Townsend C.R., Begon M., Harper J.L. Essentials of Ecology
Подождите немного. Документ загружается.

Chapter 8 Evolutionary ecology
269
0.0
1.0
0.8
0.6
0.4
0.2
51
Reef
234
0.0
1.0
0.8
0.6
0.4
0.2
0.0
1.0
0.8
0.6
0.4
0.2
Cleaner fish
No cleaner fish
(b)
(a)
(c)
Gnathiids per fishGnathiids per fishGnathiids per fish
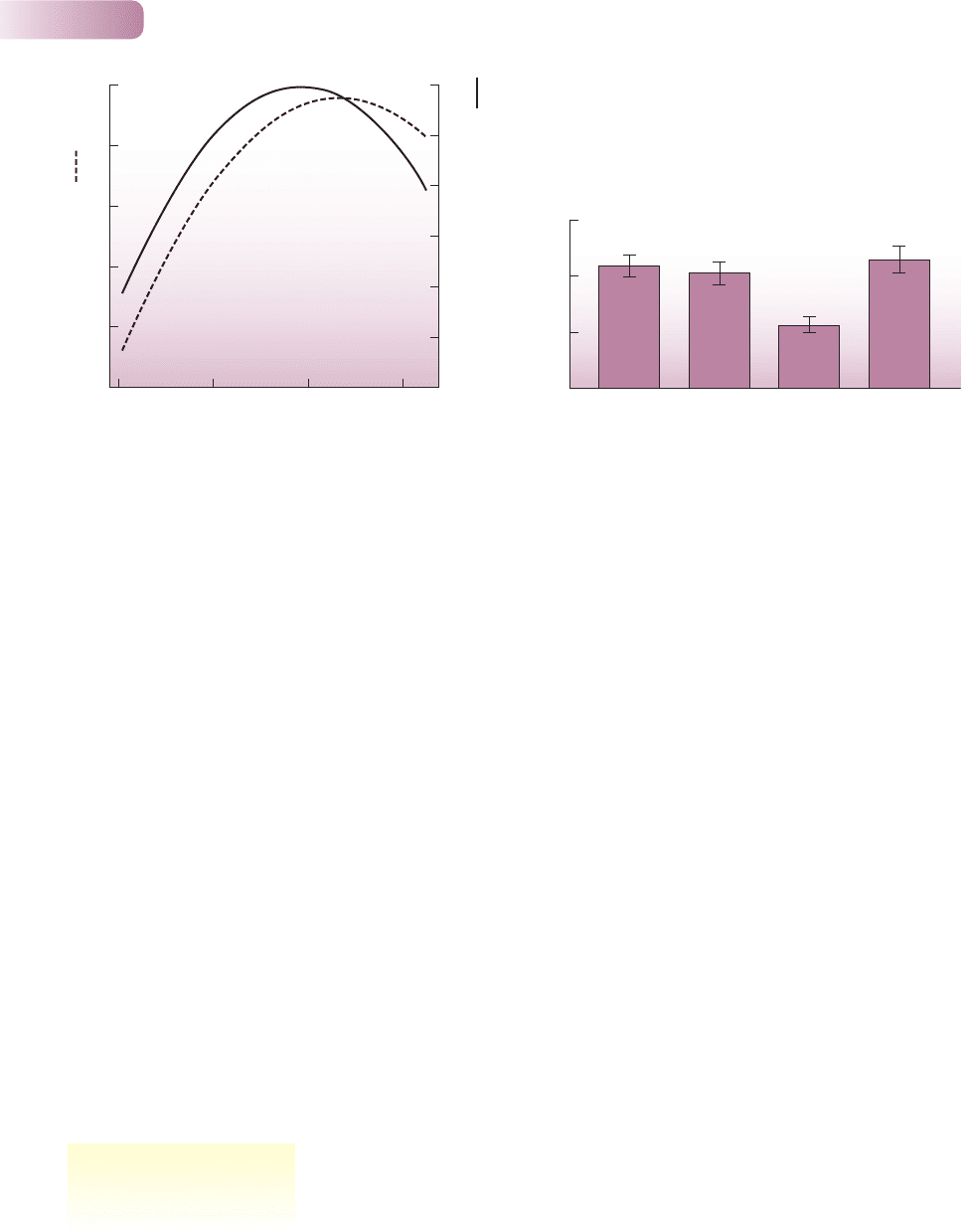
Figure 8.9
Cleaner fish really do clean their clients. The
mean number of gnathiid parasites per client,
Hemigymnus melapterus, at five reefs, from
three of which cleaners, Labroides dimidiatus,
were experimentally removed. (a) In a long-term
experiment, clients without cleaners had more
parasites after 12 days (F = 17.6, P = 0.02).
(b) In a short-term experiment, clients without
cleaners did not have significantly more parasites
at dawn after 12 hours (F = 1.8, P = 0.21),
presumably because cleaners do not feed at night.
(c) However, the difference was significant after a
further 12 hours of daylight (F = 11.6, P = 0.04).
Bars are standard errors.
AFTER GRUTTER, 1999
(a) (b)
ant–plant mutualisms...
The idea that there are mutualistic, ‘protective’ relationships between plants
and ants was put forward by Belt (1874) after observing the behavior of aggressive
ants on species of Acacia with swollen thorns in Central America. For example,
the Bull’s horn acacia (Acacia cornigera) bears hollow thorns that are used by
its associated ant, Pseudomyrmex ferruginea, as nesting sites (Figure 8.10b); its
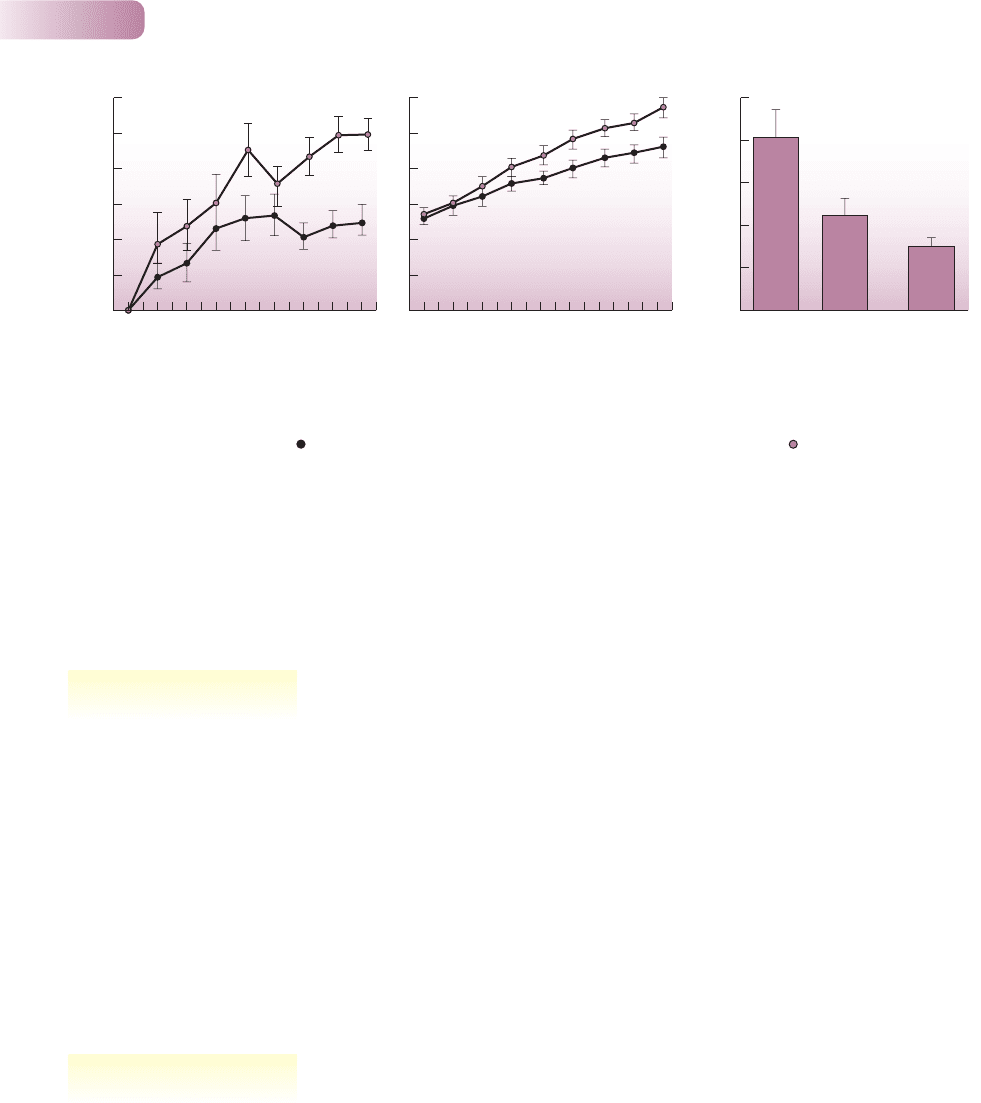
Figure 8.10
Structures of the Bull’s horn acacia
(Acacia cornigera) that attract its ant
mutualist. (a) Protein-rich Beltian bodies at
the tips of the leaflets. (b) Hollow thorns
used by the ants as nesting sites.
(a) © MICHAEL FOGDEN, OXFORD SCIENTIFIC FILMS IHY360FOM00201; (b) © C. P. HICKMAN, VISUALS UNLIMITED
9781405156585_4_008.qxd 11/5/07 14:54 Page 269
leaves have protein-rich ‘Beltian bodies’ at their tips (Figure 8.10a) which the ants
collect and use for food; and it has sugar-secreting nectaries on its vegetative parts
that also attract the ants. The ants, for their part, protect these small trees from
competitors by actively snipping off shoots of other species and also protect the
plant from herbivores – even large (vertebrate) herbivores may be deterred.
In fact, ant–plant mutualisms appear to have evolved many times (even repeatedly
in the same family of plants); and nectaries are present on the vegetative parts
of plants of at least 39 families and in many communities throughout the world.
Their precise role is not easy to establish. They clearly attract ants, sometimes in
vast numbers, but carefully designed and controlled experiments are necessary to
show that the plants themselves benefit, such as a study of the Amazonian canopy
tree Tachigali myrmecophila, which harbors the stinging ant Pseudomyrmex
concolor in specialized hollowed-out structures (Figure 8.11). The ants were
removed from selected plants. These then bore 4.3 times as many phytophagous
insects as control plants and suffered much greater herbivory, such that leaves
on plants that carried a population of ants lived more than twice as long as those
on unoccupied plants and nearly 1.8 times as long as those on plants from which
ants had been deliberately removed.
8.4.2 The culture of crops or livestock
At least in terms of geographic extent, some of the most dramatic mutualisms are
those of human agriculture. The numbers of individual plants of wheat, barley,
oats, corn and rice, and the areas these crops occupy, vastly exceed what would
have been present if they had not been brought into cultivation. The increase
in the human population since the time of hunter–gatherers is some measure of
the reciprocal advantage to Homo sapiens. Even without doing the experiment,
we can easily imagine the effect the extinction of humans would have on the
world population of rice plants or the effect of the extinction of rice plants on the
Part III Individuals, Populations, Communities and Ecosystems
270
Bottom leaves
Leaf longevity (months)
0
20
60
40
100
(b)
80
Control
(20)
Unoccupied
(17)
Experimental
(22)
Herbivory level
0.0
0.5
1.5
3.0
1988
2.5
2.0
1.0
0.0
0.5
1.5
3.0
2.5
2.0
1.0
19901989
Date
(a) Top leaves
SNJMMJSNJ
1988 19901989
Date
SNJMMJSNJ
Treatments
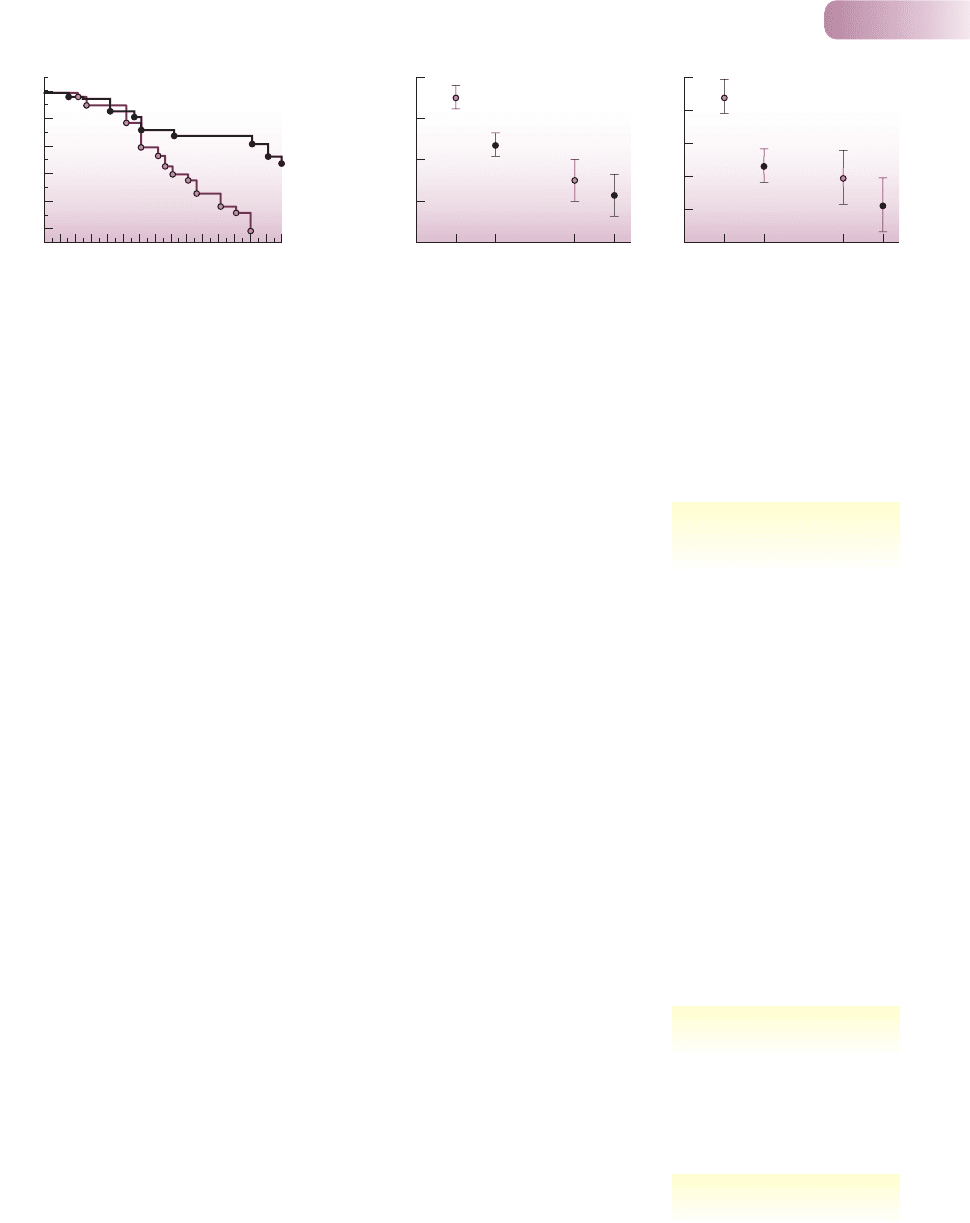
Figure 8.11
(a) The intensity of leaf herbivory (based on the cumulative proportion of leaf area removed) on plants of Tachigali myrmecophila naturally occupied
by the ant Pseudomyrmex concolor (, n = 22) and on plants from which the ants had been experimentally removed ( , n = 23). Bottom leaves
were those present at the start of the experiment and top leaves were those emerging subsequently. (b) The longevity of leaves on plants of T.
myrmecophila occupied by P. concolor (control) and from which ants were experimentally removed or from which ants were naturally absent.
Error bars ± SE.
AFTER FONSECA, 1994
. . . but do the plants benefit?
human agriculture
9781405156585_4_008.qxd 11/5/07 14:54 Page 270
population of humans. Similar comments apply to the domestication of cattle,
sheep and other mammals.
Similar ‘farming’ mutualisms have developed in termite and especially ant
societies, where the farmers may protect individuals they exploit from competitors
and predators and may even move or tend them. Ants, for example, farm many
species of aphids (homopterans) in return for sugar-rich secretions of honeydew.
The ‘flocks’ of aphids benefit through suffering lower mortality rates caused
by predators, showing increased feeding and excretion rates, and forming larger
colonies; but it would be wrong to imagine that this is a cosy relationship with
nothing but benefits on both sides: the aphids are being manipulated – is there
a cost to be entered on the other side of the balance sheet? This question has
been addressed for colonies of the aphid Tuberculatus quercicola attended by the
red wood ant Formica yessensis on the island of Hokkaido, northern Japan
(Figure 8.12). As expected, in the presence of predators, aphid colonies survived
significantly longer when attended by ants than when ants were excluded by
smearing ant repellent at the base of the oak trees on which the aphids lived
(Figure 8.12a). However, there were also costs for the aphids: in an environment
from which predators were excluded, and the effects of ant attendance on aphids
could thus be viewed in isolation, ant-attended aphids grew less well and were less
fecund than those where ants as well as predators were excluded (Figure 8.12b).
8.4.3 The dispersal of seeds and pollen
Very many plant species use animals to disperse their seeds and pollen. About
10% of all flowering plants possess seeds or fruits that bear hooks, barbs or glues
that become attached to the hairs, bristles or feathers of any animal that comes
into contact with them. They are frequently an irritation to the animal, which
often cleans itself and removes them if it can, but usually after carrying them some
distance. In these cases the benefit is to the plant (which has invested resources
in attachment mechanisms) and there is no reward to the animal.
Quite different are the true mutualisms between higher plants and the birds
and other animals that feed on fleshy fruits and disperse the seeds. Of course,
for the relationship to be mutualistic, it is essential that the animal digests only
Chapter 8 Evolutionary ecology
271
Survival rate
(a)
1002468
Days after the start of experiments
14 18 30 1 1 1122 2222 26
Ant attended
Ant excluded
0.42
0.50(b)
Season Season
0.48
0.44
0.46
10
15
13
11
12
14
1.0
0.8
0.6
0.4
0.2
0
Average hind femur
length (mm)
Average number of embryos
AFTER YAO ET AL., 2000
Figure 8.12
(a) Ant-excluded colonies of the aphid Tuberculatus quercicola were more likely to become extinct than those attended by ants (χ
2
= 15.9,
P < 0.0001). (b) But in the absence of predators (experimentally removed), ant-excluded colonies performed better than those attended by ants.
Shown are averages for aphid body size (hind femur length; F = 6.75, P = 0.013) and numbers of embryos (F = 7.25, P = 0.010), ± SE, for
two seasons (1: July 23 to August 11, 1998; 2: August 12 to August 31, 1998). Maroon circles, predator-free and ant-excluded treatment;
black circles, predator-free and ant-attended treatment.
aphids farmed by ants:
do they pay a price?
seed dispersal
fruits
9781405156585_4_008.qxd 11/5/07 14:54 Page 271

the fleshy fruit and not the seeds, which must remain viable when regurgitated
or defecated. Thick strong defenses that protect plant embryos are usually part
of the price paid by the plant for dispersal by fruit-eaters.
Many different kinds of animals have entered into pollination liaisons with
flowering plants, including humming-birds, bats and even small rodents and
marsupials (Figure 8.13). Most animal-pollinated flowers offer nectar, pollen or
Part III Individuals, Populations, Communities and Ecosystems
272
(a)
(b)
pollination
Figure 8.13
Pollinators. (a) Honeybee (Apis mellifera) on raspberry
flowers. (b) Cape sugarbird (Promerops cafer) feeding
on Protea eximia.
© H. ANGEL, NATURAL VISIONS XXIN_007_0031XX_L, AV_0258_0004
9781405156585_4_008.qxd 11/5/07 14:55 Page 272

both as a reward to their visitors. Floral nectar seems to have no value to the
plant other than as an attractant to animals and it has a cost to the plant, because
the nectar carbohydrates might have been used in growth or some other activity.
Presumably, the evolution of specialized flowers and the involvement of animal
pollinators have been favored because an animal may be able to recognize and
discriminate between different flowers and so move pollen between different
flowers of the same species but not to flowers of other species. Passive transfer of
pollen, for example by wind or water, does not discriminate in this way and is
therefore much more wasteful. On the other hand, where the vectors and flowers
are highly specialized, as is the case in many orchids, virtually no pollen is wasted
even on the flowers of other species.
The pollinators par excellence are, without doubt, the insects. Pollen is a
nutritionally-rich food resource and in the simplest insect-pollinated flowers,
pollen is offered in abundance and freely exposed to all and sundry. The plants
rely for pollination on the insects being less than wholly efficient in their pollen
consumption, carrying their spilt food with them from plant to plant. In more
complex flowers, nectar (a solution of sugars) is produced as an additional or
alternative reward. In the simplest of these, the nectaries are unprotected, but,
with increasing specialization, nectaries are enclosed in structures that restrict
access to the nectar to just a few visitor species. This range can be seen within the
family Ranunculaceae. In the simple flower of Ranunculus ficaria the nectaries
are exposed to all visitors, but in the more specialized flower of R. bulbosus there
is a flap over the nectary, and in Aquilegia the nectaries have developed into
long tubes and only visitors with long probosces (tongues) can reach the nectar.
Unprotected nectaries have the advantage of a ready supply of pollinators, but
because these pollinators are unspecialized they transfer much of the pollen to
the flowers of other species. Protected nectaries have the advantage of efficient
transfer of pollen by specialists to other flowers of the same species, but are reliant
on there being sufficient numbers of these specialists.
Charles Darwin (1859) recognized that a long nectary, as in Aquilegia, forced
a pollinating insect into close contact with the pollen at the nectary’s mouth.
Natural selection may then favor even longer nectaries, and as an evolutionary
reaction, the tongues of the pollinator would be selected for increasing length:
reciprocal coevolution. Nilsson (1988) deliberately shortened the nectary tubes
of the long-tubed orchid Platanthera and showed that the flowers then produced
many fewer seeds – presumably because the pollinator was not forced into a
position that maximized the efficiency of pollination.
8.4.4 Mutualistic gut inhabitants
Most of the mutualisms discussed so far have depended on patterns of behavior,
where neither species lives entirely ‘within’ its partner. In many other mutualisms,
one of the partners is a unicellular eukaryote or bacterium that is integrated more
or less permanently into the body cavity or even the cells of its multicellular
partner. The microbiota occupying parts of various animals’ alimentary canals
are the best known extracellular symbionts.
The crucial role of microbes in the digestion of cellulose by vertebrate herbivores
has long been appreciated, but it now appears that the gastrointestinal tracts of
all vertebrates are populated by a mutualistic microbiota. Protozoa and fungi are
Chapter 8 Evolutionary ecology
273
insect pollinators: from
generalists to ultraspecialists
the vertebrate gut
9781405156585_4_008.qxd 11/5/07 14:55 Page 273

usually present but the major contributors to these ‘fermentation’ processes are
bacteria. Their diversity is greatest in regions of the gut where the pH is relatively
neutral and food retention times relatively long. In small mammals (e.g. rodents,
rabbits, hares), the cecum is the main fermentation chamber, whereas in larger
non-ruminant mammals such as horses the colon is the main site. In ruminants,
like cattle and sheep, and in kangaroos and other marsupials, fermentation occurs
in specialized stomachs (see Figure 3.24).
The basis of the mutualism is straightforward. The microbes receive a steady
flow of substrates for growth in the form of food that has been eaten, chewed
and partly homogenized. They live within a chamber in which the pH and, in
endotherms, temperature are regulated and anaerobic conditions are maintained.
The vertebrate hosts, especially the herbivores, receive nutrition from food that
they would otherwise find, literally, indigestible. The bacteria produce short-chain
fatty acids (SCFAs) by fermentation of the host’s dietary cellulose and starches
and of the endogenous carbohydrates contained in host mucus and sloughed
epithelial cells. SCFAs are often a major source of energy for the host: for example,
they provide more than 60% of the maintenance energy requirements for cattle
and 29–79% of those for sheep (Stevens & Hume, 1998). The microbes also con-
vert nitrogenous compounds (amino acids that escape absorption in the midgut,
urea that would otherwise be excreted by the host, mucus and sloughed cells)
into ammonia and microbial protein, conserving nitrogen and water; and they
synthesize B vitamins. The microbial protein is useful to the host if it can be
digested – in the intestine by foregut fermenters and following coprophagy (eating
their own feces) in hindgut fermenters – but ammonia is usually not useful and
may even be toxic to the host.
8.4.5 Mycorrhizas
Most higher plants do not have roots, they have mycorrhizas – intimate mutualisms
between fungi and root tissue. Plants of only a few families, such as the Cruciferae,
are exceptions. Broadly, the fungal networks in mycorrhizas capture nutrients from
the soil, which they transport to the plants in exchange for carbon. Many plant
species can live without their mycorrhizal fungi in soils where neither nutrients
nor water are ever limiting, but in the harsh world of natural plant communities,
the symbioses, if not strictly obligate, are nonetheless ‘ecologically obligate’: that
is, necessary if the individuals are to survive in nature (Buscot et al., 2000).
Generally, three major types of mycorrhiza are recognized. Arbuscular
mycorrhizas are found in about two-thirds of all plant species, including most
non-woody species and tropical trees. Ectomycorrhizal fungi form symbioses with
many trees and shrubs, dominating boreal and temperate forests and also some
tropical rain forests. Finally, ericoid mycorrhizas are found in the dominant plant
species of heathland.
In ectomycorrhizas (ECMs), infected roots are usually concentrated in the
litter layer of the soil. Fungi form a sheath of varying thickness around the roots.
From there, hyphae radiate into the litter layer, extracting nutrients and water
and also producing large fruiting bodies that release enormous numbers of
wind-borne spores. The fungal mycelium also extends inward from the sheath,
penetrating between the cells of the root cortex to give intimate cell-to-cell con-
tact with the host and establishing an interface with a large surface area for the
Part III Individuals, Populations, Communities and Ecosystems
274
ectomycorrhizas
9781405156585_4_008.qxd 11/5/07 14:55 Page 274
exchange of the products of photosynthesis, soil water and nutrients between
the host plant and its fungal partner.
The ECM fungi are effective in extracting the sparse and patchy supplies
of phosphorus and especially nitrogen from the forest litter layer. Carbon flows
from the plant to the fungus, very largely in the form of simple hexose sugars:
glucose and fructose. Fungal consumption of these may represent up to 30% of
the plants’ net rate of photosynthate production. The plants, though, are often
nitrogen-limited, since in forest litter there are low rates of nitrogen mineraliza-
tion (conversion from organic to inorganic forms), and inorganic nitrogen is itself
mostly available as ammonia. It is therefore crucial for forest trees that ECM
fungi can access organic nitrogen directly through enzymic degradation, and
utilize ammonium as a preferred source of inorganic nitrogen. Nonetheless, the
idea that this relationship between the fungi and their host plants is mutually
exploitative rather than ‘cosy’ is emphasized by its responsiveness to changing
circumstances. ECM growth is directly related to rate of flow of hexose sugars
from the plant. But when the direct availability of nitrate to the plants is high,
either naturally or through artificial supplementation, plant metabolism is directed
away from hexose production (and export) and towards amino acid synthesis.
As a result the ECM degrades: the plants seem to support just as much ECM as
they appear to need.
Arbuscular mycorrhizas (AMs) do not form a sheath but penetrate within
the roots of the host. Roots become infected from mycelium present in the soil
or from germ tubes that develop from asexual spores, which are very large and
produced in small numbers: a striking contrast with the ECM fungi. Initially, the
fungus grows between host cells but then enters them and forms a finely branched
intracellular ‘arbuscule’.
There has been a tendency to emphasize facilitation of the uptake of phosphorus
as the main benefit to plants from AM symbioses (phosphorus is a highly immobile
element in the soil, and is therefore frequently limiting to plant growth), but the
truth appears to be more complex than this, with benefits demonstrated, too, in
nitrogen uptake, pathogen and herbivore protection, and resistance to toxic metals
(Newsham et al., 1995). Certainly, there are cases where the inflow of phosphorus
is strongly related to the degree of colonization of roots by AM fungi. This has
been shown for the bluebell, Hyacinthoides non-scripta, as colonization pro-
gresses during its phase of subterranean growth from August to February through
to its above-ground photosynthetic phase thereafter (Figure 8.14a). Indeed, blue-
bells cultured without AM fungi are unable to take up phosphorus through their
poorly branched system of roots (Merryweather & Fitter, 1995).
On the other hand, a set of experiments examined the growth of the annual
grass Vulpia ciliata ssp. ambigua (Figure 8.14b) in which seedlings of Vulpia were
grown with an AM fungus (Glomus sp.), with the pathogenic fungus Fusarium
oxysporum, with both, and with neither. Growth was not enhanced by Glomus
alone, but growth was harmed by Fusarium in the absence of Glomus. When
both were present, growth returned to normal levels. Clearly, the mycorrhiza did
not benefit the phosphorus economy of the Vulpia, but it did protect it from the
harmful effects of the pathogen.
The key difference appears to be that Vulpia, unlike the bluebell, has a highly
branched system of roots (Newsham et al., 1995). Plants with finely branched roots
have little need for supplementary phosphorus capture, but development of that
Chapter 8 Evolutionary ecology
275
arbuscular mycorrhizas
a range of benefits?
it depends on the species
9781405156585_4_008.qxd 11/5/07 14:55 Page 275
same root architecture provides multiple points of entry for plant pathogens. In
such cases AM symbioses are therefore likely to have evolved with an emphasis on
plant protection. By contrast, root systems with few lateral and actively growing
meristems are relatively invulnerable to pathogen attack, but these root systems
are poor foragers for phosphorus. Here, AM symbioses are likely to have evolved
with an emphasis on phosphorus capture.
8.4.6 Fixation of atmospheric nitrogen in
mutualistic plants
The inability of most plants and animals to fix atmospheric nitrogen is one of
the great puzzles in the process of evolution, since nitrogen is in limiting supply
in many habitats. However, the ability to fix nitrogen is widely though irregu-
larly distributed amongst both the eubacteria (‘true’ bacteria) and the archaea
(Archaebacteria), and many of these have been caught up in tight mutualisms
with distinct groups of eukaryotes. The best known, because of the huge agri-
cultural importance of legume crops, are the rhizobia, which fix nitrogen in
the root nodules of most leguminous plants and just one non-legume, Parasponia
(a member of the family Ulmaceae, the elms).
The establishment of the liaison between rhizobia and legume plants proceeds
by a series of reciprocating steps. The bacteria occur in a free-living state in the
soil and are stimulated to multiply by root exudates and cells that have been
sloughed from roots as they develop. In a typical case, a bacterial colony develops
Part III Individuals, Populations, Communities and Ecosystems
276
Sept 1
–1
1
2(a)
0
60
50
40
30
20
10
0
–2
Dec 1 Mar 1 Jun 1
(b)
*
–Fus
–Glm
0
200
300
100
–Fus +Fus
–Glm
+Fus
+GlmDate +Glm
Mean root length (cm)
P inflow (pmol m
–1
s
–1
) ( )
Percentage root length colonized by AM fungi ( )
Figure 8.14
(a) Curves fitted to rates of phosphorus inflow (dashed line, left axis) and root colonization by arbuscular mycorrhiza (AM) fungi (solid line, right
axis) in the bluebell, Hyacinthoides non-scripta, over a single growing season. Phosphorus uptake appears to be strongly linked to root colonization
by the fungi. (b) The effects of a factorial combination of Fusarium oxysporum (Fus, a pathogenic fungus) and an AM fungus, Glomus sp. (Glm) on
growth (root length) of Vulpia plants. Values are means of 16 replicates per treatment; bars are standard errors; the asterisk signifies a significant
difference at P < 0.05 in a Fisher’s pairwise comparison. In this case, the benefit provided by AM fungi seems not to be an improvement in nutrient
uptake but protection against the pathogen.
(A) AFTER MERRYWEATHER & FITTER, 1995; NEWSHAM ET AL., 1995; (B) AFTER NEWSHAM ET AL., 1994, 1995
mutualisms of rhizobia and
leguminous plants:
several steps to a liaison
9781405156585_4_008.qxd 11/5/07 14:55 Page 276
on the root hair, which then begins to curl and is penetrated by the bacteria. The
host responds by laying down a wall that encloses the bacteria and forms an
‘infection thread’, which grows within the host root cortex, and within which
the rhizobia proliferate. Rhizobia in the infection thread cannot fix nitrogen, but
some are released into host cells in a developing ‘nodule’, where, surrounded
by a host-derived peribacteroid membrane, they differentiate into ‘bacteroids’
that can fix nitrogen. Meanwhile, a special vascular system develops in the host,
supplying the products of photosynthesis to the nodule tissue and carrying away
fixed-nitrogen compounds to other parts of the plant.
The costs and benefits of this mutualism need to be considered carefully.
From the plant’s point of view, we need to compare the energetic costs of
alternative processes by which supplies of fixed nitrogen might be obtained.
The route for most plants is direct from the soil as nitrate or ammonium ions.
The metabolically cheapest route is the use of ammonium ions, but in most
soils ammonium ions are rapidly converted to nitrates by microbial activity
(nitrification). The energetic cost of reducing nitrate from the soil to ammonia
is about 12 mol of adenosine triphosphate (ATP, the cell’s energy currency) per
mole of ammonia formed. The mutualistic process (including the maintenance
costs of the bacteroids) is energetically slightly more expensive to the plant: about
13.5 mol of ATP. However, we must also add the costs of forming and main-
taining the nodules, which may be about 12% of the plant’s total photosynthetic
output. It is this that makes nitrogen fixation energetically inefficient. Energy,
though, may be much more readily available for green plants than nitrogen. A
rare and valuable commodity (fixed nitrogen) bought with a cheap currency
(energy) may be no bad bargain. On the other hand, when a nodulated legume
is provided with nitrates (i.e. when nitrate is not a rare commodity) nitrogen
fixation declines rapidly.
On the other hand, the mutualisms of rhizobia and legumes (and other nitrogen-
fixing mutualisms) must not be seen as isolated interactions between bacteria
and their own host plants. In nature, legumes normally form mixed stands in
association with non-legumes. These are potential competitors with the legumes
for fixed nitrogen (nitrates or ammonium ions in the soil). The nodulated legume
sidesteps this competition by its access to its unique source of nitrogen. It is in this
ecological context that nitrogen-fixing mutualisms gain their main advantage.
Where nitrogen is plentiful, however, the energetic costs of nitrogen fixation
often put the plants at a competitive disadvantage.
Figure 8.15, for example, shows the results of a classic experiment in which
soybeans (Glycine soja, a legume) were grown in mixtures with Paspalum, a
grass. The mixtures either received mineral nitrogen, or were inoculated with
Rhizobium, or received both. The experiment was designed as a ‘replacement
series’, which allows us to compare the growth of pure populations of the grass
and legume with their performances in the presence of each other. In the pure
stands of soybean, yield was increased very substantially either by inoculation
with Rhizobium, or by application of fertilizer nitrogen, or by receiving both.
The legumes can use either source of nitrogen as a substitute for the other. The
grass, however, responded only to the fertilizer. Hence, when the species com-
peted in the presence of Rhizobium alone, the legume contributed far more to the
overall yield than did the grass: over a succession of generations, the legume
would have outcompeted the grass. When they competed in soils supplemented
Chapter 8 Evolutionary ecology
277
costs and benefits of rhizobial
mutualisms
interspecific competition:
a classic ‘replacement series’
9781405156585_4_008.qxd 11/5/07 14:55 Page 277
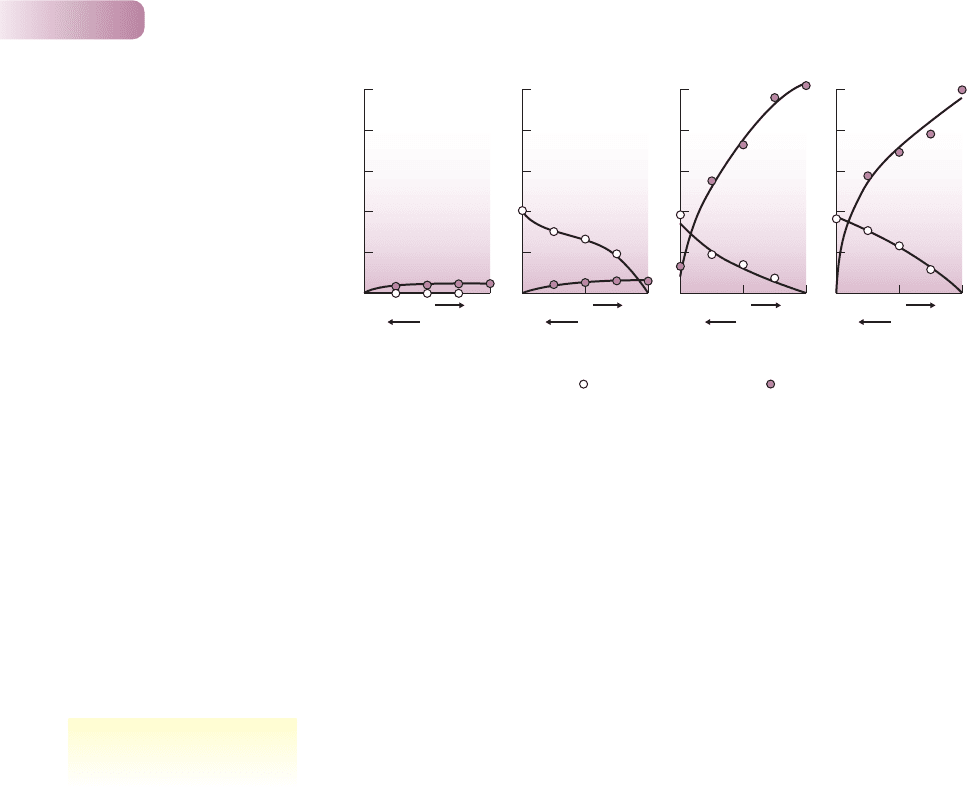
with fertilizer nitrogen, however, whether or not Rhizobium was also present, it
was the grass that made the major contribution: long term, it would have out-
competed the legume.
Quite clearly, then, it is in environments deficient in nitrogen that nodulated
legumes have a great advantage over other species. But their activity raises the
level of fixed nitrogen in the environment. After death, legumes augment the level
of soil nitrogen on a very local scale with a 6–12-month delay as they decompose.
Thus, their advantage is lost – they have improved the environment of their
competitors, and the growth of associated grasses will be favored in these local
patches. Hence, organisms that can fix atmospheric nitrogen can be thought of as
locally suicidal. This is one reason why it is very difficult to grow repeated crops
of pure legumes in agricultural practice without aggressive grass weeds invading
the nitrogen-enriched environment. It may also explain why leguminous herbs or
trees usually fail to form dominant stands in nature.
Grazing animals, on the other hand, continually remove grass foliage, and the
nitrogen status of a grass patch may again decline to a level at which the legume
is once more at a competitive advantage. In a stoloniferous legume, such as white
clover, the plant is continually ‘wandering’ through the sward, leaving behind it
local grass-dominated patches, whilst invading and enriching with nitrogen new
patches where the nitrogen status has become low. The symbiotic legume in such
a community not only drives its nitrogen economy but also some of the cycles that
occur within its patchwork (Cain et al., 1995).
We end this section, then, on a theme that has recurred repeatedly. To under-
stand the ecology of mutualistic pairs, we must look beyond those species to the
wider community of which they are part.
Part III Individuals, Populations, Communities and Ecosystems
278
–R –N +R –N –R +N
0
10
20
50
40
30
4
0
0
8
2
4
4
0
0
8
2
4
4
0
0
8
2
4
4
0
0
8
2
4
Dry weight per container (g)
G
P
G
P
G
P
G
P
+R +N
Figure 8.15
The growth of soybeans (Glycine soja, G, ) and a grass (Paspalum, P, ) grown alone and in mixtures
with and without nitrogen fertilizer (N) and with and without inoculation with nitrogen-fixing Rhizobium (R).
The plants were grown in pots containing 0–4 plants of the grass together with 0–8 plants of Glycine.
Thus, moving left to right on the horizontal axis, the treatments are zero Paspalum (0P) and 8 Glycine
(8G), 1P with 6G, 2P with 4G, 3P with 2G and, finally, 4P with 0G. The vertical scale on each figure shows
the mass of plants of the two species in each container. −R −N, no Rhizobium and no fertilizer; +R −N,
inoculated with Rhizobium but no fertilizer; −R +N, no Rhizobium but nitrate fertilizer was applied; +R +N,
inoculated with Rhizobium and nitrate fertilizer was supplied. When the two species competed in the
presence of nitrogen-fixing Rhizobium and without fertilizer, the soybeans (with their mutualistic
relationship to Rhizobium) performed best, but in the presence of nitrogen fertilizer (with or without the
Rhizobium) the grass outperformed the soybeans.
AFTER DE WIT ET AL., 1966
the shifting balance between
nitrogen-fixers and non-fixers
9781405156585_4_008.qxd 11/5/07 14:55 Page 278
