian terranes involve staurolite,
garnet, and kyanite, whereas in low-P Buchan regional ter-
ranes and contact aureoles cordierite and andalusite are dom-
inant phases. Compare Figure 14.1. Redrawn from Dymoke
and Sandiford (1992) and Spear (1993).
P
T
“
N
o
r
m
a
l
”
g
e
o
t
h
e
r
m
S
o
l
i
d
u
s
Buchan
And
Ky
Sil
b
a
c
c
b
Granulite
facies
Granitic
Diapir
(a)
(b)
(c)
(d)
Fertile source rock
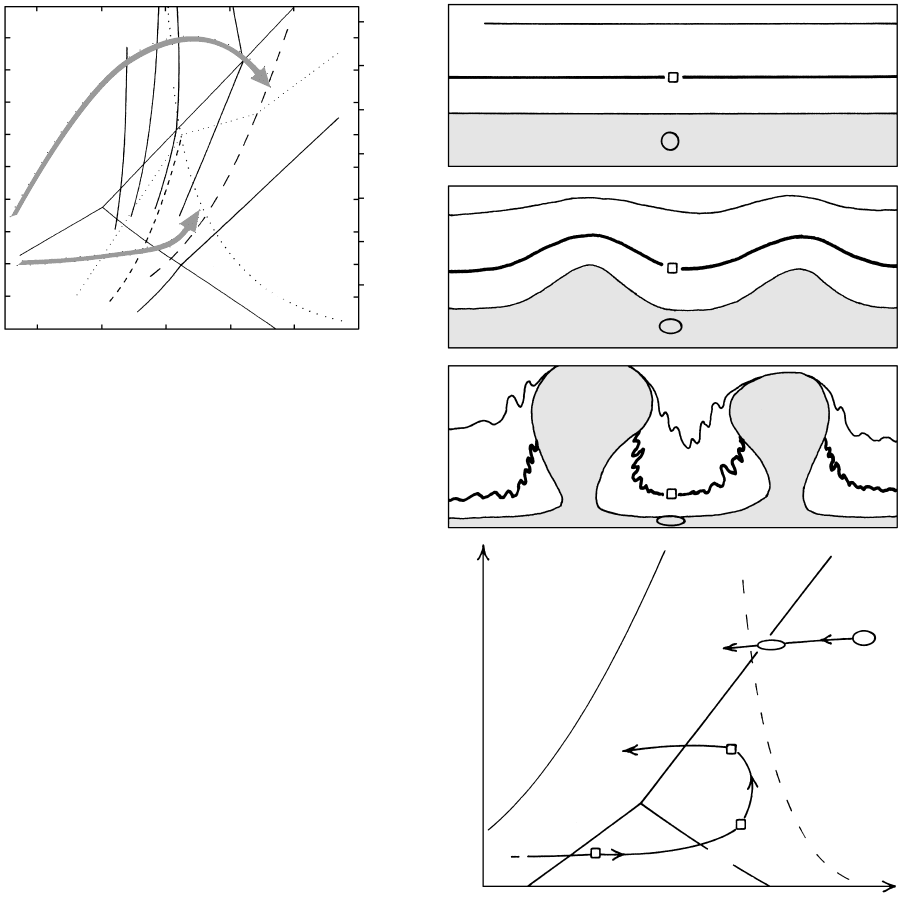
18.20 Geologic model and schematic P–T diagrams for Buchan meta-
morphism. Redrawn from Warren and Ellis (1996). (a)–(c) show
progressive steps in growth of hypothetical buoyant granitic
magma diapirs from a fertile source layer near the base of the con-
tinental crust, with heat possibly inserted from an underlying
mafic magma underplate. These three diagrams were inspired
by laboratory experiments on “gravity tectonics” by Ramberg
(1981). Note the deepening of a reference volume (open square)
of the wallrock surrounding the ascending diapirs and the sub-
horizontal elongation of a reference volume (open circle and
ellipses) of the source layer. (d) Schematic diagram showing
P–T paths of reference volumes from upper model. The wall-
rock follows a counter-clockwise path similar to Buchan terranes,
while the extended and flattened source layer follows a retro-
grade path similar to foliated and lineated granulite-facies rocks.
collisional orogens. The prograde part of the Buchan
path is nearly isobaric through the andalusite field and
to peak T in the sillimanite field; this trajectory is read-
ily explained in terms of heating by copious volumes of
intruded granitoid magma (Barton and Hanson, 1989),
these likely being a product of introduction of mantle-
derived mafic magma into the lower crust to drive par-
tial melting. But the subsequent upturn of the path to
higher P followed by isobaric cooling in an counter-
clockwise direction has proven to be a major hurdle in
interpretations of Buchan metamorphism.
Warren and Ellis (1996) have proposed a novel ex-
planation based on return flow of country rocks sur-
rounding ascending granitoid diapirs (Figure 18.20; see
also Figure 9.15). Field relations around plutons com-
monly indicate the country rock moved downward in
response to the pluton making room for itself in the
shallow crust. Where the volume of intruded plutons is
large relative to intervening country rock the down-
warping in rim synclines can be considerable, if the re-
sults of laboratory models can be extrapolated to the
real crust. Following initial heating of the wallrock in a
regional contact aureole, continued rise of the diapiric
magma causes increased depression of the synclinal
wallrock to its maximum P. After the thermal pertur-
bation, possibly induced by mafic underplating at the
base of the crust, has died, the metamorphosed coun-
try rocks thermally relax along an essentially isobaric
trajectory back to a normal geotherm. The lack of
overall crustal thickening that results in significant
isostatic rebound and unroofing precludes develop-
ment of a clockwise path.
An intriguing facet of the crustal model in Figure
18.20 is the subhorizontal extension that takes place in
