Труды Всемирного конгресса Международного общества солнечной энергии - 2007. Том 2
Подождите немного. Документ загружается.

Proceedings of ISES Solar World Congress 2007: Solar Energy and Human Settlement
672
curves were recorded for the two alloys in EdMPNTf
2
N
and compared with the pure nickel (99.99% goodfellow) in
the same conditions. All the systems reach an almost
constant value (AISI 304: -0.32÷-0.35 V; AISI 1018: -0.53
÷ -0.56V; Pure Nickel: -0.40÷-0.45V) after about 9 hours of
immersion. The highest voltage value corresponds to the
stainless steel AISI 304, confirming its ‘nobler’ behavior in
comparison to both pure Nickel and carbon steels.
Tafel Plots and Rpol measurements. These measurements
provide an insight on the corrosion current densities of the
alloys in ILs environment. After the registration of the
OCV curve, Polarization Resistance (R
pol
) measurements
were performed in the range of ± 25 mV respect to OCV. In
a few minutes after the R
pol
scan the potential returned to
OCV value. At this point, the anodic Tafel plot was
recorded starting from – 50 mV up to +250 mV respect
OCV. The cathodic Tafel plot was registered
independently, repeating the above described procedure
with fresh fluid, a new WE and performing first the OCV
scan. The scan rate for Rpol and Tafel plots was 0.166 mV
s
-1
.
The extrapolated quantities from Tafel plots are
summarized in Table 2 and they are of the same order
obtained for a similar IL
8,9
. The slopes of the linear portion
of the Tafel plots (β
a
and β
c,
) were used to calculate
corrosion current densities from Polarization Resistance
measurements, which result 1,17 μA cm
-2
for AISI 1018
and 0,024 μA cm
-2
for AISI 304. Superimposed events
could influence the β values, such as different oxidation
state of the metals, the presence of several oxidants, etc.,
leading the observation of a mixed potential.
TABLE 2: TAFEL PLOTS EXTRAPOLATED PARAMET-
ERS
Alloy I
corr
A
(μA cm
-2
)
I
corr
C
(μA cm
-2
)
β
a
(mV d
-1
)
β
c
(mV d
-1
)
304 0,014 0,014 143 280
1018 1,22 0, 239 127 496
4. DISCUSSION
The results reported here show a complex behavior of the
different combinations of substrate and IL. In general, we
believe that the high reactivity of these systems can be at
least in part attributed to dissolved oxygen. It appears that
ILs can dissolve much more oxygen than water, as shown
by a recent work by Brennecke et al.
10
, which estimates the
dissolved oxygen in several ionic liquids, including
methyl-tri-butylammonium(Tf
2
N), similar to those
employed in this work. According to this study, the Henry
constant expressed in pressure units is about 1000 bar at 25
°C. In comparison, the Henry’s constant of oxygen in water,
expressed as function of gas concentration and partial
pressure (Eq. 1), is 1.28 10
-3
mol l
-1
bar
-1
at 25 °C:
1−
⋅= piCi
conc
H (1)
Thus, the oxygen concentration, obtained considering the
atmospheric partial pressure of the gas equal to 0.21 bar, is
2.7 10
-4
M. Since a dm
3
of water contains 55.51 moles, the
molar fraction of oxygen is something about 4.86 10
-6
, that
gives rise, according to the equation (2), to an Henry’s
constant of 43210 bar, a pressure 40 times higher to
dissolve in water the same amount of oxygen that dissolves
in IL.
For the application studied here the laws that govern the
electrochemical processes are expressed in terms of molar
concentrations. This is not only a formal question. The
electrochemistry is not concerned of the relative amount of
the solvent and the electroactive species since the solvents
only acts as a support for the reaction. The
electrochemical phenomena are only affected by the density
of the molecules of the electroactive species and of the
kinetic of the process. The number of active molecules
reaching the electrode surface is only related to the
volumetric concentration and also on some combined
properties which are function of both the electrolyte and the
electroactive species (e.g. the diffusion coefficient). In
terms of molar fraction, the ratio of the solubility of the
oxygen in the ionic liquid and in water is large:
43210/1000=43, but in terms of volume concentration the
situation is different, because the number of molecules of
solvents per volume unit is different. We have earlier
calculated that the oxygen concentration in water is around
0.27 mM or 8.64 mg l
-1
. For the IL, considering that the
density of EdMPNTf
2
N is 1.41 g cm
-3
and a molar weight
is 396.37 g mol
-1
we can conclude that the number of mol/l
is around 3.5, much lower than the 55.5 for water. From the
3 SOLAR COLLECTOR TECHNOLOGIES AND SYSTEMS
673
inverse Henry’s law, expressed in term of molar fraction, X
i
,
and partial pressure, p
i
, of the i
th
gas:
1−
⋅= XipiH
X
(2)
and considering an oxygen partial pressure of 0.21 bar, we
get that the molar fraction of dissolved gas is 2.1 10
-4
. From
the definition of molar fraction and considering the number
of mol of IL per liter, the concentration of the dissolved
oxygen is around 7 10
-4
mol l
-1
or 22.4 mg l
-1
; approximately
three times larger than in water. Such a concentration is
sufficiently high to justify the occurrence of electrochemical
phenomena due to the oxygen. The β values could be also
affected by oxidant agents diffusion controlled mechanism at
the electrode interface, thus activation polarization
contributions seems to be not negligible. This fact probably
is also the cause of the high β values in the cathodic plots,
where the only reduced agents could be protons from
residual water. The determination of the corrosion rates by
such electrochemical approach needs the definition of the
charge number Z, that, at this moment, can only be a
hypothesis. Nevertheless, the comparison of the corrosion
current densities derived by the R
pol
method and to the
corrosion rates derived by Tafel plots extrapolation shows
good agreement. Such element provides a confirmation of
the reliability of the performed measurements.
5. CONCLUSIONS
The results reported in the present study show that the
resistance to corrosion of steel substrates in contact with
the Ionic Liquids investigated is still not satisfactory
enough for practical applications. Among the investigated
Ionic Liquids only the EdMPNTf
2
N does not decompose at
the working temperature of the trough collectors in
presence of air and even though the electrochemistry
estimated corrosion current densities at room temperature
are low, especially for AISI 304, the dissolved oxygen in
this ionic liquid is not negligible at 220°C, and it is the
probable cause of the observed localized corrosion after the
open vessels immersion tests. Considering the important
amount of oxygen that dissolves in ionic liquids, a better
resistance to corrosion is likely to be obtainable in absence
of air (i.e. nitrogen purged atmosphere), which is a
condition that can be obtained also in practical conditions
in a sealed heat transfer system in a solar concentration
plant. This will be the objective of further investigation in
this field.
6. ACKNOWLEDGMENTS
We gratefully acknowledge financial support provided by EC,
Project FP6-2003-INCO-MPC2 (STREP) Contract Number:
015434, REACt, Self-sufficient Renewable Energy Air-
Conditioning system for Mediterranean countries.
7. REFERENCES
(1) L. Moens, D.M. Blake, D.L. Rudnicki, M.J. Hale,
“Advanced thermal storage fluids for solar parabolic trough
systems”, J. Solar Energy Eng, 125, 112–116, 2003.
(2) C. P. Fredlake, J.M. Crosthwaite, D.G. Hert, S.N.V.K.
Aki, J.F. Brennecke, “Thermophysical properties of
Imidazolium-based Ionic Liquids”, J. Chem. Eng. Data,
49, 954–964, 2004
(3) M.E. Van Valkenburg, R.L. Vaughn, M. Williams, J.S.
Wilkes, “Thermochemistry of Ionic Liquid heat-transfer
fluids”, Thermochim. Acta, 425,181-188, 2005
(4) M. Kosmulski, J. Gustafsson, J.B. Rosenholm,
“Thermal stability of low temperature ionic liquids
revisited”, Thermochim. Acta, 412, 47–53, 2004
(5) U. Bardi, S.P. Chenakin, S. Caporali, A. Lavacchi,I.
Perissi, A. Tolstoguzov, “Surface modification of
industrial alloys induced by long-term interaction with
an ionic liquid”, Surf. Interface Anal. , 38, 1768-1772,
2006
(6) S. Chowdhury, R. S. Mohan and J. L. Scott, “Reactivity
of Ionic Liquids”, Tetrahedron, 63, 2363-2389, 2007
(7) K.J. Baranyai, G.B. Deacon, D.R. MacFarlane, J.M.
Pringle, J.L. Scott, “Thermal degradation of Ionic
Liquids at elevated temperature”, Aust. J. Chem,
57,145-147, 2004
(8) I. Perissi, U. Bardi, S. Caporali, A. Lavacchi, “High
temperature corrosion properties of Ionic Liquids”,
Corros. Sci., 48, 2349-2362, 2006
(9) M. F .Arenas, R.G. Reddy, “Corrosion of Steel in Ionic
Liquids”, J. Min. Met., 39, 81-91, 2003
(10) J.L. Anthony, J.L. Anderson, E.J. Maginn, J.F.
Brennecke, “Anion effects on gas solubility in Ionic
Liquids”, J. Chem. Phys. B, 109, 6366-6374, 2005
EXPERIMENTAL RESEARCH ON THE ALL-GLASS EVACUATED TUBE
SOLAR AIR COLLECTOR
Hong Liang
Institute of Electrical Engineering, CAS
Beijing 100080, China
Hongwei7600@vip.sina.com
ABSTRACT
This paper is focus on the research of the thermal
performance of all glass-evacuated tube solar air
collectors by experiment. Two sections composed of inlets
and outlets are formed by the inserted pipes with the
target to heat the forced convection air by heat exchange
with the vacuum pipes’ inside surface. Solar radiance,
work fluid flow rate, the static pressure difference
between inlet and outlet, the temperature of ambient and
inlet and outlet and ambient wind speed were measured in
this experiment, for they are the factors that can effect the
instance efficiency of solar air collector. The testing was
finished based on the methods introduced in
ASHRAE93-86 and ISO9806-2 standard.
1. INTRODUCTION
The requirement of energy in China is dramatically
increasing with the rapid development of its economy.
Fossil fuel consumption caused many widely known
problems like environmental pollution and climate warm
up. Nowadays, the entire worlds are discussing how to
protect the environment and to solve the energy lack by use
of renewable energy. Solar is one of the renewable energy
sources with most potential for its large amount and easy
collection.
In China, air conditioning energy demand accounts 65% of
the total architectural energy consumption. How to use
solar air heater instead of the conventional energy to meet
the heating and cooling demand in winter and summer now
becomes the main research field.
Furthermore, all kinds of solar air heater can be widely
utilized in the fields of agricultural crops drying, sea water
desalination and building heating and cooling. This paper
presented the experimental research results of the all glass
evacuated tube solar air collector inserted with metal pipes.
Those tests are based on the standards introduced in
ASHRAE 93-1986 and ISO 9806-1.
2. THEORETICAL ANALYSIS
2.1 Transient Efficient Test
Inlet air temperature from 4 points with equivalent interval
spaces was measured as follows under sunny weather:
(1) Total insulations on the evacuated tube solar air heater
surface G;
(2) Diffuse insolation on the vacuum tube solar air heater
surface G
d
;
(3) Incidence angle
θ ;
(4) Surrounding temperature
t
;
(5) Ambient wind speed u;
(6) Solar air heater inlet temperature
t ;
(7) Solar air heater outlet temperature
t
;
(8) Inlet air mass flow rate
m
;
(9) Outlet air flow rate
m
.
3 SOLAR COLLECTOR TECHNOLOGIES AND SYSTEMS
675
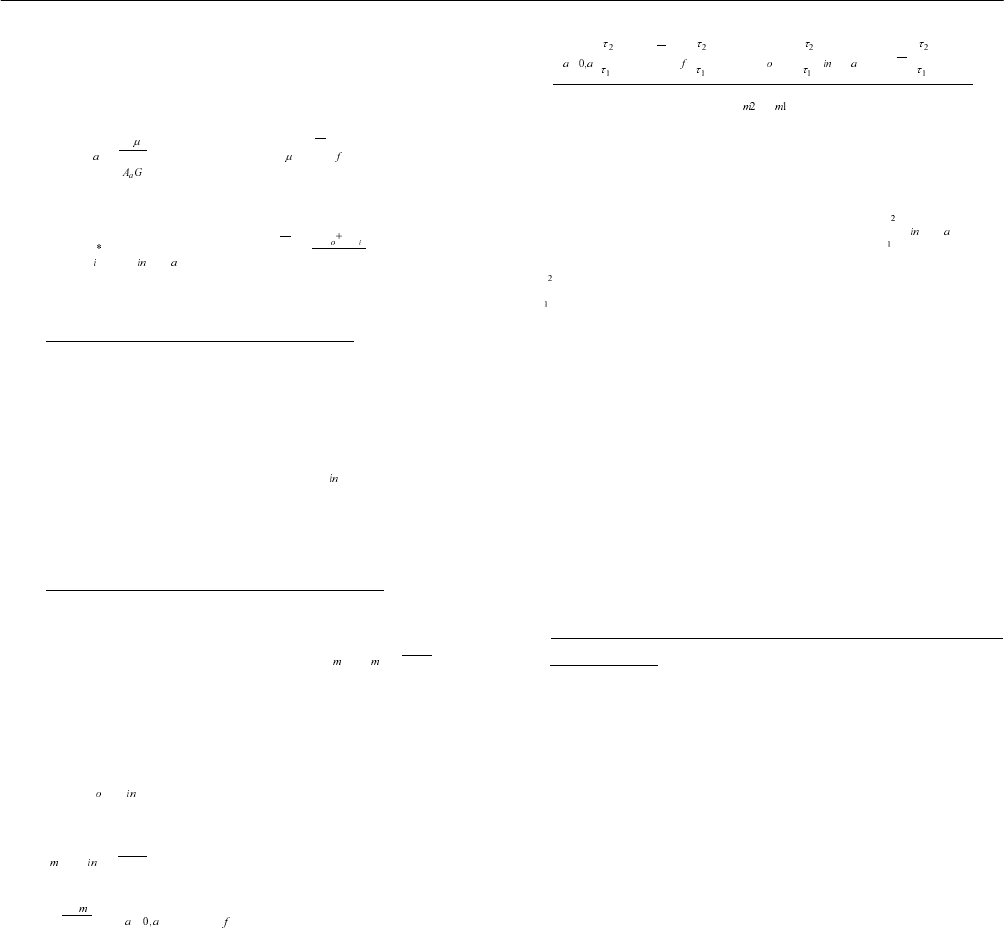
Transient efficiency is calculated from the following
equations:
η
=
q
˗
q
=
mc TΔ
˗
()/TttG=−
;
2
mm
m
=
2.2 Solar Air Collector Pressure Drop Test
Inlet and outlet air pressure drop which is crucial to the
solar collector’s characteristic is an important design
variable. The test is measured between flow rate of
0.01m
3-
0.03 m
3
Variables including
t
,
qv
and
PΔ
are
measured and the pressure drop
PΔ
vs. flow rate curves
are plotted.
2.3 Solar Air Collector Thermal Capacity Test
Variables including are measured G,
2
T
tt
Δ
=+
,
in
t
,
o
t , m
, based on the following equations:
Tt tΔ= −
2
T
tt
Δ
=+
dt
CAGmC
d
η
τ
=−
()
aina
TAUt tΔ− −
Two static state transient characteristics are calculated and
the two static equations are integrated coming to the following
thermal capacity:
1
()
2
AGdmCTdAUttd Td
C
tt
ητ τ τ τ
⎡⎤
−Δ− −+Δ
⎢⎥
⎣⎦
=
−
∫∫ ∫ ∫
Curves are plotted based on the experimental results
including ˄t
in
̢t
a
˅,T and G. The space area between
two static stats are separately as:
()ttd
τ
τ
τ
−
∫
,
Td
τ
τ
τ
Δ
∫
.
The efficient thermal capacity can be calculated from the
experimental data.
The thermal capacity results from three experimental are:
C
1
=9733.6J/K; C2= 6317J/K; C3=10356J/K.
The solar air heater capacity is defined as the arithmetic
average of the three values above: C=8802.6J/K.
3. EXPERIMENTAL APPARATUS AND THE MEA-
SUREMENT
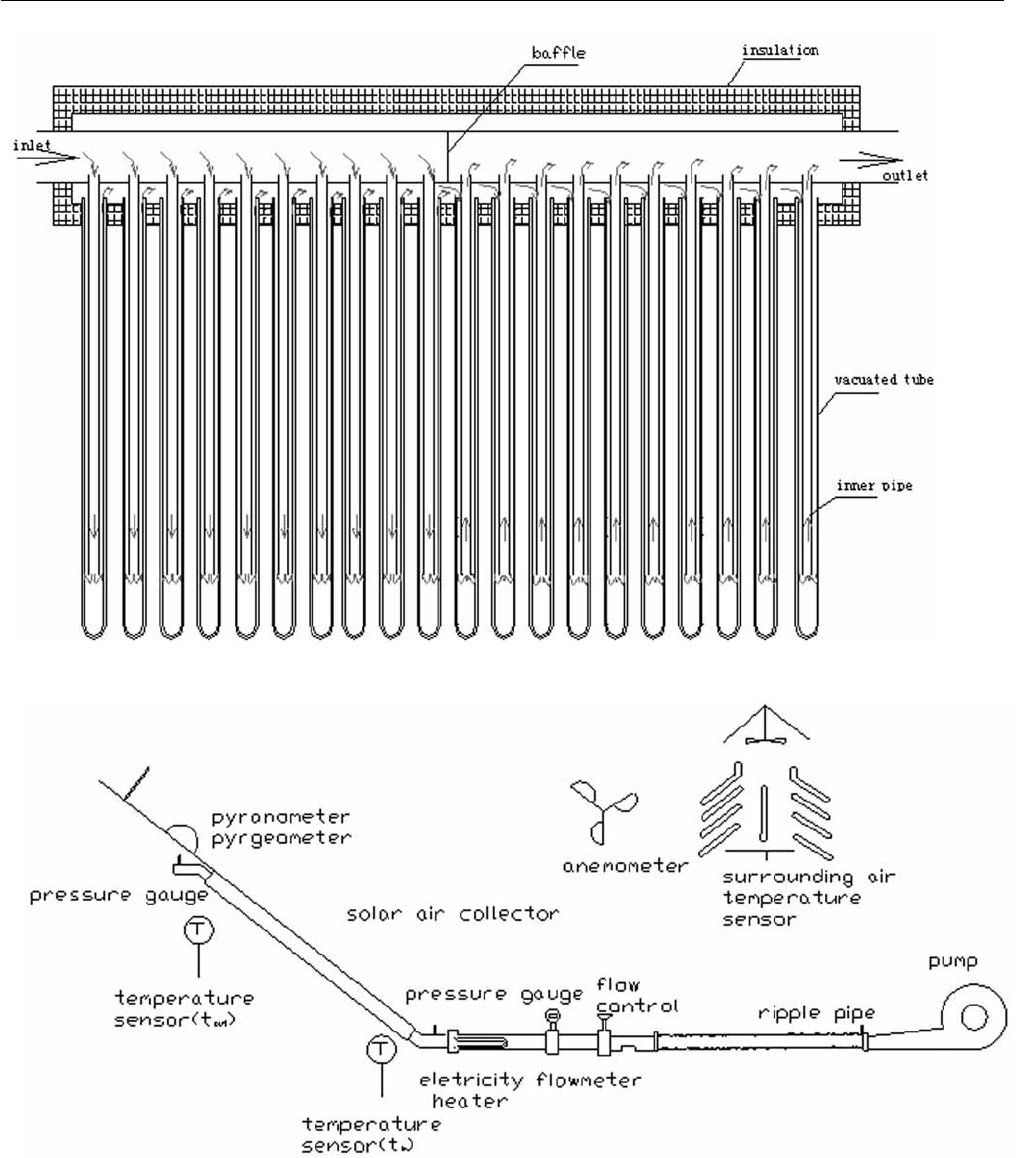
The all-glass evacuated tube solar air collector is composed
of vacuum tube, inserted metal tubes and the header box.
The vacuum tubes and header box are separated into two
sections with inlet and outlet by the inserted metal tubes. It
absorbs heat through air convection. The structural
schematic of the all –glass evacuated tube solar air heater
and the experimental apparatus schematic are as following
Fig. 1 and Fig. 2.
The test of this solar air heater has been finished in
Beijing during Jun-Aug 2006.Experimental results are
shown in Fig. 3.
Proceedings of ISES Solar World Congress 2007: Solar Energy and Human Settlement
676
Fig. 1: Schematic of the all-glass evacuated tube solar air collector.
Fig. 2: Schematic of the experimental apparatus.
3 SOLAR COLLECTOR TECHNOLOGIES AND SYSTEMS
677
\ [
1 1/12 1/13 1/14 1/15 1/16 1/17
7
¨
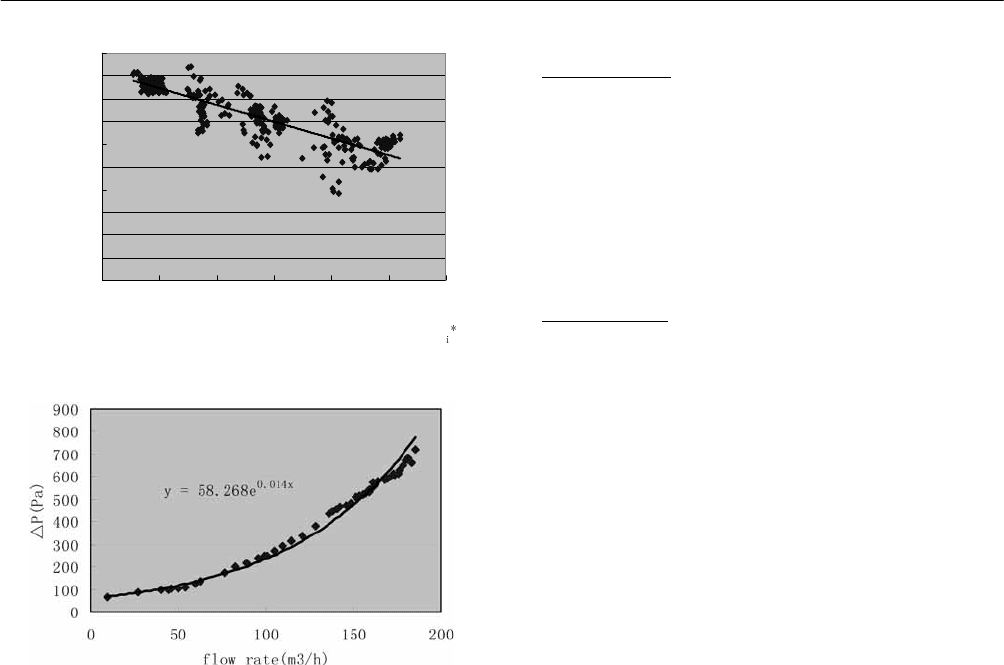
Fig. 3: Transient efficiency equitation.
Fig. 4: Pressure drop V.S. mass flow rate.
4. CONCLUSION
From the tests finished, it can be found the thermal specific
of air is too small to establish static test state. Even the flow
rate tiny change can cause obvious difference of
temperature and pressure. Dust accumulated on vacuum
tubes’ surface also disturb the testing accuracy.
5. REFERENCES
(1) A. Ucar M. InallˈThermal and exergy analysis of solar
air collectors with passive augmentation techniques
International Communications in Heat and Mass
Transfer 33 (2006) 1281̢1290
(2) Performance analysis of new-design solar air collectors
for drying applications Suleyman Karsli ˈ
Renewable Energy
(3) Efficiency investigation of a new-design air solar plate
collector used in a humidification̢dehumidification
desalination process ˈ Mahmoud Ben-Amara, Imed
Houcine*,Aman-Allah Guizani, Mohammed Maalejˈ
Renewable Energy 30 (2005)

INTEGRAL-TYPE SOLAR WATER HEATER USING LOOP HEAT PIPE
B.J. Huang, P.E. Yang , J. H. Wang, J. H. Wu
New Energy Center, Department of Mechanical Engineering
Taiwan University, Taipei, Taiwanm, China
bjhuang@seed.net.tw
ABSTRACT
Taiwan University has been devoted to the development of
low-cost LHP (loop hat pipe). A new manufacturing
process for low-cost LHP has been developed that leads to
a cost down to less than 10 USD for a 100W LHP. The
present study utilizes the low-cost LHP to develop a new
type of solar water heater. A thermosyphon which is
thermally bonded with a solar absorber plate is used to
absorb solar energy and transfer the heat upward to the
evaporator of the LHP. From that, the heat is conducted
downward to the condenser of the LHP which is immersed
in a hot water tank beneath the solar collector. The solar
water heater thus can be designed in integral type with
streamline shape which is easy to install and has a good
outlook as well as high efficiency. A prototype was
designed and fabricated in the present study.
A preliminary heat transfer test for a single unit of
thermosyphon-LHP combination shows that it is capable of
transferring the absorbed energy downward to the LHP
condenser immersed in a hot water tank. The overall
thermal resistance from the absorber plate to water is
0.34
o
C/W, and the thermal resistance of the LHP is
0.16
o
C/W. Two prototypes of the solar water heaters with
50 liters and 80 liters hot water tanks were designed
according to the results of the feasibility test. In addition,
an automatic monitoring system was designed and set up
for the performance test of these two solar water heaters
based on the test standard CNS B7277. The measured
overall thermal resistance from solar absorber plate to
water is 0.369
o
C/W. The daily solar water heater efficiency
is 0.503. The present study has shown that the application
of low-cost LHP in solar water heater is feasible and cost
competitive.
1. INTRODUCTION
Solar hot water heater is important in renewable energy
application. There are three types of commonly-used solar
water heaters: namely, forced-circulation system,
natural-circulation system (known as thermosyphon), and
constant temperature-intermittent output system (known as
once-through system). Among them, the thermosyphon
solar water heater is the most popular one since no
circulation pump or regulating valve is needed. However,
in order to prevent heat loss during night time or raining
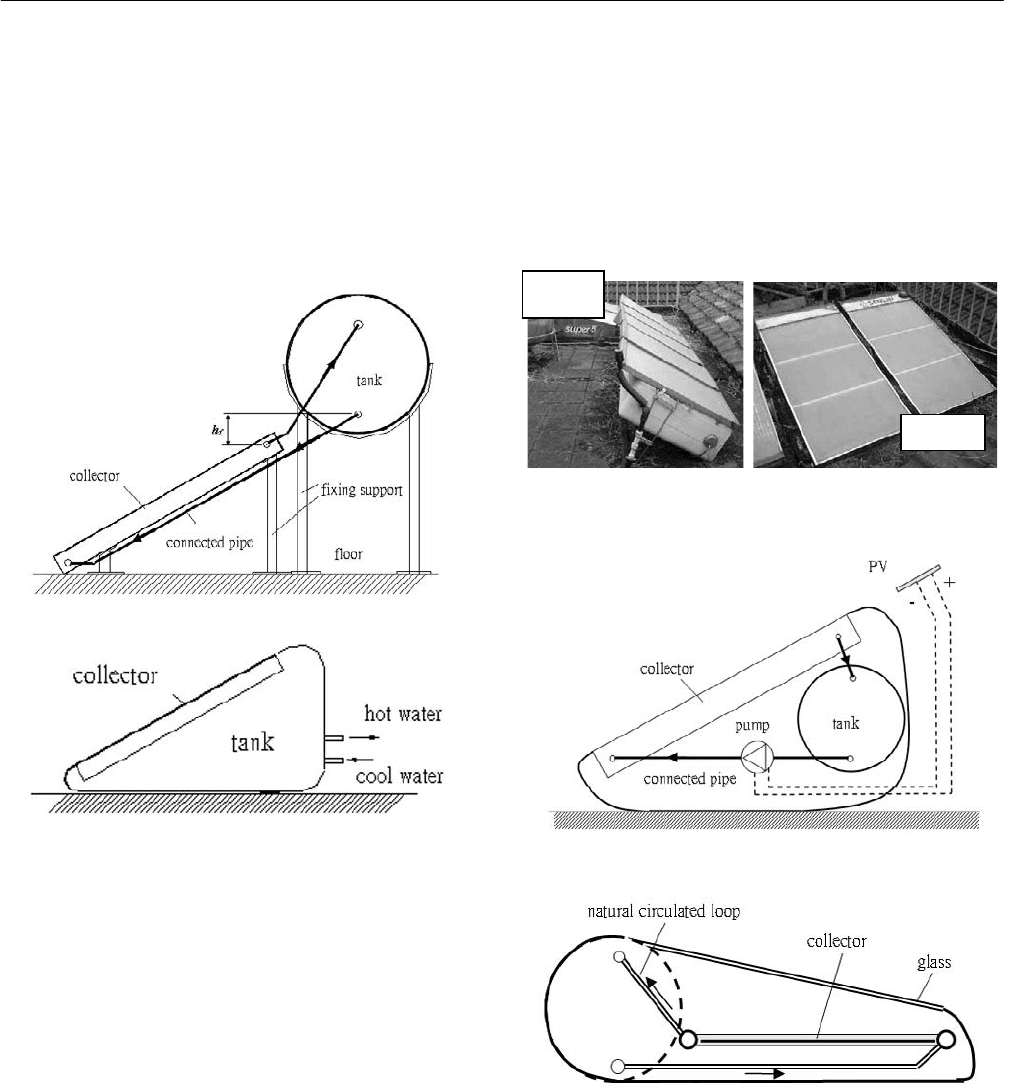
days caused by reverse-flow phenomenon, the water tank
must be placed upon the collector [1]. In addition, the
whole solar water heater is manufactured at factory in three
separate parts: solar collector, tank, and supporting frame
(Figure 1). The solar water heater is then installed by
assembling the three separate parts together on site,
including frame fixing on roof and cold and hot water
piping. The installation cost is thus time consuming and at
high cost.
The design of integral-type solar water heater (ISWH) as
shown in Figure 2 can greatly simplify the installation since
the whole system can be manufactured in factory with good
quality control. The whole system is then moved to the
3 SOLAR COLLECTOR TECHNOLOGIES AND SYSTEMS
679
rooftop and just placed on the floor without additional
fixing work. A few solar water heaters of this type were in
market. However, the thermal efficiency is low since the
downward heat transfer design is poor, usually with daily
thermal efficiency < 0.4. The poor integral-type solar water
heater cannot survive in Taiwan solar market since the
government subsidy program asks all products to have
daily solar thermal efficiency larger than 0.5.
Fig. 1: Natural-circulation solar water heater.
Fig. 2: Integral-type solar water heater design.
Two companies in Taiwan had ever marketed the integral-
type product about 10 years ago, but failed (Figure 3).
Brand A was designed in forced-circulation type, with
water capacity 75 liters which is placed under the solar
collector. The water pump is driven by a solar PV to
circulate the water through solar collector to tank (Figure 4).
This product failed due to the reliability problem of
solar-pumping system.
Brand B is designed in thermosyphon type with water
capacity 150 liters. The solar collector is placed nearly on
floor in order to keep it below the tank (Figure 4). The
vertical distance between the tank inlet and the collector
outlet thus becomes negative and causes flow reversal at
night or cloudy days. The solar energy collection efficiency
is thus very low, < 0.40, and the product cannot survive on
the market. Since then, the integral-type solar water heater
disappeared from Taiwanese market. However, the
installation process of both brand A and B had been
simplified a great deal. Brand A (5 modules, 375 liters)
takes only two man-hour to install. Brand B (300 liters,
single module) is too heavy (>60kg) and took about 6
man-hour to install on the roof.
Fig. 3: Failed ISWH products.
Fig. 4: Failed ISWH design.
The present study intends to utilize the low-cost LHP to
develop a new integral-type solar water heater. Two
prototypes were designed and fabricated in the present
study.
Brand A
Brand B
Proceedings of ISES Solar World Congress 2007: Solar Energy and Human Settlement
680
2. PRELIMINARY TEST OF THERMOSYPHON-LHP
In order to prove that the low-cost LHP can achieve
downward heat transfer to the water tank from solar
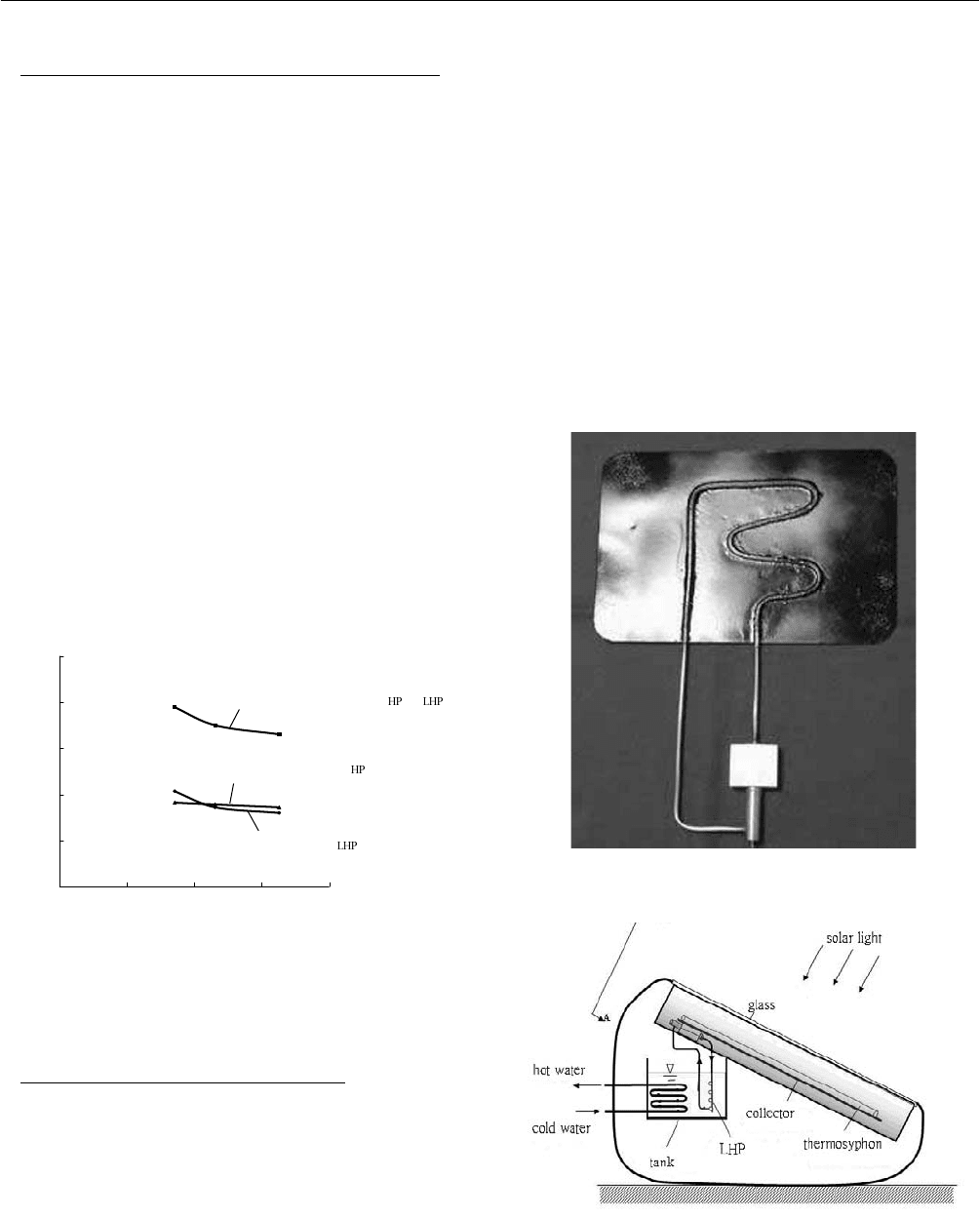
collector, a thermosyphon-LHP experiment was first carried
out. A single unit of thermosyphon-LHP device was
designed. The device includes a single strip of tube-in-sheet
solar collector (0.12m
2
) with a thermosyphon, a LHP and a
water tank set beneath the collector. An electric film heater
is adhered on the solar collector to simulate solar heating in
both summer and winter. The thermosyphon is thermally
bonded with the solar absorber plate and transfers the heat
upward to the evaporator of the LHP. The heat is then
conducted downward to the condenser of the LHP which is
immersed in a hot water tank beneath the solar collector.
The test results (Figure 5) shows that the thermosyphon-
LHP configuration is capable of transferring the absorbed
energy downward to the LHP condenser immersed in a hot
water tank beneath the collector. The overall thermal
resistance from the absorber plate to water is 0.34
o
C/W, and
the thermal resistance of the LHP is 0.16
o
C/W.
0
0.1
0.2
0.3
0.4
0.5
0 50 100 150 200
Power(W)
Thermal resistance(
к
/W)
system thermal resistance(R +R )
LHP thermal resistance(R )
thermosyphon resistance(R )
Fig. 5: Thermal resistance measurement in a single
thermosyphon-LHP device.
3. LHP-BASED SOLAR WATER HEATER
Applying the low-cost LHP (Figure 6) [2] to solar water
heater, we can effectively transfer the heat downward to the
tank from the solar collector. A thermosyphon which is
thermally bonded with a solar absorber plate is used to
absorb solar energy and transfer the heat upward to the
evaporator of the LHP. From that, the heat is conducted
downward to the condenser of the LHP which is immersed
in a hot water tank beneath the solar collector. The solar
water heat thus can be designed in integral type with
streamline shape which is easy to install and has a good
outlook as well as high efficiency. Figure 7 shows the
design concept. Two models, 80L(Figure 8) and 50L
(Figure 9), were designed.
ISWH-1 was designed for feasibility study to validate the
downward heat transfer capability. ISWH-1 uses 1m
2
solar
collector and 4 sets of LHP. The tank is of closed-type
which can sustain the tape water pressure. ISWH-2 is
designed with compact and rectangular configuration with
open-type tank.
Fig. 6: Low-cost LHP.
Fig. 7: LHP-based solar water heater.
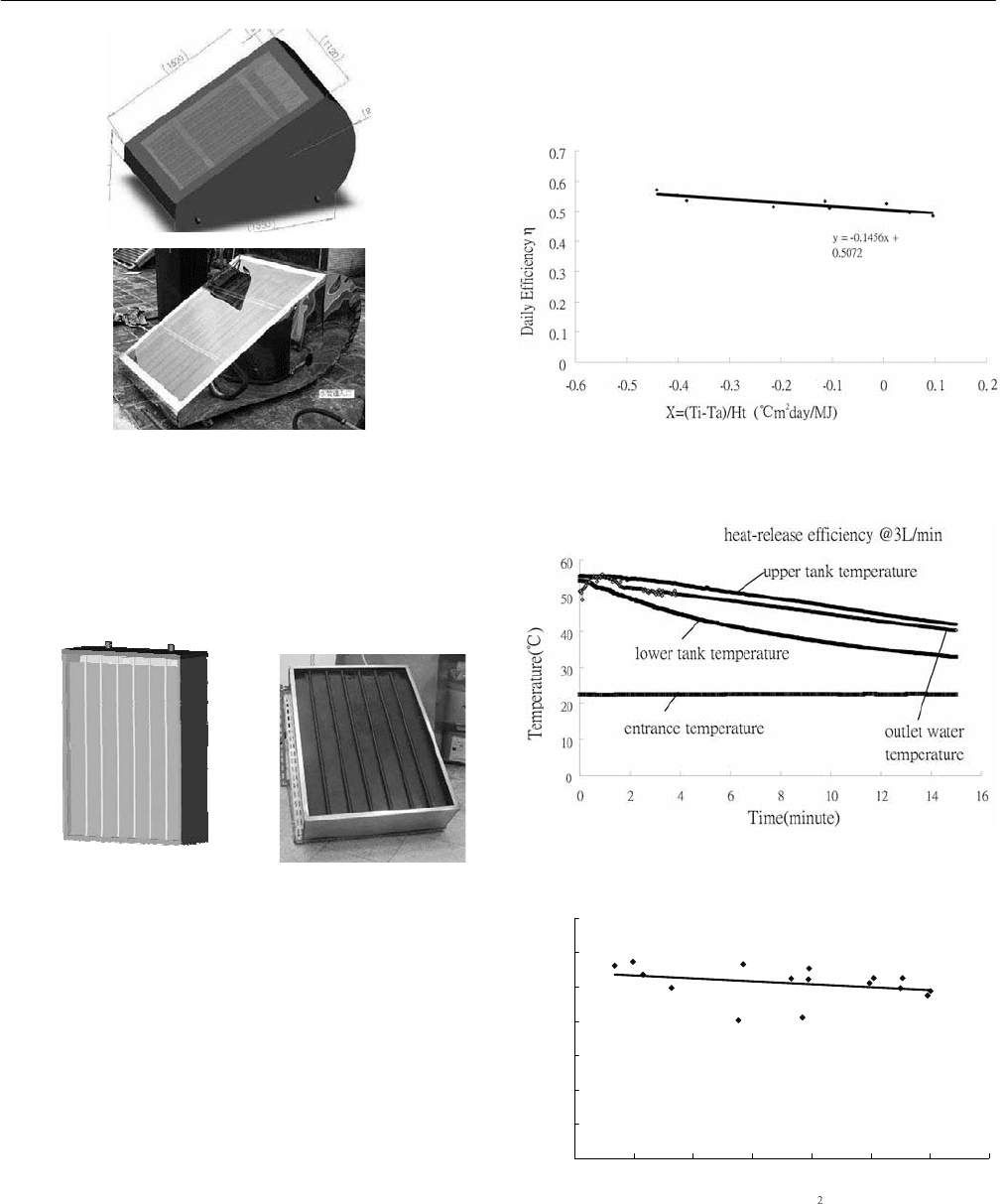
3 SOLAR COLLECTOR TECHNOLOGIES AND SYSTEMS
681
Fig. 8: ISWH-1.
The solar collector area of ISWH-2 is 0.5m
2
. ISWH-2 uses
2 sets of LHP. The daily solar energy collection efficiency
is measured according to the outdoor test standard
CNSB7277 [3].
Fig. 9: ISWH-2.
The daily solar energy collection efficiency is 0.503 (Fig.
10) and the heat releasing efficiency of hot water supply is
0.77 as shown in Figure 11. Both exceed the requirement of
the subsidy program. The measured overall thermal
resistance from solar absorber plate to water is 0.369
o
C/W.
ISWH has been proved at the first time to be able to utilize
low-cost LHP as the downward heat transfer element and
obtain a good efficiency.
In order to understand whether the LHP can perform
normally in the long run, we have carried out a long-term
outdoor experiment. The result indicates that LHP can
function normally; moreover, the daily solar energy
collection efficiency is 0.501 (Fig 12) for the test period of
3 months.
Fig. 10: ISWH-2 thermal efficiency.
Fig. 11: Heat releasing test of the tank.
̌ʳːʳˀ˃ˁ˄ˆˌˇ̋ʳʾʳ˃ˁˈ˃ˆ
˃
˃ˁ˄
˃ˁ˅
˃ˁˆ
˃ˁˇ
˃ˁˈ
˃ˁˉ
˃ˁˊ
ˀ˃ˁˈ ˀ˃ˁˇ ˀ˃ˁˆ ˀ˃ˁ˅ ˀ˃ˁ˄ ˃ ˃ˁ˄ ˃ˁ˅
˫ːʻ˧˼ˀ˧˴ʼ˂˛̇ʳʳʻк̀ ˷˴̌˂ˠ˝ʼ
˘˹˹˼˶˼˸́˶̌ʳӯ
Fig. 12: ISWH-2 thermal efficiency in the long run.
