Schlick T. Molecular Modeling and Simulation: An Interdisciplinary Guide
Подождите немного. Документ загружается.

280 9. Force Fields
i
j
k
l
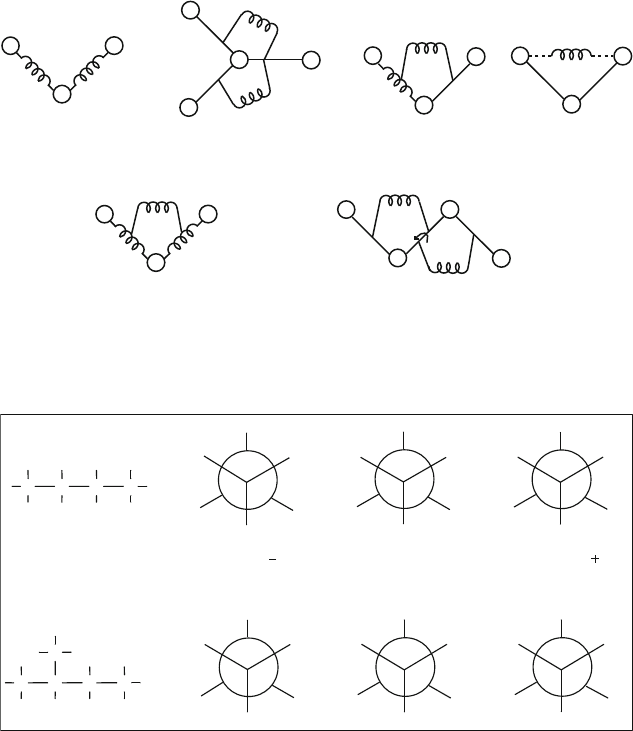
stretch/stretch
bond angle/dihedral/bond angle
stretch/bend/stretch
bend/bend
stretch/bend
UB
i
i
i
i
i
j
j
j
j
j
k
k
k
k
k
l
Figure 9.7. Schematic illustrations for various cross terms involving bond stretching, angle
bending, and torsional rotations.
n - butane
C
2-methylbutane
H
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
H
HH
H
H
H
H
H
H
H
H
H
H
H
H
HHH
H
H
gauche
trans
(anti)
gauche
CCC
CCCC
C
Figure 9.8. Torsional orientations for n-butane and 2-methylbutane, as illustrated by
Newman projections viewed along the central C–C bond. Note that while n-butane has
the global minimum at the trans (or anti) configuration, the gauche states of 2-methylbu-
tane are lower in energy than the corresponding trans (symmetrical) state since the methyl
groups are better separated and hence the molecule is less congested.
It is commonly used in molecular mechanics force fields, like CHARMM. The
potential is a simple harmonic function of the interatomic (not bond) distance ρ,
between atoms i and k in the bonded ijk sequence (see Figure 9.7):
E
ρ
(ρ)=S [ρ − ¯ρ] . (9.22)

9.5. Torsional Potentials 281
9.5 Torsional Potentials
9.5.1 Origin of Rotational Barriers
Torsional potentials are included in potential energy functions since all the
previously described energy contributions (nonbonded, stretching, and bending
terms) do not adequately predict the torsional energy of even a simple molecule
such as ethane. In ethane, the C–C bond provides an axis for internal rotation of
the two methyl groups.
Although the origin of the barrier to internal rotation has not been resolved
[472, 1010, 1360],
4
explanations involve the relief of steric congestion and opti-
mal achievement of resonance stabilization (another quantum-mechanical effect
arising from the interactions of electron orbitals) [1010]. This latter consideration
represents a newer hypothesis. For a long time, the primary interactions that give
rise to rotational barriers have been thought to be repulsive interactions, caused
by overlapping of bond orbitals of the two rotating groups [1002].
In ethane (C
2
H
6
), for example, the torsional strain about the C–C bond is
highest when the two methyl groups are nearest, as in the eclipsed or cis state,
and lowest when the two groups are optimally separated, as in the anti or trans
state (see Figure 9.8 and the related illustration for n-butane in Figure 3.13 of
Chapter 3). Without a torsional potential in the energy function, the eclipsed form
for ethane is found to be higher in energy than the staggered form, but not suf-
ficiently higher to explain the observed staggered preference [24]. See [27]fora
meticulous theoretical treatment of the torsional potential of butane, showing that
a large basis set combined with a treatment of electron correlations in ab initio
calculations is necessary to obtain accurate equilibrium energies relative to the
anti conformation.
Accounting accurately for rotational flexibility of atomic sequences is impor-
tant since rotational states affect biological reactivity. Such torsional parameters
are obtained by analyzing the energetic profile of model systems as a function of
the flexible torsion angle. The energy differences between rotational isomers are
used to parameterize appropriate torsional potentials that model internal rotation.
9.5.2 Fourier Terms
The general function used for each flexible torsion angle τ has the form
E
τ
(τ)=
n
V
n
2
[1 ± cos nτ] , (9.23)
where n is an integer. For each such rotational sequence described by the tor-
sion angle τ, the integer n denotes the periodicity of the rotational barrier, and
4
Goodman et al. [472] refer to the barrier origin as “the Bermuda Triangle of electronic the-
ory”, reflecting a complex interdependence among three factors: electronic repulsion, relaxation
mechanisms, and valence forces.

282 9. Force Fields
V
n
is the associated barrier height.Thevalueofn used for each such torsional
degree of freedom depends on the atom sequence and the force field parameteri-
zation (see below). Typical values of n are 1, 2, 3 (and sometimes 4). Other values
(e.g., n =5, 6) are used in addition by some force fields (e.g., CHARMM [805]).
A reference torsion angle τ
0
may also be incorporated in the formula, i.e.,
E
τ
(τ)=
n
V
n
2
[1 + cos(nτ −τ
0
)] . (9.24)
Often, τ
0
= 0orπ and thus the cosine expression of form (9.23) suffices; this is
because eq. (9.24) can be reduced to the form of eq. (9.23) in such special cases,
from relations like:
1+cos(nτ −π)=1− cos(nτ) .
Experimental data obtained principally by spectroscopic methods such as
NMR, IR (Infrared Radiation), Raman, and microwave, each appropriate for var-
ious spectral regions, can be used to estimate barrier heights and periodicities in
low molecular weight compounds. According to a theory developed by Pauling
[972], potential barriers to internal rotation arise from exchange interactions of
electrons in adjacent bonds; these barriers are thus similar for molecules with the
same orbital character. This theory has allowed tabulations of barrier heights as
class averages [871, 898, 1152, 1153]. Since barriers for rotations about various
single bonds in nucleic acids and proteins are not available experimentally, they
must be estimated from analogous chemical sequences in low molecular weight
compounds.
9.5.3 Torsional Parameter Assignment
In current force fields, these parameters are typically assigned by selecting several
classes of model compounds and computing energies as a function of the torsion
angle using ab initio quantum-mechanical calculations combined with geometry
optimizations. The final value assigned in the force field results from optimization
of the combined intramolecular and nonbonded energy terms to given experimen-
tal vibrational frequencies and measured energy differences between conformers
of model compounds.
This procedure often results in several Fourier terms in the form (9.23), that is,
several {n, V
n
} pairs for the same atomic sequence; see examples in Table 9.3.
Moreover, parameters for a given quadruplet of atoms may be deduced from
more than one quadruplet entry. For example, the quadruplet C1
–C2
–C3
–O3
in nucleic acid sugars may correspond to both a C1
–C2
–C3
–O3
entry and
a –C–C– entry, the latter designating a general rotation about the endocyclic
sugar bond; here, designates any atom. When general rotational sequences are
involved (e.g., –C–N–), CHARMM may list a pair of {V
n
,τ
0
} values (i.e.,
different τ
0
for the same rotational term); only one τ
0
is used when a specific
quadruplet atom sequence is specified.

9.5. Torsional Potentials 283
Twofold and Threefold Sums
Twofold and threefold potentials are most commonly used (see Figure 9.9 for
an illustration). Inclusion of a one-fold torsional term as well is a force-field
dependent choice.
A threefold torsional potential exhibits three maxima at 0
o
, 120
o
, and 240
o
and three minima at 60
o
, 180
o
, and 300
o
. Ethane has a simple 3-fold torsional
energy profile, as seen in the 3-fold energy curve in Figure 9.9, with all maxima
energetically equivalent and corresponding to the eclipsed or cis form, and all
minima corresponding to the energetically equivalent staggered or trans form.
In related hydrocarbon sequences, the local minima may not be of equal
energies. For n-butane, for example, the anti (or trans) conformer is roughly
1 kcal/mol lower in energy than the two gauche states. In 2-methylbutane, the
gauche states are lower in energy than the anti state due to steric effects. The
torsional conformations of these molecules are illustrated in Figure 9.8.
In general, the gauche forms may be higher in energy than the trans form, as
in n-butane [1153], or lower in energy, as in 2-methylbutane due to steric effects.
Certain X–C–C–Y atomic sequences where the X or Y atoms are electronega-
tive like O or F (e.g., 1-2-difluoroethane, certain O–C–C–O linkages in nucleic
acids) [532] also have lower-in-energy gauche conformations, but this is due to a
fundamentally different effect than present in hydrocarbons — neither steric, nor
dipole/dipole — called the gauche effect [532].
Reproduction of Cis/Trans and Trans/Gauche Energy Differences
A combination of a twofold and a threefold potential can be used to reproduce
both the cis/trans and trans/gauche energy differences. Figure 9.9 illustrates the
case for different parameter combinations of the twofold and threefold parame-
ters. One interaction is modeled after the O–C–C–O sequence in nucleic acids
(e.g., O3
–C3
–C2
–O2
in ribose) [937], showing a minimum at the trans state.
The other is a rotation about the phosphodiester bond (P–O) in nucleic acids,
showing a shallow minimum at the trans state. Note that for small molecules, tor-
sional potentials with n =1, 2, 3, 4 and 6 are often used to reproduce torsional
frequencies accurately.
To see how to combine twofold and threefold torsional potentials, for example,
denote the experimental energy barrier by ΔV , the total empirical potential en-
ergy function as E,andletE
τ
represent the following combination of twofold
and threefold torsional terms:
E
τ
=
V
2
2
[1 + cos 2τ]+
V
3
2
[1 + cos 3τ] . (9.25)
The torsional parameters V
2
and V
3
for a given rotation τ are then computed from
the relations:
ΔV
cis/trans
= E
τ =0
o
− E
τ =180
o
= V
3
+[E − E
τ
]
τ =0
o
− [E − E
τ
]
τ =180
o
,
(9.26)
284 9. Force Fields
ΔV
trans/gauche
= E
τ =180
o
− E
τ =60
o
=(3V
2
)/4+[E − E
τ
]
τ =180
o
− [E − E
τ
]
τ =60
o
,
(9.27)
since
V
3
= E
τ
τ =0
o
− E
τ
τ =180
o
and
3
4
V
2
= E
τ
τ =180
o
− E
τ
τ =60
o
.
Thus, Cartesian coordinates of the molecule must be formulated in terms of τ.
A simplification can be made by assuming fixed bond lengths and bond angles
and calculating nonbonded energy differences only. In theory, then, every differ-
ent parameterization of the nonbonded coefficients requires an estimation of the
torsional potentials V
2
and V
3
to produce a consistent set.
This general requirement in development of consistent force fields explains why
energy parameters are not transferable from one force field to another.
In general, many classes of model compounds must be used to represent
the various torsional sequence in proteins and nucleic acids. Since rotational
barriers in small compounds have little torsional strain energy compared with
large systems, rotational profiles are routinely computed for a series of substi-
tuted molecules. Substituted hydrocarbons are used, for example, to determine
rotational parameters for torsion angles about single C–C bonds in saturated
species.
50 100 150 200 250 300 350
0
0.5
1
1.5
2
2.5
3
O
P
O5'
C5'
50 100 150 200 250 300 350
0
1
2
3
4
O3'
C3'
C2'
O2'
2 fold
3 fold
sum
τ (dihedral angle) [deg.]
Dihedral angle energy [kcal/mol]
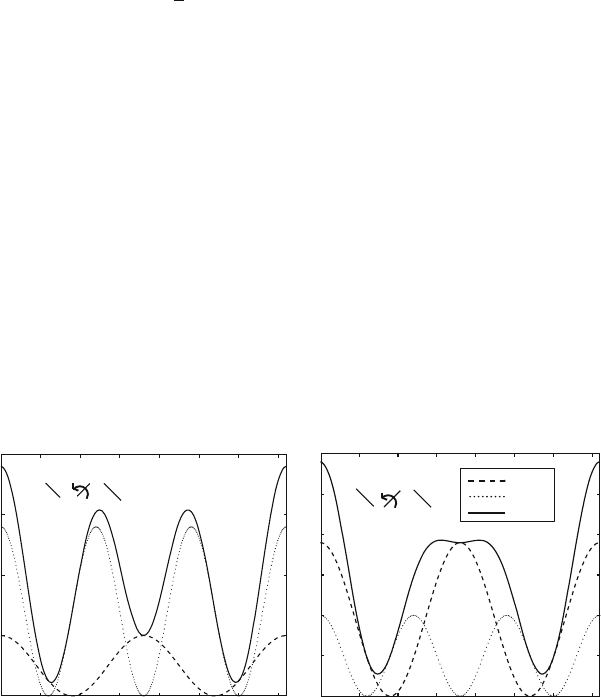
Figure 9.9. Twofold and threefold torsion-angle potentials and their sums for an O–C–C–O
rotational sequence in nucleic-acid riboses (V
2
= 1.0 and V
3
= 2.8 kcal/mol, reproducing
the known trans/gauche energy difference [937]) and a rotation about the phosphodiester
(P–O) bond in nucleic acids (V
2
= 1.9 and V
3
= 1.0 kcal/mol, from CHARMM [805]).

9.5. Torsional Potentials 285
Model Compounds
Examples of model compounds used for assigning torsional parameters to nucleic
acids and proteins include the following (see Figure 9.10).
• Hydrocarbons like ethane, propane,andn-butane, and the more crowded
environments of 2-methylbutane and cyclohexane: for rotations about sin-
gle C–C bonds in saturated species (i.e., each carbon is approximately
tetrahedral), such as C–C–C–C, H–C–C–H, and H–C–C–C;
• The ethylbenzene ring: for rotations about CA–C bonds in ring systems
(CA denotes an aromatic carbon);
• Alcohols like methanol and propanol: to model H–C–O–H, C–C–O–H,
H–C–C–O, and C–C–C–O sequences (e.g., in the amino acids serine and
threonine);
C
2
H
6
Ethane
C
3
H
8
Propane
C
4
H
10
n-Butane
C
5
H
12
2-Methylbutane
C
6
H
12
Cyclohexane
C
8
H
10
Ethylbenzene
CH
3
OH
Methanol
CH
3
CH
2
CH
2
OH
Propanol
C
C
C
C
C
C
C
C
CC
C
CC
C
C
OC
C
SCCC
CH
3
SCH
2
CH
2
CH
3
Methyl Propyl Sulfide
CSC
CH
3
SCH
3
Dimethyl Sulfide
NC
NH
2
CH
3
Methylamine
CC
CH
3
CHO
Ethanal (acetaldehyde)
CNC
CH
3
NHCHO
n-Methyl formamide
O
O
CCC
O
CC
C
CC
C
C
CCCC
CC
Figure 9.10. Model compounds for determining torsional parameters for various atomic
sequences in biomolecules. Illustrated in bold is τ : the bond about which the τ rotation
occurs, or the complete τ sequence (3 bonds), as needed for an unambiguous definition.
286 9. Force Fields
• Sulfide molecules (e.g., methyl propyl sulfide): to model rotations
involving sulfur atoms (e.g., C–C–C–S, C–C–S–C, and H–C–S–C) as in
methionine, or dimethyl disulfide (C–S–S–C), to model disulfide bonds in
proteins;
• Model amine, aldehydes, and amides (e.g., methylamine, ethanal,
N-methyl formamide): for sequences involving nitrogens such as
H–C–N–H, H–C–C=O, and H–C–N–C.
See [842] for a comprehensive description of such a parameterization for peptides
based on ab initio energy profiles.
In the CHARMM and AMBER force fields, the parameters n and V
n
are
highly environment dependent. Furthermore, more than one potential form can
be used for the same bond about which rotation occurs; these different torsion-
angle terms may be weighted proportionately. Some examples of torsion angle
parameters from the AMBER [265] and CHARMM [805, 809]forcefieldsare
given in Table 9.3.
Table 9.3. Parameters for selected torsional potentials from the AMBER (first row for each
sequence) [218] and CHARMM (second row of that sequence) [415, 805]forcefields.
Barrier heights are in units of kcal/(mol rad
2
), angles are in radians, and represents
any atom.
SEQUENCE
V
1
2
τ
0
V
2
2
τ
0
V
3
2
τ
0
DESCRIPTION
–C–C– 1.4 0 alkane C–C (e.g., Lys
0.2 0 or Leu C
α
–C
β
–C
γ
–C
δ
)
O4
–C1
–N9–C8 2.5 0 purine glycosyl
1.1 0 C1
–N9 rotation (A, G)
C–C–S–C 1.0 0 rotation about C–S
0.24 π 0.37 0 in Met
O3
–P–O5
–C5
1.2 0 0.5 0 P–O5
rotation in
1.2 π 0.1 π 0.1 π nucleic-acid backbone
9.5.4 Improper Torsion
Harmonic ‘improper torsion’ terms, also known as ‘out-of-plane bending’ poten-
tials, are often used in addition to the terms described above to improve the overall
fit of energies and geometries. They can enforce planarity or maintain chirality
about certain groups. The potential has the form
E
χ
(χ)=(V
/2) χ
2
, (9.28)
9.5. Torsional Potentials 287
i
j
k
l
i
j
j
l
i
k
’
’
’
Wilson angle
improper/improper
Figure 9.11. Wilson angle definition (left) and geometry for a cross improper/improper
torsion term (right).
where χ is the improper Wilson angle. It is defined for the four atoms i, j, k, l for
which the central j is bonded to i, l, and k (see Figure 9.11) as the angle between
bond j–l and the plane i–j–k.
The following are examples of atom centers used to define sequences of atoms
counted in improper torsion potentials (taken from CHARMM):
• Polypeptide backbone nitrogen and carbonyl carbons attached to the C
α
atom (i.e., N and C in the sequence –N–C
α
–C=O), for maintaining
planarity of the groups around the peptide bond;
• Terminal side-chain carbons in the CO
2
unit of the Asp and Glu amino
acids;
• Terminal side-chain carbons in the O=C–NH
2
unit of the Asn and Gln
amino acids;
• Terminal side-chain carbon in the NH
2
–C–NH
2
unit of the Arg amino acid;
• Various carbons and nitrogens in the side-chain rings of the Phe; Tyr, and
Trp amino acids (not used in all CHARMM versions);
• Glycosyl nitrogen (N1 in pyrimidines or N9 in purines) in nucleic acid
bases;
• Exocyclic nitrogen of the NH
2
unit attached to the aromatic ring of the
adenine and cytosine bases in nucleic acids (N6 in adenine and N4 in
cytosine).
9.5.5 Cross Dihedral/Bond Angle and Improper/Improper
Dihedral Terms
Cross terms are used in certain force fields (e.g., CVFF used in the In-
sight/Discover program) to model various relationships between quadruplet
sequences (see Figure 9.11) or to couple torsion angles with bond angles or torsion
angles with bond lengths (see Figure 9.7). For example, the association between
a torsion angle and related bond angles can take the form
E
τθθ
(τ,θ,θ
)=KV
τθθ
cos τ [θ −
¯
θ][θ
−
¯
θ
] . (9.29)

288 9. Force Fields
This kind of potential couples the two bending motions (e.g., symmetric and
antisymmetric wagging of the two methyl groups in ethane) and has an important
affect on spectra (vibrational frequencies). A cross term that relates the dihedral
angle to one bond angle can, however, have instead a large effect on geometry
(with a small effect on spectra):
E
τθ
(τ,θ)=KV
τθ
cos τ [θ −
¯
θ] . (9.30)
(More generally, cos τ above may be replaced by a Fourier series in the form of
eq. (9.23)oreq.(9.24)).
This torsion/bend effect may be important to reproduce fine features like a
small C–C–H bond-angle opening in the eclipsed configuration in ethane relative
to the staggered configuration.
Torsion/stretch potentials can similarly be used for fine tuning, for exam-
ple to reproduce a bond-stretching tendency for ethane in the eclipsed torsional
configuration (relative to the staggered-configuration bond length) [30].
The association between neighboring Wilson angles, in which the two centers
(j and j
) are bonded, can be represented as:
E
χχ
(χ, χ
)=V
χχ
χχ
. (9.31)
9.6 The van der Waals Potential
9.6.1 Rapidly Decaying Potential
The van der Waals potential for a nonbonded distance r
ij
has the common 6/12
Lennard-Jones form in macromolecular force fields:
E
LJ
(r
ij
)=
−A
ij
r
6
ij
+
B
ij
r
12
ij
, (9.32)
where the attractive and repulsive coefficients A and B depend on the type of
the two interacting atoms. The attractive r
−6
term originates from quantum me-
chanics, and the repulsive r
−12
term has been chosen mainly for computational
convenience. Together, these terms mimic the tendency of atoms to repel one an-
other when they are very close and attract one another as they approach an optimal
internuclear distance (Figure 9.12).
As mentioned in the last chapter, the 6/12 Lennard Jones potential represents
a compromise between accuracy and computational efficiency; more accurate fits
over broader ranges of distances are achieved with the Buckingham potential [30,
766]: −A
ij
/r
6
ij
+ B
ij
exp(−B
ij
r
ij
).
The steep repulsion has a quantum origin in the interaction of the electron
clouds with each other, as affected by the Pauli-exclusion principle, and this
combines with internuclear repulsions.

9.6. The van der Waals Potential 289
The weak bonding attraction is due to London or dispersion force (in quantum
mechanics this corresponds to electron correlation contributions). Namely, the
rapid fluctuations of the electron density distribution around a nucleus create a
transient dipole moment and induce charge reorientations (dipole-induced dipole
interactions), or London forces.
Note that as r →∞, E
LJ
→ 0 rapidly, so the van der Waals force is short
range. For computational convenience, van der Waals energies and forces can be
computed for pairs of atoms only within some “cutoff radius”.
9.6.2 Parameter Fitting From Experiment
Parameters for the van der Waals potential can be derived by fitting parameters
to lattice energies and crystal structures, or from liquid simulations so as to re-
produce observed liquid properties [265]. From crystal data, minimum contact
distances {r
i
}of atom radii (see below) can be obtained. Liquid simulations, such
as by Monte Carlo, are typically performed on model liquid systems (e.g., ethane
and butane) to adjust empirical minimum contact distances {r
i
} (see below) so as
to reproduce densities and enthalpies of vaporization of the liquids.
9.6.3 Two Parameter Calculation Protocols
To determine the attractive and repulsive coefficients for each {ij} pair, two main
procedures can be used for each different type of pairwise interaction (e.g., C–C,
C–O).
Energy Minimum/Distance Procedure (V
ij
,r
0
ij
)
In the first approach, coefficients can be obtained by requiring that an energy min-
imum V
ij
will occur at a certain distance, r
0
ij
, equal to the sum of van der Waals
radii of atoms i and j. This requirement between the minimum energy and
distances produces the relations:
A
ij
=2(r
0
ij
)
6
V
ij
(9.33)
and
B
ij
=(r
0
ij
)
12
V
ij
. (9.34)
The latter equation can be written using eq. (9.33)intermsofA
ij
and r
0
ij
as
B
ij
=
A
ij
2
(r
0
ij
)
6
. (9.35)
The van der Waals radii can be calculated from the measured X-ray contact
distances — which reflect the distance of closest approach in the crystal. These
contact distances are appreciably smaller than the sum of the van der Waals radii
of the atoms involved. This relationship — between the X-ray contact distances,
which crystallographers refer to as “van der Waals radii”, and the molecular
mechanics meaning of van der Waals radii — was recognized in the early days of
molecular mechanics [28,1345] but still may not be widely appreciated.
