Zuo-Guang. Ye Advanced Dielectric Piezoelectric and Ferroelectric Materials: Synthesis, Characterisation and Applications
Подождите немного. Документ загружается.

Handbook of dielectric, piezoelectric and ferroelectric materials500
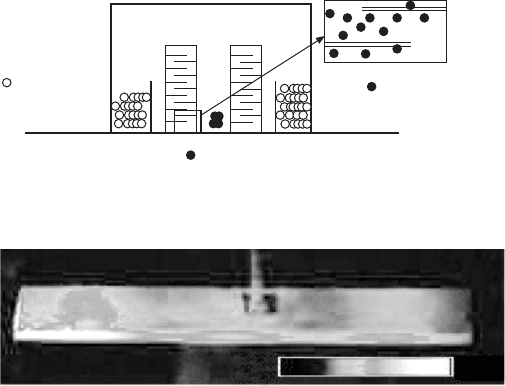
Cu-embedded multilayer transformers are now being developed. Figure
16.18 illustrates an experimental setup for sintering Cu-electrode-embedded
multilayer transformers in a reduced N
2
atmosphere. Figure 16.19 shows co-
fired multilayer transformers with pure Ag (right) and Cu (left) electrodes
sintered at 900 °C. The transformer performance will be reported in a successive
paper.
16.8 Summary and conclusions
• There are three loss origins in piezoelectrics: the dielectric, elastic and
piezoelectric losses. The 180
o
and non-180
o
domain wall motions contribute
primarily to the extensive dielectric and elastic losses, respectively.
• Heat generation occurs in the sample uniformly under an off-resonance
mainly due to the intensive dielectric loss, while heat is generated primarily
at the vibration nodal points via the intensive elastic loss under a resonance.
In both cases, the loss increase originates from the extensive dielectric
loss change with electric field and/or stress.
• In a ‘hard’ piezoelectric PZT, the mechanical quality factor Q
m
for the
k
33
mode is only 1/5 of that of the k
31
mode. We integrated the loss
anisotropy with an FEM software code and obtained more accurate
simulation results for the piezo-transducer designing.
• Actuator materials: doping rare-earth ions into PZT–Pb(Mn,X)O
3
(X =
Sb, Nb) ceramics increases the maximum vibration velocity up to 1m/s,
Fe
3
O
4
(s)
PbO (s)
Al (s)
UHP N
2
,
p
O
2
= 10
–6
atm.
16.18
Experimental setup for sintering Cu-electrode-embedded
multilayer transformers in a reducing N
2
atmosphere.
16.19
Multilayer co-fired transformer with hard PZT and Cu (left) or
pure Ag (right) electrode, sintered at 900 °C (Penn State trial
products).
23.83 58.87
WPNL2204
Loss mechanisms and high-power piezoelectric components 501
which corresponds to one order of magnitude higher energy density than
conventionally commercialized piezo-ceramics. To obtain high-power
density/high-vibration velocity materials, domain wall immobility/
stabilization via the positive internal bias field seems to be essential,
rather than the local domain wall pinning effect.
• Transducer materials: the Sb, Li and Mn-substituted 0.8Pb(Zr
0.48
Ti
0.52
)O
3
–
0.16Pb(Zn
1/3
Nb
2/3
)O
3
–0.04Pb(Ni
1/3
Nb
2/3
)O
3
ceramics showed the value
of k
p
= 0.57, Q
m
= 1502 (planar mode), d
33
= 330 pC/N,
εε
3
T
0
/
= 1653
and the maximum vibration velocity = 0.58 m/s at 31-mode. Low-
temperature sinterable ‘hard’ piezoelectrics were also synthesized based
on the Sb, Li and Mn-substituted ceramics of 0.8Pb(Zr
0.5
Ti
0.5
)O
3
–
0.16Pb(Zn
1/3
Nb
2/3
)O
3
–0.04Pb(Ni
1/3
Nb
2/3
)O
3
, by adding CuO and Bi
2
O
3
,
giving rise to k
p
= 0.56, Q
m
(31-mode) = 1023, d
33
= 294 pC/N, ε
33
/ε
0
=
1282 and tanδ = 0.59%, and vibration velocity 0.41 m/s.
• Cu embedded multilayer piezo-transformers were trial-manufactured under
a low temperature sintering process (900 °C for 2 hours).
16.9 Future trends
Since the above conclusions have been derived only from a limited number
of PZT-based soft and hard piezoelectrics, it is too early to generalize these
conclusions. Further investigations are highly required, including:
• determination of the three (dielectric, elastic and piezoelectric) losses
for various piezoceramics;
• determination of loss anisotropy for piezoceramics;
• loss origin clarification through dynamic domain observations, and the
modeling of loss mechanisms in piezoelectrics;
• high-power density piezoelectric actuators and transducer developments.
16.10 Acknowledgement
Part of this research was supported by the Office of Naval Research through
the grant no. N00014-96-1-1173 and N00014-99-1-0754.
16.11 References
1. K. H. Haerdtl, Ceram. Int l., 8, 121–127 (1982).
2. T. Ikeda, Fundamentals of Piezoelectric Materials Science (Ohm Publication Co.,
Tokyo, 1984), p. 83.
3. N. Setter ed., Piezoelectric Materials in Devices (2002).
4. K. Uchino and S. Hirose, IEEE-UFFC Trans., 48, 307–321 (2001).
5. N. Bhattacharya and K. Uchino, IEEE-UFFC Trans. (2006) [on review].
6. J. Zheng, S. Takahashi, S. Yoshikawa, K. Uchino and J. W. C. de Vries, J. Amer.
Ceram. Soc., 79, 3193–3198 (1996).
WPNL2204
Handbook of dielectric, piezoelectric and ferroelectric materials502
7. N. Uchida and T. Ikeda, Jpn. J. Appl. Phys., 6, 1079 (1967).
8. K. Uchino, Piezoelectric Actuators and Ultrasonic Motors (Kluwer Academic Publ.,
Boston, 1997), p. 197.
9. M. Umeda, K. Nakamura and S. Ueha, Jpn. J. Appl. Phys., 38, 3327–3330 (1999)
10. S. Hirose, M. Aoyagi, Y. Tomikawa, S. Takahashi and K. Uchino, Proc. Ultrasonics
Intl 95, Edinburgh, pp. 184–187 (1995).
11. S. Tashiro, M. Ikehiro and H. Igarashi, Jpn. J. Appl. Phys., 36, 3004–3009 (1997).
12. S. Takahashi and S. Hirose, Jpn. J. Appl. Phys., 32, 2422–2425 (1993).
13. K. Uchino, J. Zheng, A. Joshi, Y. H. Chen, S. Yoshikawa, S. Hirose, S. Takahashi
and J. W. C. de Vries, J. Electroceramics, 2, 33–40 (1998).
14. J. Ryu, H. W. Kim, K. Uchino and J. Lee, Jpn. J. Appl. Phys., 42, No. 3, 1–4 (2003).
15. Y. Gao, K. Uchino and D. Viehland, J. Appl. Phys., 92, 2094–2099 (2002).
16. K. Uchino, Ferroelectric Devices (Marcel Dekker, Inc., New York, 2000), p. 63.
17. Park, S.-H., S. Ural, C.-W. Ahn, S. Nahm and K. Uchino, Jpn. J. Appl. Phys., 45,
2667–2673 (2006).
WPNL2204

503
17.1 Introduction
Bi
2
O
3
–ZnO–Nb
2
O
5
(BZN)-based pyrochlore ceramics are lead-free dielectrics
with high permittivity, low loss and low sintering temperatures. BZN dielectric
ceramics were first developed in the 1970s by Chinese researchers for low
sintering temperature multilayer ceramic capacitors (MLCC) (ZP Wang et
al., 1985; Li et al., 1986). However, the very complex chemical compositions
and unknown complicated phase structures frustrated the early attempts to
make these promising low-sintering dielectrics in practical applications for
MLCC. In the late 1980s, BZN compositions were picked up and studied by
Yan and coworkers in Bell Lab (MF Yan et al., 1990; Ling, 1990). Their
studies involved much simplified chemical compositions using a two-step
powder process technology by mixing together calcined powders of BZN
and Bi
2
O
3
–NiO–Nb
2
O
5
(BNN). The resulting phase structures were still
complex and unclear. From the late 1980s up to now, Yao’s group has been
studying the Bi
2
O
3
–ZnO–Nb
2
O
5
(BZN) system and related dielectric materials
systematically. From their early studies (DH Liu et al., 1993; H Wang et al.,
1994a,b, 1995, 1996a,b, 1997; XL Wang et al., 1997; Cai et al., 1994a,b, c;),
the cubic pyrochlore structure was first recognized as a main phase in this
system in the compositions around Bi
3x
Zn
2–2x
Nb
2–x
O
7
(x = 0.45–0.55), while
another main phase Bi
2
(Zn
2/3
Nb
4/3
)O
7
with low symmetry in this system was
identified as orthorhombic then monoclinic pyrochlore. The PDF file 54-971
for (Bi
1.5
Zn
0.5
)(Zn
0.5
Nb
1.5
)O
7
cubic pyrochlore and 54-972 for Bi
2
(Zn
1/3
Nb
2/3
)
2
O
7
pyrochlore were added to the International Center of Diffraction
Data. The continuous investigation has been focused on the structure–property
relations including phase diagram, phase equilibrium and dielectric property
optimization. Those fundamental works provide a useful understanding of
the BZN material system and accelerate the studies for dielectric properties
improvements and potential applications.
With the recent development in low-temperature co-fired ceramic (LTCC)
devices, the demands for new materials that can be co-fired with base metal
17
Bismuth-based pyrochlore dielectric
ceramics for microwave applications
HONG WANG and X YAO,
Xi’an Jiaotong University, China
WPNL2204

Handbook of dielectric, piezoelectric and ferroelectric materials504
electrodes at lower temperatures (normally less than 1000 °C) have significantly
increased in the past decade. Bismuth-based pyrochlore dielectrics thus attracted
more and more attention due to their excellent dielectric properties and
lower sintering temperatures and have become promising candidates for
LTCC and microwave passive components since late 1990s (Cann et al.,
1996; Mergen et al., 1996, 1997). High-performance BZN-based temperature
stable dielectrics with low sintering temperature below 940 °C were developed
for multilayer ceramic capacitors (MLCC) (M Chen et al., 1998; Du et al.,
2001). Recent publications reported on the formation, stability, processing
windows and crystallographic characterization in this system as well as
successful manufacturing of prototype devices including LC filters and LTCC
components (H Wang, 1999a, 2004a; Randall, 2003; Zanetti, 2004). The
high tunability found in BZN cubic pyrochlore thin films makes this material
a new candidate for microwave tunable devices which may replace the
conventional (Ba
x
Sr
1–x
)TiO
3
thin film (Ren et al., 2001). Potential applications
of BZN dielectrics include their use in MLCC, tunable filters, phase shifters,
and electrically steerable antennas.
This chapter aims to review the highlights of the bismuth-based pyrochlore
dielectrics developed so far and discuss the strategy for tailoring both structure
and performance towards applications.
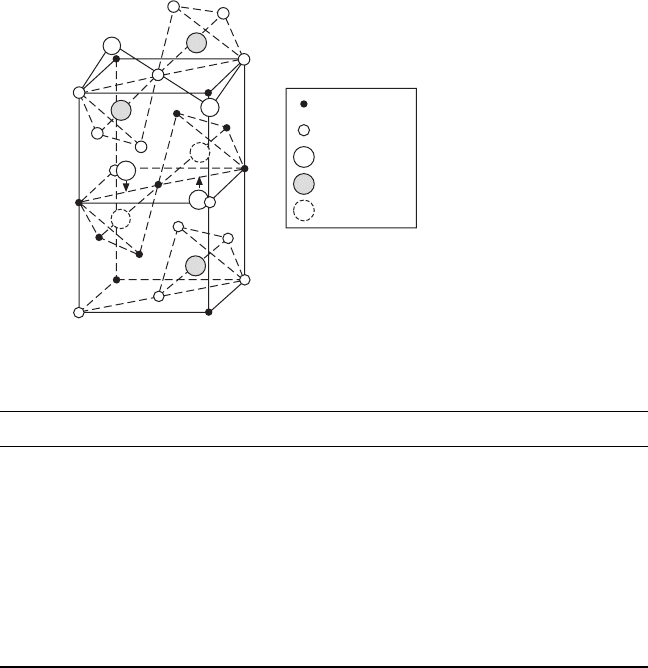
17.2 Crystal structures in the BZN system
The general formula of oxide pyrochlores can be written as A
2
B
2
O
6
O′ with
four crystallographically non-equivalent ions which are A (site 16d), B (site
16c), O (site 48f), and O′ (site 8b) (Subramanian, 1983). The space group of
an ideal pyrochlore structure is
Fd3m O
h
–
7
and there are eight formula
units per unit cell (Z = 8). Figure 17.1 shows the schematic of 1/4 unit cell
of pyrochlore structure. The pyrochlore can be regarded as a derivative
structure from a defective fluorite structure with anion vacancies on 8a sites.
Table 17.1 gives the atomic coordinate data of an ideal cubic pyrochlore.
Owing to the existence of vacancies on 8a site, the 48f anions thus have a
balance shift towards the two neighboring B cations. The A cations (usually
with ~1 Å ionic radius) are eight coordinated and are located within
scalenohedra (distorted cubes) that contains two equally spaced O′ anions at
a slightly shorter distance from the central cations (A
2
O
6
′
O
2
). The smaller B
cations (~ 0.6 Å ionic radius) are six coordinated and are located within
trigonal antiprisms (distorted octahedral, BO
6
) with all the six anions at
equal distances from the central cation. Thus the pyrochlore structure can be
described as a 3D network with the corner-sharing BO
6
octahedra and the
eight coordinated A cations (A
2
O
6
′
O
2
) locating in the interstices of BO
6
network (see Fig. 17.2).
The main crystal structures in the BZN ternary system were revealed as a
cubic pyrochlore (α)
Fd3m O
h
–
7
, Z = 8 and a low symmetry pyrochlore (β),
WPNL2204
Bismuth-based pyrochlore dielectric ceramics 505
which was indexed as orthorhombic first, then determined as monoclinic
zirconolite-like pyrochlore (C2/c –
C
h2
6
) (H Wang et al., 1994b; XL Wang et
al., 1997; Levin et al. 2002b).
17.2.1 Crystal structure of cubic pyrochlore
The cubic pyrochlores in the BZN ternary system were first observed in the
region of Bi
x
Zn
2/3
Nb
4/3
O
4+3x/2
(1 ≤ x ≤ 10/6), Bi
x
Zn
8/3–x
Nb
4/3
O
6+x+2
(1 ≤ x ≤
10/6), and Bi
x
Zn
2–2x/3
Nb
2–x/3
O
7
(1.5 ≤ x ≤ 1.8) (DH Liu et al., 1993). It was
found to exist in a wide region around the composition of Bi
1.5
ZnNb
1.5
O
7
(H Wang et al., 1994b, 1996a; XL Wang et al., 1997), while the ideal pyrochlore
composition Bi
2
(Zn
1/3
Nb
2/3
)
2
O
7
was identified as a low symmetry pyrochlore
(H Wang et al., 1996b, 1997; XL Wang et al., 1997).
The structure of Bi
1.5
ZnNb
1.5
O
7
cubic pyrochlore was studied using X-
ray diffraction (H Wang et al., 1994b, 1995). By comparing the theoretically
B [
16c
]
A [
16d
]
O [
48f
]
O′ [
8b
]
Vacancy [
8a
]
17.1
Schematic of pyrochlore structure (1/4 unit cell).
Table 17.1
Pyrochlore (A
2
B
2
O
6
O′) structural data (origin on the B site)
Ion Location Site symm. Coordinates
16A 16d D
3d
(0,0,0;0,1/2,1/2;1/2,0,1/2;1/2,1/2,0) +
1/2,1/2,1/2; 1/2,1/4,1/4;1/4,1/2,1/4;1/4,1/4,1/2
16B 16c D
3d
0,0,0;0,1/4,1/4;1/4,0,1/4;1/4,1/4,0
48O 48f C
2v
x
,1/8,1/8;–
x
,7/8,7/8;1/4–
x
,1/8,1/8;3/4+
x
,7/8,7/8
1/8,
x
,1/8;7/8,–
x
,7/8;1/8,1/4–
x
,1/8;7/8,3/4+
x
,7/8
1/8,1/8,
x
;7/8,7/8,–
x
;1/8,1/8,1/4–
x
;7/8,7/8,3/4+
x
8O
′
8b T
d
3/8,3/8,3/8;5/8,5/8,5/8
x
for regular octahedra: 0.3125 (5/16)
x
for regular cube: 0.375 (3/8)
WPNL2204
Handbook of dielectric, piezoelectric and ferroelectric materials506
calculated X-ray diffraction patterns with the observed ones, the chemical
formula of this material was determined as a stuffed (Bi
1.5
Zn
0.5
)(Zn
0.5
Nb
1.5
)O
7
pyrochlore with disordered cation distribution (H Wang et al., 1995). The
lattice parameter was refined by the Rietveld method as a = 10.555 Å.
Combining with Raman spectroscopy, the site occupation of
(Bi
1.5
Zn
0.5
)(Zn
0.5
Nb
1.5
)O
7
was found to occur in such a way that Zn
2+
is apt
to occupy the B site first and then enters the A site after the B site is fully
occupied (H Wang et al., 2003).
Levin et al. (2002a) have carried out a careful structural investigation on
the Bi
1.5
ZnNb
1.5
O
7
composition using combined electron, X-ray and neutron
powder diffraction techniques.
Their results showed small amounts of ZnO
existing in addition to the main cubic pyrochlore phase of Bi
1.5
ZnNb
1.5
O
7
.
The single pyrochlore phase forms at the composition Bi
1.5
Zn
0.92
Nb
1.5
O
6.92
.
Rietveld refinements using neutron powder diffraction data confirmed an
average pyrochlore structure A
2
B
2
O
6
O’ (
Fd3m O
h
–
7
; a = 10.5616(1) Å)
with both Bi and Zn mixed on the A-sites. Refinements also revealed significant
local deviations from the ideal pyrochlore arrangement which were caused
by apparent displacive disorder on both the A and O′ sites. The best fit was
obtained with a disordered model in which the A-cations were randomly
displaced by ~0.39 Å from the ideal eight-fold coordinated positions. The
refined structural parameters of (Bi
1.5
Zn
0.5
)(Zn
0.5
Nb
1.5
)O
7
is presented in
Table 17.2.
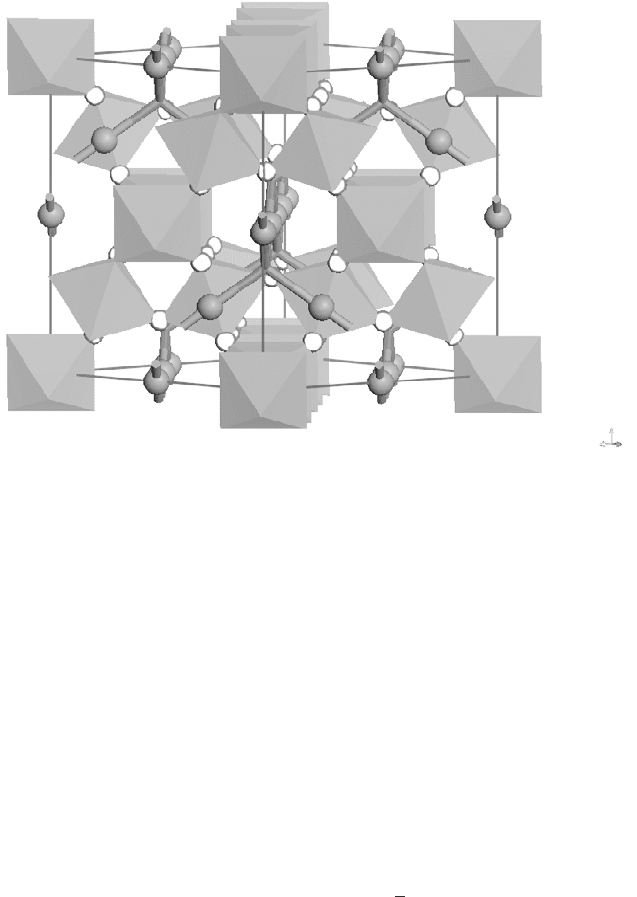
Z
XY
17.2
Crystal structure of the BZN cubic pyrochlore (BO
6
network, 䊉 A
site; octahedral: B site; 䊊 O atom).
WPNL2204

Bismuth-based pyrochlore dielectric ceramics 507
17.2.2 Crystal structure of monoclinic zirconolite-like
structure
The low-symmetry phase in BZN system was identified as orthorhombic
first and then as monoclinic pyrochlore by using X-ray diffraction technology
on ceramic powder (XL Wang et al., 1997; H Wang et al., 2001a). Attempts
to grow the single crystal of this low-symmetry phase were not successful
because this β phase is incongruent (H Wang et al., 2001b). Levin et al.
(2002b) used electron, X-ray and neutron diffraction to investigate the β
phase in detail and obtained useful structural information of Bi
2
Zn
2/3
Nb
4/3
O
7
composition. The crystal structure of Bi
2
Zn
2/3
Nb
4/3
O
7
(see Fig. 17.3) was
thus determined as a monoclinic zirconolite-like structure (space group C2/
c –
C
h2
6
, a = 13.1037(9) Å, b = 7.6735(3) Å, c = 12.1584(6) Å, β = 101.318(5)°].
According to the structural refinement using neutron diffraction data, Nb
preferentially occupies the six-fold coordinated sites in octahedral sheets
parallel to the (001) planes, while Zn is statically distributed between the
two half-occupied (5+1)-fold coordinated sites near the centres of six-membered
rings of the [Nb(Zn)O
6
] octahedral. The Nb/Zn cation layers alternate along
the c-axis with Bi-layers, in which Bi cations occupy both eight- and seven-
fold coordinated sites.
16.2.3 Non-stoichiometric pyrochlores and local
crystal chemistry
The pyrochlore with the formula A
2
B
2
O
7
is often written as A
2
B
2
O
6
O′ to
distinguish the oxygen atoms in the two different networks of BO
6
and A
2
O′.
The ‘defect’ pyrochlores form easily since the network of A
2
O′ can be partially
occupied or even completely absent. In the bismuth-based pyrochlores, the
composition Bi
1.5
ZnNb
1.5
O
7
was shown to be a two-phase mixture of pyrochlore
and ZnO. The single-phase cubic pyrochlore structure was obtained at the
composition Bi
1.5
Zn
0.92
Nb
1.5
O
6.92
where A sites were assumed to be occupied
by a disordered mixture of Bi
3+
, Zn
2+
and vacancies (Levin et al., 2002a).
Table 17.2
Room temperature structural parameters of (Bi
1.5
Zn
0.5
)(Zn
0.5
Nb
1.5
)O
7
pyrochlore (Melot
et al.
, 2006)
Atom Site Occ.
xyzU
Bi/Zn 96
g
0.125/0.035 0.4689(1) 0.5174(2) 0.5174(2) 1.55(3)
Nb/Zn 16
c
0.750/0.250 0 0 0 1.32(2)
O′ 96
g
0.0767 0.3443(2) 0.3443(2) 0.3770(4) 2.2(1)
O48
f
1 0.31996(4) 1/8 1/8 2.20*
*
U11
, 3.30(3);
U22
=
U33
1.64(2);
U23
, 0.90(2). SG (space group).
Fd3m
(No. 227,
origin 2)
a
= 10.5555(5) Å,
χ
2
= 1.872. Anisotropic parameters are presented for O.
WPNL2204
Handbook of dielectric, piezoelectric and ferroelectric materials508
The single phase cubic pyrochlores were found mostly in the non-stoichiometric
compositions, such as Bi
1.50
Zn
0.45
(Zn
0.46
Nb
1.54
)O
7
, Bi
1.50
Zn
0.45
(Zn
0.48
Nb
1.52
)O
7
,
Bi
1.55
Zn
0.42
(Zn
0.49
Nb
1.51
)O
7
, Bi
1.60
Zn
0.40
(Zn
0.53
Nb
1.47
)O
7
, and Bi
1.63
Zn
0.35
(Zn
0.53
Nb
1.47
)O
7
, with Zn
2+
being partially absent on A sites while the B sites
being fully occupied by Nb
5+
and Zn
2+
(Vanderah et al., 2005). The structural
study using Raman spectroscopy revealed that the site occupation of Bi
1.5–
2y
Zn
1+2y
Nb
1.5
O
7–y
(–0.3 ≤ y ≤ 0.3) could be explained as Bi
1.5–2y
Zn
0.5+2y
(Zn
0.5
Nb
1.5
)O
7–y
with Zn
2+
occupying the B site first and then entering the A
site after the B site was fully occupied (H Wang et al., 2003; Du et al.,
2004a). The ZnO deficiency compared to Bi
1.5
ZnNb
1.5
O
7
was also confirmed
by stoichiometric study on the compositions Bi
3+y
Zn
2–x
Nb
3–y
O
14–x–y
(–0.11 ≤
y ≤ 0.14, –0.03 ≤ y ≤ 0.31) (Tan et al., 2005). Local crystal chemistry and
structure diffused scattering on (Bi
1.5–α
Zn
0.5–β
)(Zn
0.5–γ
Nb
1.5–δ
)O
7–1.5α–β–γ–2.5δ
and (Bi
1–x
Y
x
)(M
III
Nb
V
)O
7
(M = Fe
3+
, In
3+
) were carefully studied by using
electron diffraction technique (Withers et al., 2004; Y Liu et al., 2006, 2007;
Somphon et al., 2006). The fundamental underlying crystal chemistry of
bismuth-based pyrochlore is based on a strong local displacive disorder in
the A
2
O′ network which gives rise to the dielectric relaxation in the cubic
bismuth-based pyrochlores (Withers et al., 2004; Vanderah et al., 2005; Y
Liu et al., 2007). The displacive disorder in the (Bi
1.5
Zn
0.5
)(Zn
0.5
Nb
1.5
)O
7
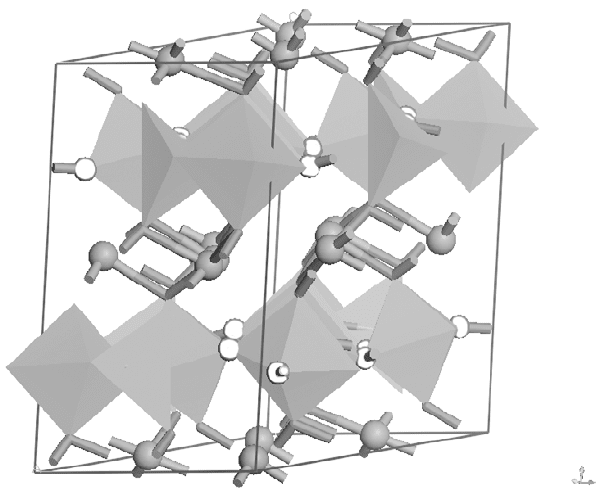
,
Z
X
Y
17.3
Crystal structure of the monoclinic zirconolite-like phase. (BO
6
network, 䊉 A site; Octahedral: B site; 䊊 O atom)
WPNL2204
Bismuth-based pyrochlore dielectric ceramics 509
(Bi
1.5
Zn
0.5
)(Zn
0.5
Sb
1.5
)O
7
and (Bi
1.5
Zn
0.5
) (Zn
0.5
Sb
1.5
)O
7
pyrochlores was
studied by time-of-flight neutron powder diffraction (Melot et al., 2006).
Although the precise nature of the disorder in the three pyrochlores is quite
similar, the reported dielectric constants of the three compounds are related
to the extent of local displacement, and (Bi
1.5
Zn
0.5
)(Zn
0.5
Nb
1.5
)O
7
with the
largest extent of local atomic displacement of A and O′ is reported to have
the highest dielectric constant.
17.3 Phase equilibrium and phase relation of
BZN pyrochlores
17.3.1 Phase equilibrium and phase diagram
The phase equilibrium, phase formation, and phase relations in the Bi
2
O
3
–
ZnO–Nb
2
O
5
ternary system were investigated (H Wang et al., 1998; Kim
et al., 2002; Tan et al., 2005; Vanderah et al., 2005). The ceramic samples in
a triangular area (see Fig. 17.4) around the cubic pyrochlore phase
Bi
1.5
ZnNb
1.5
O
7
with the compositions of (Bi
3x
Zn
2–3x
)(Zn
x
Nb
2–x
)O
7
(0 ≤ x ≤
2/3), (Bi
2–x
Zn
x
)(Zn
(5–x)/15
Nb
(10+x)/15
)
2
O
7–0.3x
(0 ≤ x ≤ 2), (Bi
2–2x
Zn
x
)(Zn
(1–x)/
3
Nb
(2+x)/3
)
2
O
7–x
(0 ≤ x ≤ 1) and some other compositions around the ‘ideal’
pyrochlore composition Bi
2
(Zn
1/3
Nb
2/3
)O
7
were selected and prepared by
conventional powder processing technique (H Wang et al., 1998). Quenching
technology was adopted to keep the high-temperature phase of the samples
after they reached the equilibrium. Based on X-ray diffraction analysis and
thermal analysis, the phase formation was studied from 550 °C to 1050 °C
with a temperature increment of 50 °C. The isothermal phase diagrams have
been obtained. The pure α phase region and β phase region were determined
in different temperatures, while the α–β co-existing phase was found to exist
between the two single phases region. The phase diagrams of pyrochlores in
the Bi
2
O
3
–ZnO–Sb
2
O
5
(Miles et al., 2006), Bi
2
O
3
–NiO–Nb
2
O
5
(Valant et
al., 2005) and Bi
2
O
3
–ZnO–Ta
2
O
5
(Khaw et al., 2007) ternary systems have
also been elaborated.
17.3.2 Phase formation of cubic pyrochlore and
monoclinic pyrochlore
The phase formation study was carried out by X-ray diffraction (XRD)
technique and thermal analysis (XL Wang et al., 1997; H Wang, 1998).
The
results show that Bi
2
O
3
, ZnO and Nb
2
O
5
do not react at 450 °C in the BZN
system. X-ray diffraction lines of the 24Bi
2
O
3
·
ZnO phase were observed at
500 to 550 °C. The diffraction lines of Bi
2
O
3
disappeared at 550 °C while
those of 24Bi
2
O
3
·
ZnO increased. This indicates that Bi
2
O
3
reacts with ZnO
WPNL2204
