Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.

646
Chapter 14: Phase Diagrams
Fig. 14.10.
(continued)
~/:'(u
BoY2Cuo5 \
(
Bo4YCu30x
t
40
Mol ~. l.Jq I 80
3 ~iq.2 "BaEu02" 60 100
"~---BaO CuOx
~/2(Y2O3)
BaY2Cu05 ~, ,., ..
ci
Ba4YC
t
40
"BaCu02" 60 Mol g "Liq.1 80 100
..=-- BaO CuOx
(continued)
D. Representative Phase Diagrams 647
Fig. 14.10.
(continued)
1/2(Y203)
Bou
(g)
...... \,,,,co,o,
9 ,
_
. .
///
- _ _ ..." "'-..,.~
Ba4YCu;~)x .""
Liq_.,'2
40 "BaCu02" 60 Mol % \ Liq.1 80 100
BaO CuOx
Y203
~ 1/2(Y203)
BeY2CuO 5
(h) --~- -
B C
V
40 60 Mol % 80
4--BaO
11o48oo. i
100
CuOx
(continued)
648 Chapter 14: Phase Diagrams
Fig. 14.10.
(continued)
(i)
Y20~I~
BAY20"4 .... ".. "... i; aY2Cu05 1/2(Y2031
"
Os
\ 1i 274~
\
\
\
\ u
V V V
40 60 Mol % 80 100
9 ,.--- BaO
CuOx
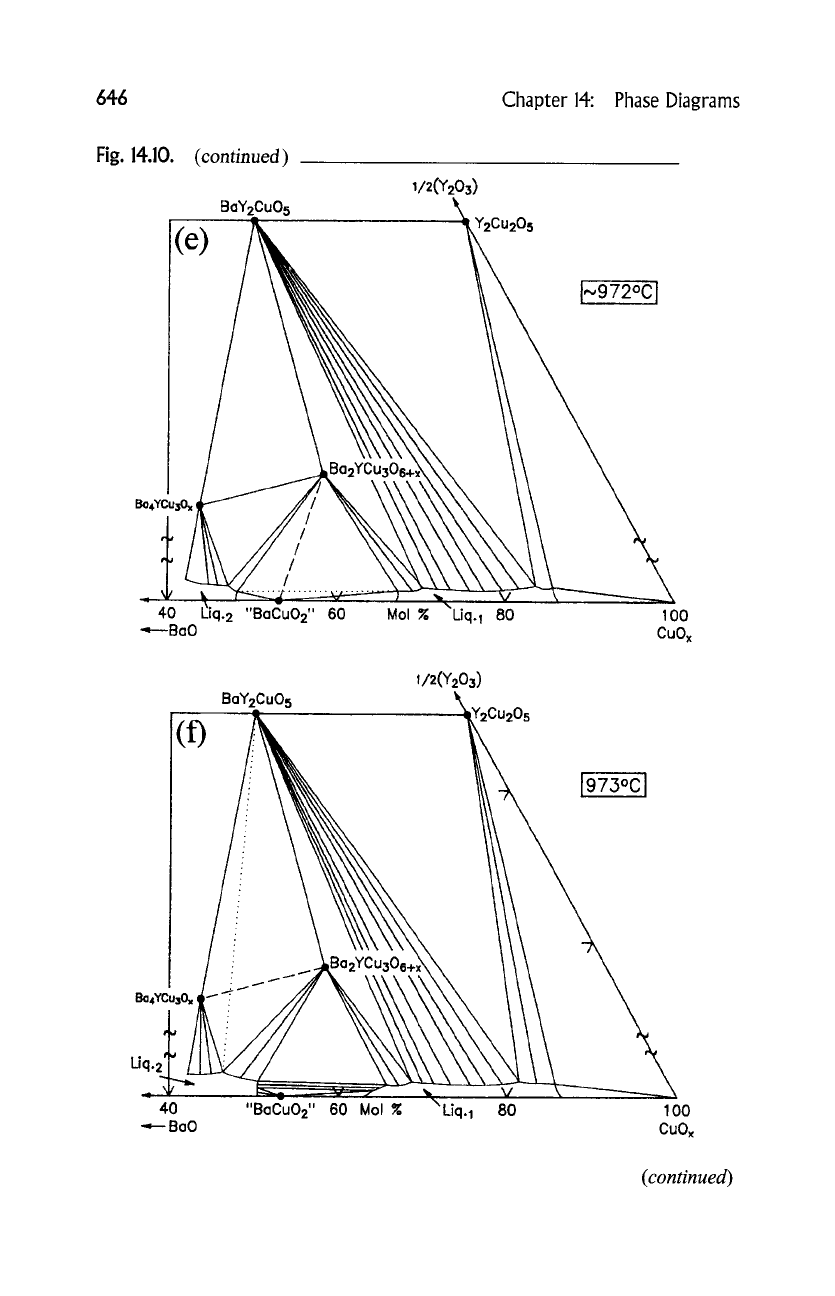
(a) 923~ "BaCuO2" + CuO + Ba2YCu306+ x ---, L 1 +
0 2
(b) 947~ BazYCu306+ x + CuO --+ BaYzCuO 5 + L 1 + O 2
(c) 967~ BaYzCuO 5 + CuO
--+ YzCu205
-'t- L 1 + O 2
(d) 971~ Ba4YCu30 x + "BaCuO2" ---, BazYCu306+ x + L 2 +
O 2
(e) 972~ "BaCuO2" + BazYCu306+ x --+ L 1 + L 2 --t- O 2
(f) 973~ Ba4YCu30 x + BazYCu306+x --+ BaYzCuO 5 + L2 + O2
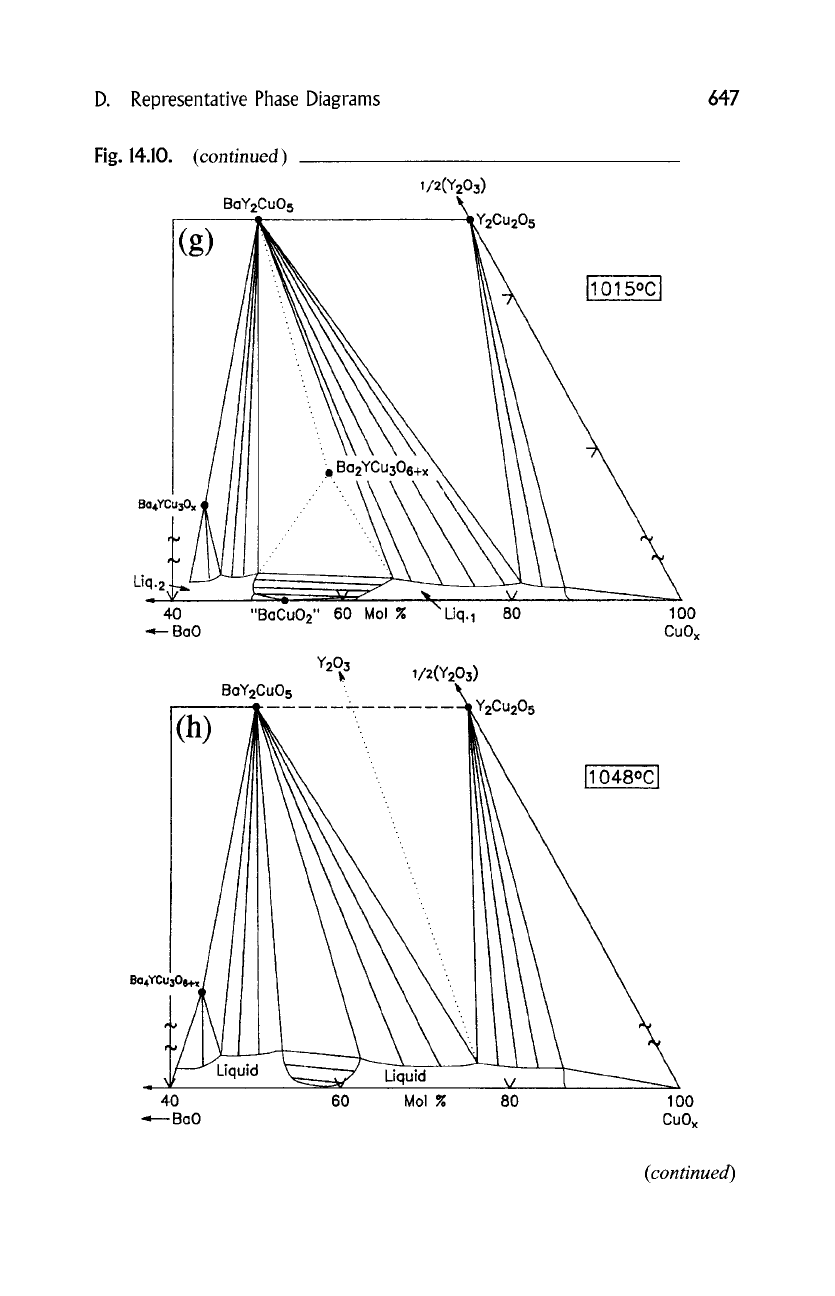
(g) 1015~ BazYCu306+x --+ BaYzCuO5 + L1 + L2 + O2
(h) 1048~ BaYzCuO 5 + Y2Cu205 --+ Y203 --t- L1 + O2
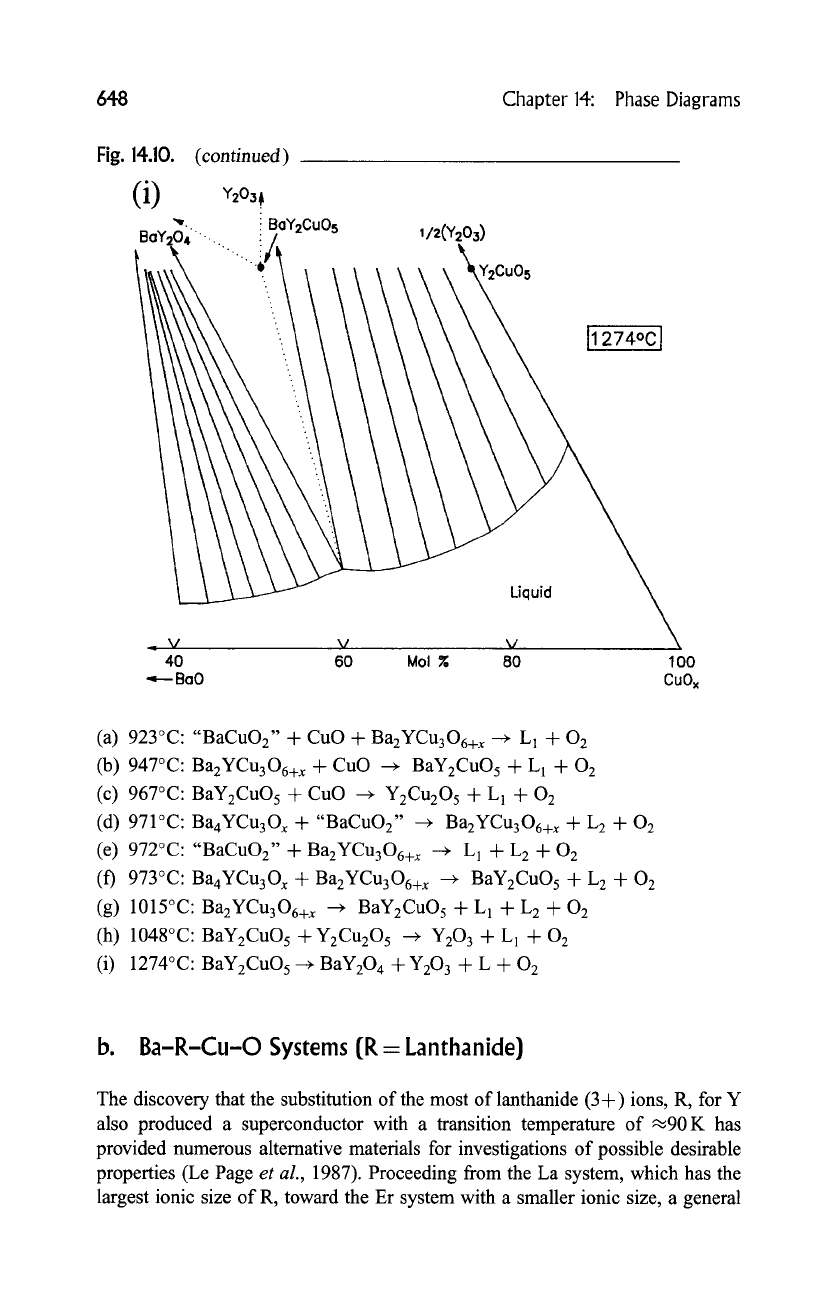
(i) 1274~ BaYzCuO 5 --+ BaY204 + Y203 + L + O 2
b. Ba-R-Cu-O Systems (R = Lanthanide)
The discovery that the substitution of the most of lanthanide (3+) ions, R, for Y
also produced a superconductor with a transition temperature of ~90 K has
provided numerous altemative materials for investigations of possible desirable
properties (Le Page
et al.,
1987). Proceeding from the La system, which has the
largest ionic size of R, toward the Er system with a smaller ionic size, a general

D. Representative Phase Diagrams 649
trend of phase formation, solid solution formation, and phase relationship was
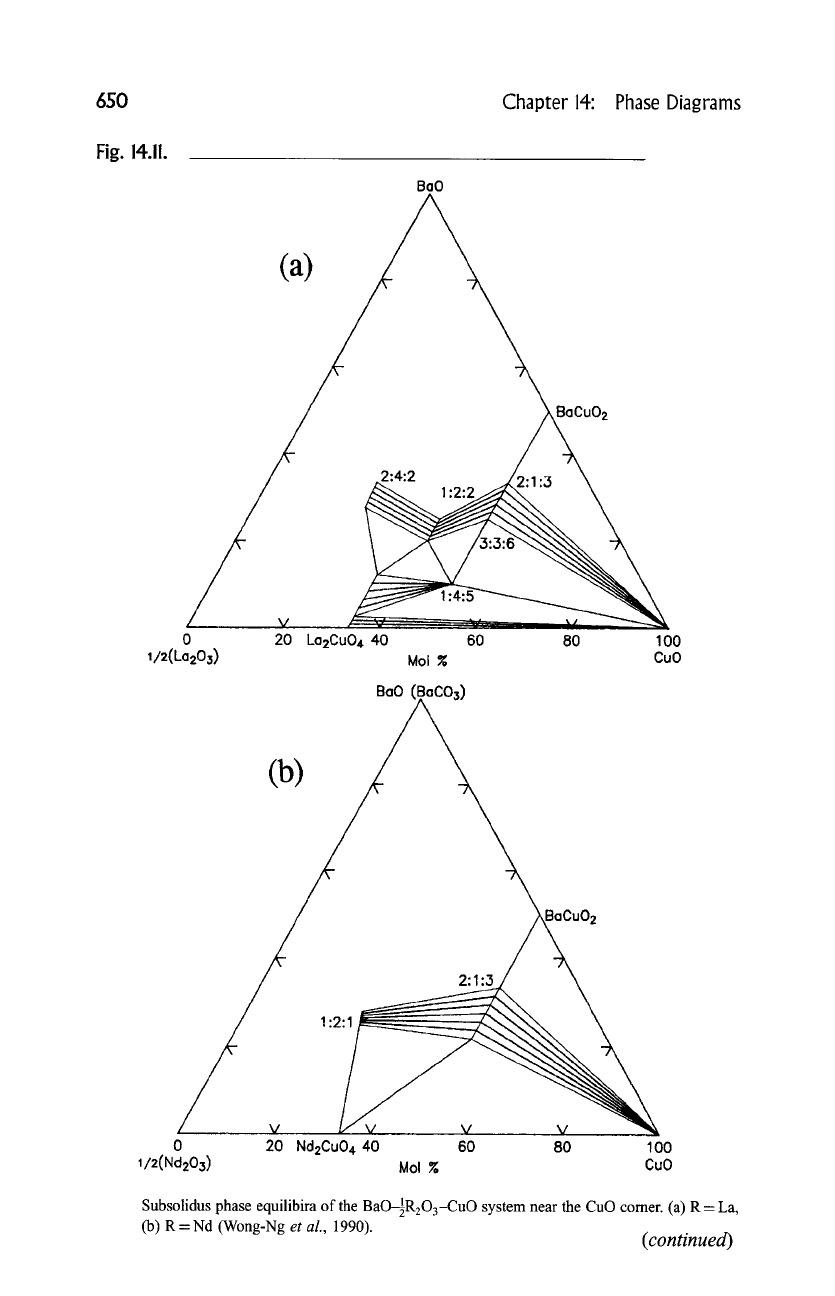
found to be correlated with the ionic size of R. The ternary phase compatibility
diagrams of the systems BaO(BaCO3)-89 and BaO(BaCO3)-89
CuO in the vicinity of the CuO corners (most relevant to the processing of the
high-T c materials), where R- La, Nd, Sm, Eu, Gd, Er, are shown schematically in
Fig. 14.11 (a) to (f) (Wong-Ng
et al.,
1990). Features of the progressive changes
in the appearance of these temary diagrams near the CuO comer include the
following: (1) The La system has the largest number of ternary compounds and
solid-solution series; this number decreases as the size of R decreases. (2) The
superconductor phase, Ba2RCu306+x, for the first half of the lanthanide family,
that is, R- La, Nd, Sm, Eu, and Gd, which are relatively larger in size, exhibit a
solid solution of
Ba2_zRl+zCu306+ x
with a range of formation that decreases as
the size of R decreases; this solid-solution region terminates at Dy and beyond,
where the superconductor phase assumes a point stoichiometry. The size
compatibility between Ba 2+ and R 3+ is a predominant factor governing the
formation of this solid solution. As the mismatch between R 3+ and Ba 2+
increases, the range of substitution decreases.The approximate upper limit of
the solid solution range ofz
of Ba2_zRl+zCu306+x
are La" 0.7, Nd" 0.7, Sm" 0.7,
Eu" 0.5, and Gd" 0.2, (3) A trend is observed regarding the tie-line connections
between BaR2CuO 5, CuO, the superconductor phases
Ba2_zRl+zCu306+x,
and
the binary phase R2CuO 4, or R2Cu2Os; note that the binary phase R2CuO 4 is
replaced by the binary phase R2Cu205 after the tie-line connection changes.
More complete diagrams of the systems with R- La, Nd are shown in Figs.
14.12 and 14.13, respectively. It is within the Ba-La-Cu-O system that the first
30 K high-T c phase in polycrystalline form,
BaxLas_xCusOs(3_y),
was discovered
by Bednorz and Mfiller (1986). The isothermal section of the Ba-La-Cu-O
system (Klibanow
et al.,
1988) shows a total of five solid solutions:
Ba2+xLa4_2xCu2_xOlo_2x
(242), BaLa4CusO13+x (145),
BaxLa2_xCuOa_(x/2)+ ~
(021), and Ba l+xLaz_xCuzo6_(x/2
) (122),
and a solid solution
Ba3+xLa3_xCu6014ix that spans from the 213 composition to the 336 composi-
tion. The limits of most of these solid solutions have not been quantified. The
solubility limits for
Baz+xLa4_zxCuz_xOlo_zx
were reported to be 0.15 _< x _< 0.25
[54]. The tie-line connectivity of the figure is schematic.
The ternary diagram of the Ba-Nd-Cu-O system at 890~ in air [56] is
reported in Fig. 14.13. In the barium-rich region, samples were annealed in air
with CO2 < 3 ppm. A total of three phases were found in this system. In addition
to the solid solution of the superconductor (213), Baz_xNdl_xCu307_ ~
(0.04 _< x_< 0.6) and
Baz+xNd4_zxCuz_xOlo_2x
(242) (x is negligible), a 6"1"3
phase (orthorhombic: a-3.886(2), b-3.984(2) and c-13.001(5)A) is also
found. The existence of the
Baz_xNdl+xCu3Oz-Baz+xNd4_zxCu2_xOlo_zx two-
phase field enables one to select a starting composition that leads to composite
superconductors of these two phases that are completely devoid of the minor
second phases that segregate at Baz_xNdl+xCu30~ grain boundaries after a solid-
state sintering.
650
Chapter
14:
Phase Diagrams
Fig. 14.11.
BaO
(a) ~k
//
1:2::
_~ . .. ~-~ ...- : . .'------~ ~ -~- ,.
0 20 La2CuO 4 40 60
11z(La203) Mol
2:4:2
Bao (E~aC03)
BaCu02
80
100
CuO
(b) /
///
1:2:1
0 20 Nd2CuO 4 40
~/2(Nd203)
2:1:3
_V v
60 80 1 O0
Mol % CuO
Subsolidus phase equilibira of the BaOIR203-CuO system near the CuO comer. (a) R = La,
(b) R = Nd (Wong-Ng
et al.,
1990).
(continued)
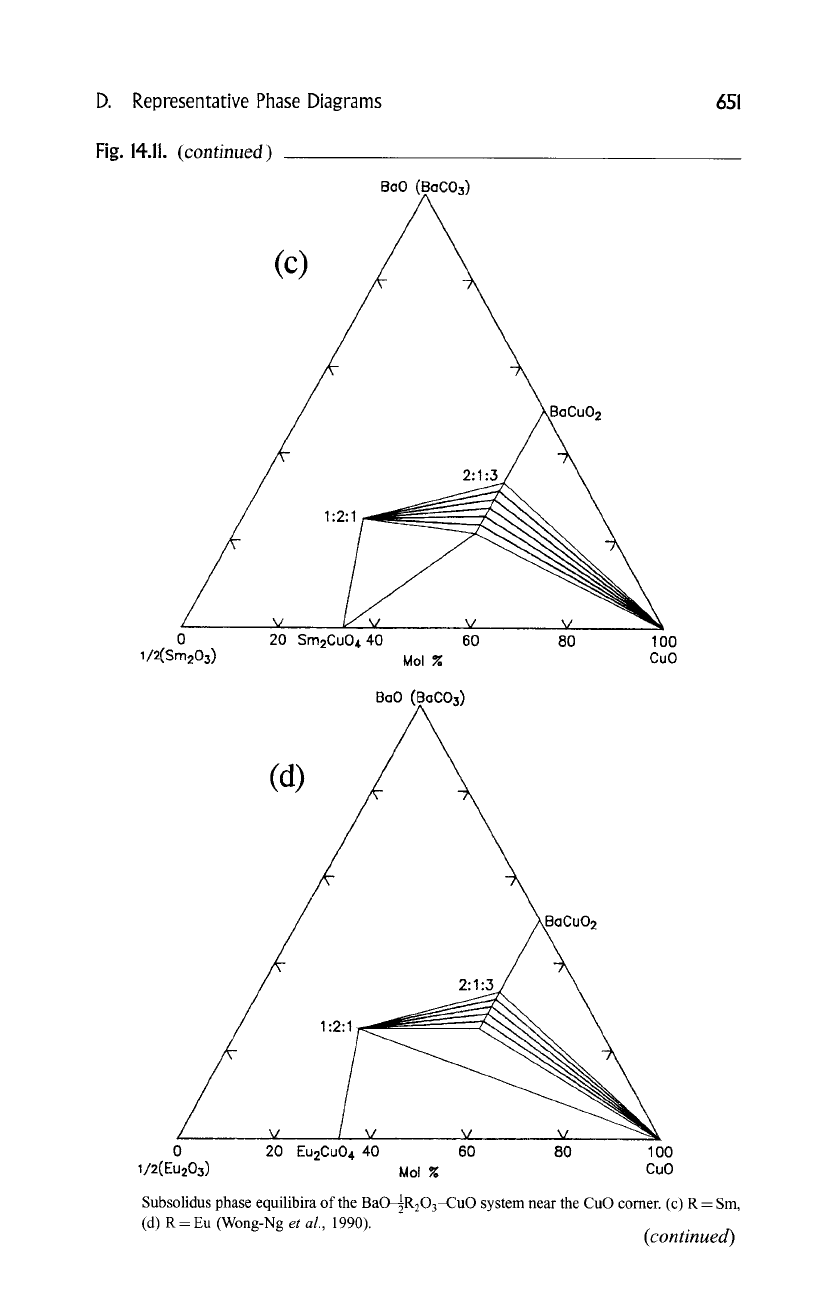
D. Representative Phase Diagrams 651
Fig. 14.11.
(continued)
Boo (E}oC03)
(c) /
v V
0
20
Sm2Cu04.
40
I/2(Sm203)
Mol %
2:1:3
v
6O
BoO (IE)aCO~)
V
80
~oo
CuO
(d)
/
/
/
1:2:1
v v
0 20 EuzCuO 4 40
I/2(Eu203)
2:1:3
v v
60 80 100
Mol %
CuO
Subsolidus phase equilibira of the BaO4R203-CuO system near the CuO comer. (c) R - Sm,
(d) R- Eu (Wong-Ng
et al.,
1990).
(continued)

652
Chapter 14: Phase Diagrams
Fig. 14.11. (continued)
BoO (I~IoC03)
(e) /
1:2:1
0 20 Gd2Cu04 40
1/2(Gd203)
Moi
%
2:1:3
V ..... V '~%~.
....
60 80 1
O0
CuO
BaO (IBaC03)
(0
/
///:
1:2:1 "
/ M ...... V
0 20
I12(Er203)
BaCu02
2:1:3
\ M _v
40 Er2CuzO 5 60 80 100
Mol % CuO
Subsolidus phase equilibria of the BaO1R203-CuO system near the CuO comer. (e) R = Gd,
and (f) R = Er (Wong-Ng
et al.,
1990).
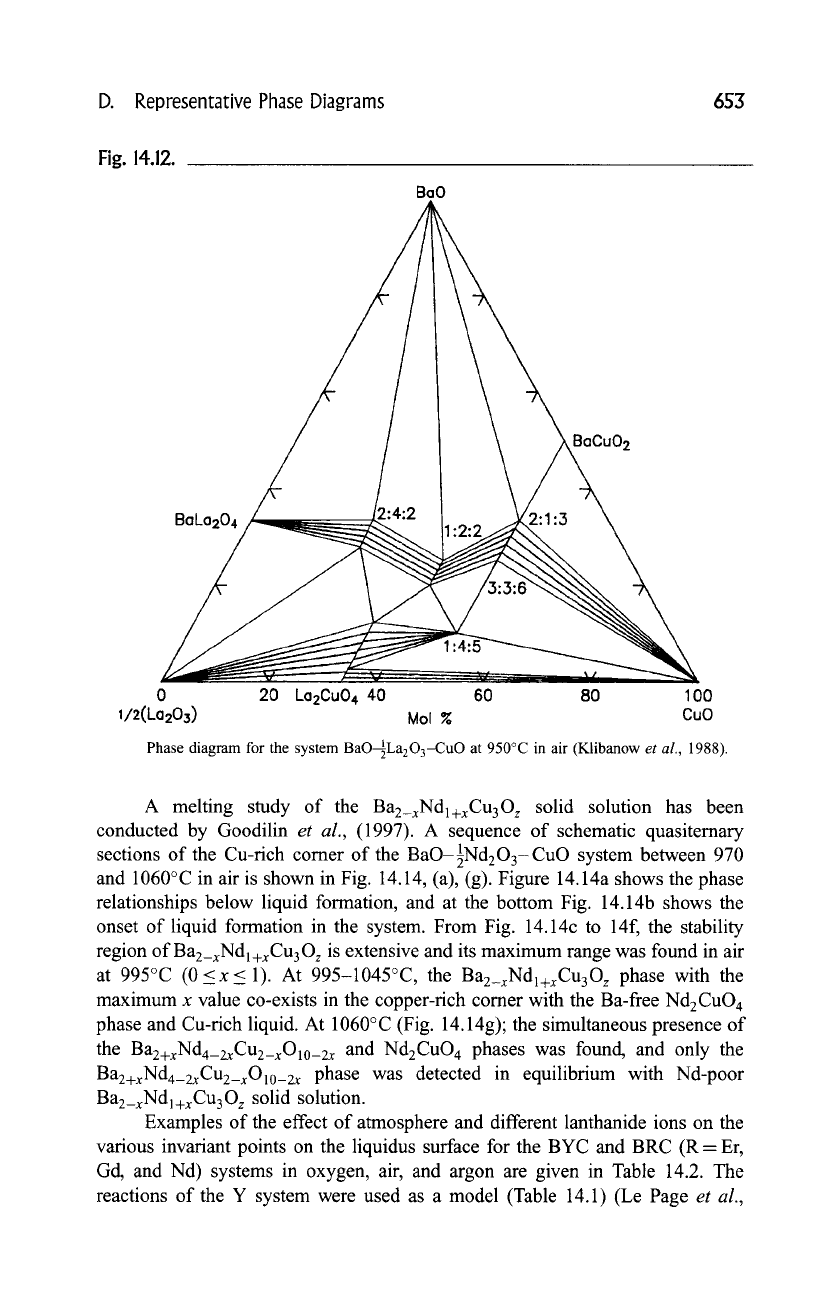
D. Representative Phase Diagrams 653
Fig. 14.12.
BaO
BaLa204
0 20 La2Cu04 40 60 80 100
1/2(La203) Mol ~ CuO
Phase diagram for the system BaO-~La203-CuO at 950~ in air (Klibanow
et al.,
1988).
A melting study of the
Ba2_xNd1+xCu30 z
solid solution has been
conducted by Goodilin
et al.,
(1997). A sequence of schematic quasiternary
sections of the Cu-rich comer of the BaO-89 system between 970
and 1060~ in air is shown in Fig. 14.14, (a), (g). Figure 14.14a shows the phase
relationships below liquid formation, and at the bottom Fig. 14.14b shows the
onset of liquid formation in the system. From Fig. 14.14c to 14f, the stability
region
of Ba2_xNdl+xCu30:
is extensive and its maximum range was found in air
at 995~ (0<x< 1). At 995-1045~ the
Bae_xNdl+xCu30 z
phase with the
maximum x value co-exists in the copper-rich comer with the Ba-free Nd2CuO 4
phase and Cu-rich liquid. At 1060~ (Fig. 14.14g); the simultaneous presence of
the
Ba2+xNdn_2xCu2_xOlo_2x
and Nd2CuO 4 phases was found, and only the
Ba2+xNda_2xCu2_xOlo_2x
phase was detected in equilibrium with Nd-poor
Ba2_xNdl+xCu30z
solid solution.
Examples of the effect of atmosphere and different lanthanide ions on the
various invariant points on the liquidus surface for the BYC and BRC (R = Er,
Gd, and Nd) systems in oxygen, air, and argon are given in Table 14.2. The
reactions of the Y system were used as a model (Table 14.1) (Le Page
et al.,
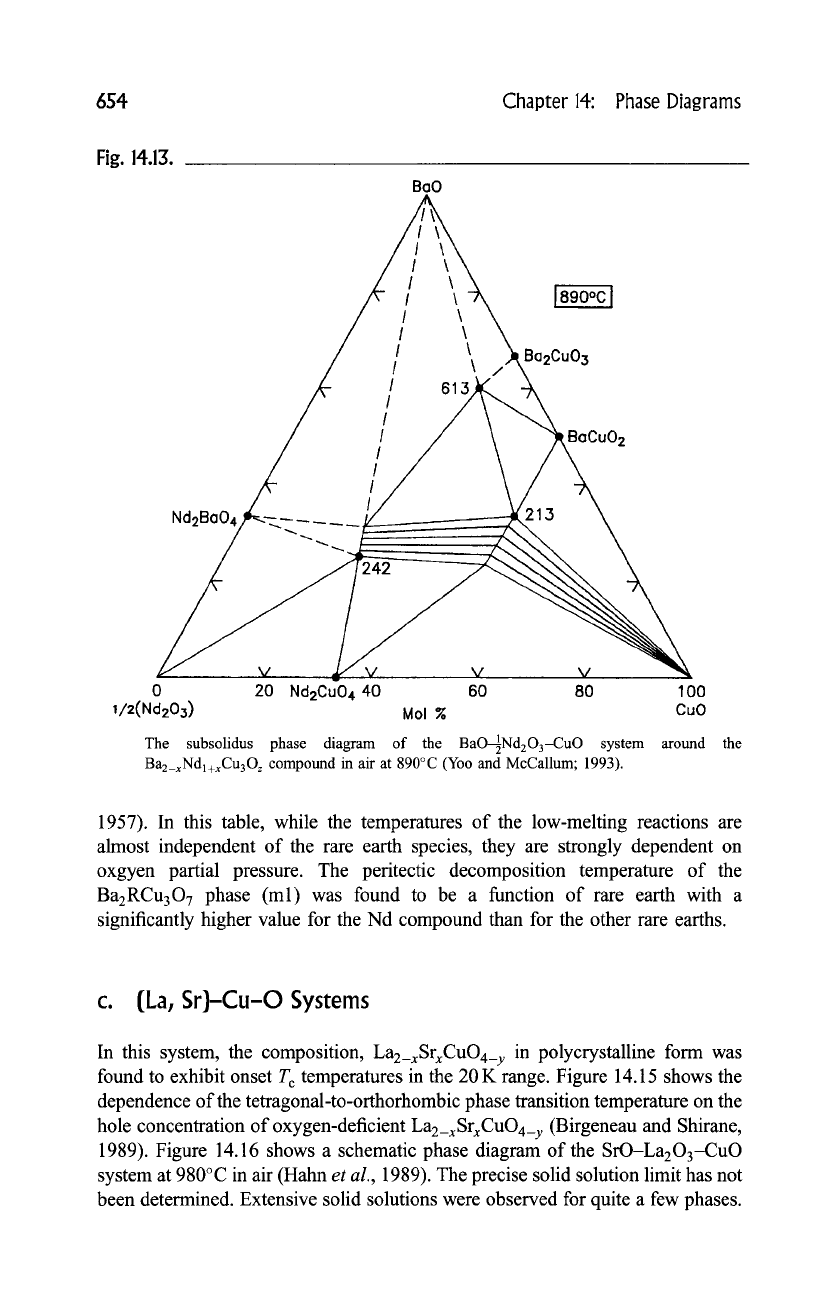
654 Chapter 14: Phase Diagrams
Fig. 14.13.
BaO
Nd2Bo04
I I ~
l~ l''-ooc'
/ \
/ \
/ \
I \ Ba2CuO 3
! 61
/
/
/
I
/
/
I/
= __ ~ ~
V V V l l
0 20
Nd2Cu04 40
60 80 100
I/2(Nd203) Mol 7, CuO
The subsolidus phase diagram of the BaO1Nd2
O3-CuO
system around the
Ba2_xNdl+xCu30z
compound in air at 890~ (Yoo and McCallum; 1993).
1957). In this table, while the temperatures of the low-melting reactions are
almost independent of the rare earth species, they are strongly dependent on
oxgyen partial pressure. The peritectic decomposition temperature of the
Ba2RCu307 phase (ml) was found to be a function of rare earth with a
significantly higher value for the Nd compound than for the other rare earths.
c. (La, Sr)-Cu-O Systems
In this system, the composition,
La2_xSrxCuO4_y
in
polycrystalline form was
found to exhibit onset T c temperatures in the 20 K range. Figure 14.15 shows the
dependence of the tetragonal-to-orthorhombic phase transition temperature on the
hole concentration of oxygen-deficient
La2_xSrxfuOa_y
(Birgeneau and Shirane,
1989). Figure 14.16 shows a schematic phase diagram of the SrO-La203-CuO
system at 980~ in air (Hahn
et al.,
1989). The precise solid solution limit has not
been determined. Extensive solid solutions were observed for quite a few phases.
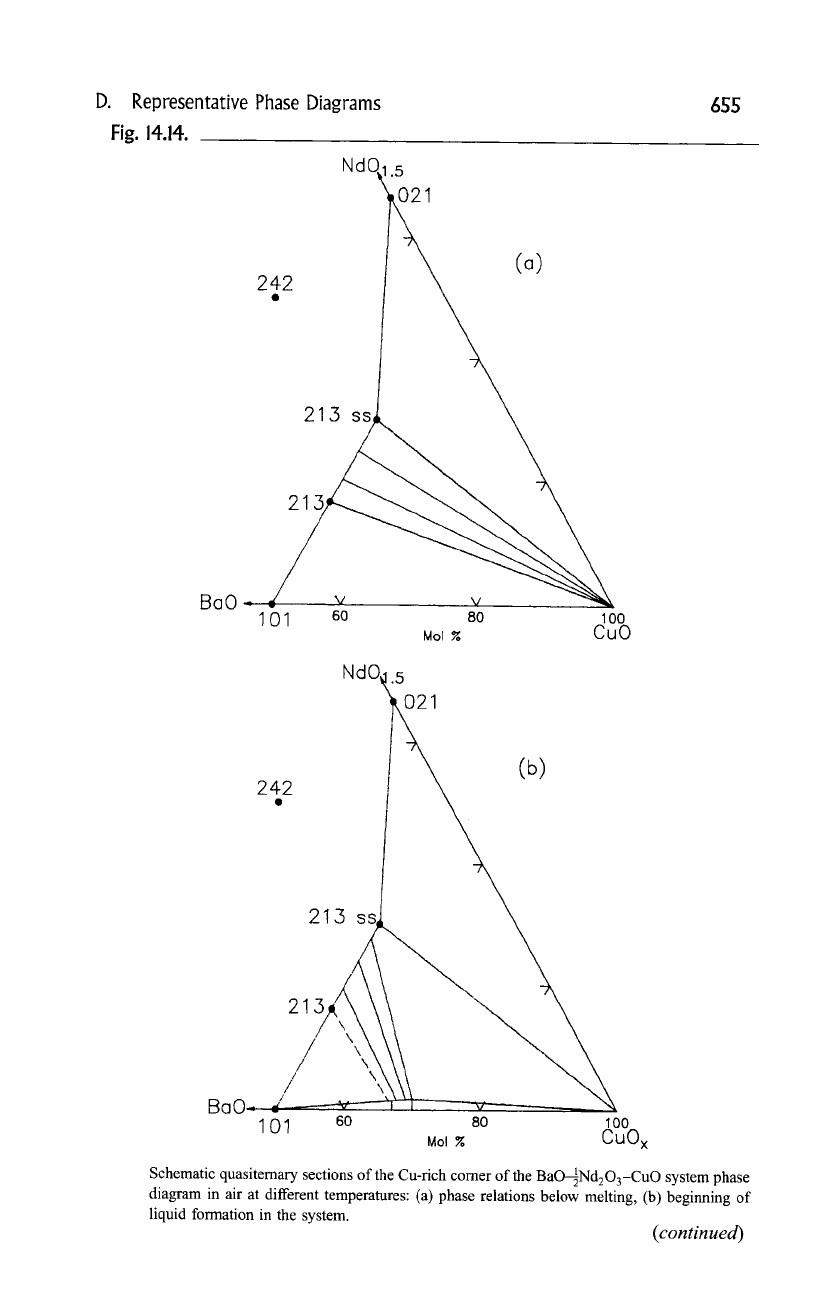
D.
Representative Phase Diagrams
Fig.
14.14.
655
NdO~.5
021
242
@
21
3/~-..~
BaO~ .~ v v .....
1 01 60 80 1 o0
Mol ~ CuO
BaC
242
@
(b)
213 s
213
/
/
/
\
101 60 80 1 O0
Mol
~ CuOx
Schematic quasiternary sections of the Cu-rich corner of the BaO-~Nd203-CuO system phase
diagram in air at different temperatures: (a) phase relations below melting, (b) beginning of
liquid formation in the system.
(continued)
