Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

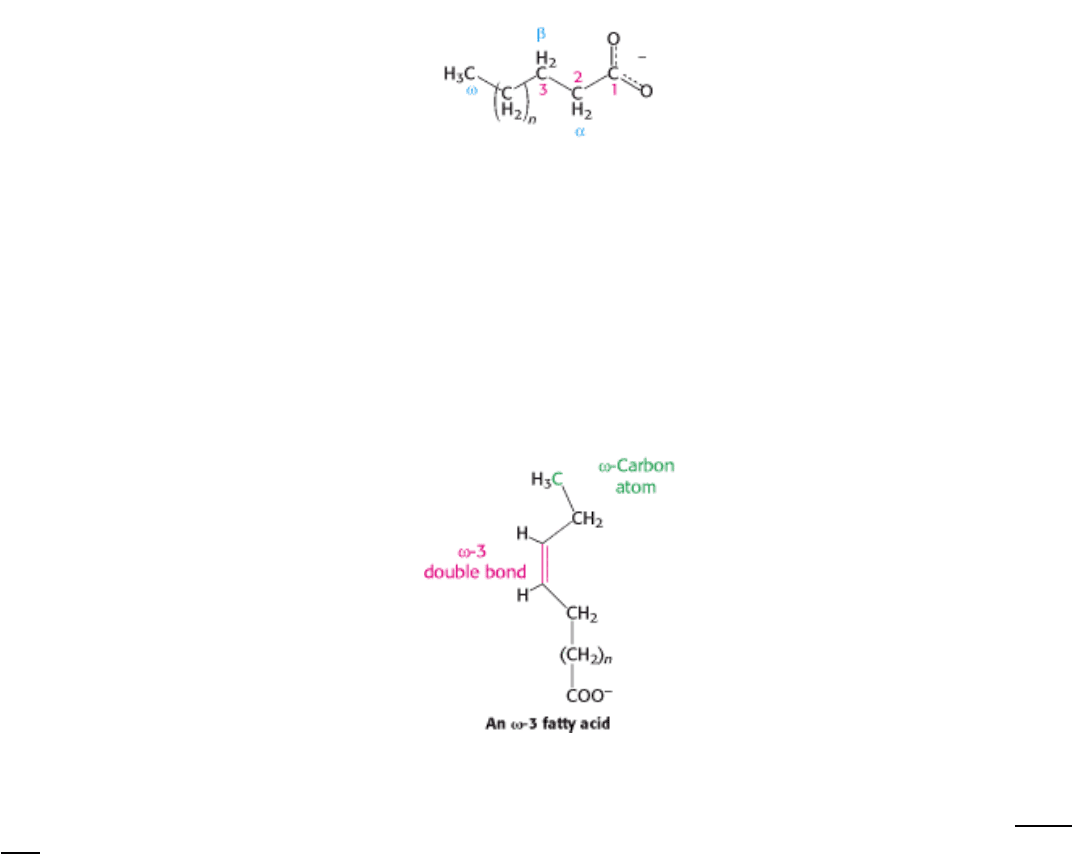
Fatty acid carbon atoms are numbered starting at the carboxyl terminus, as shown in the margin. Carbon atoms 2 and 3
are often referred to as α and β, respectively. The methyl carbon atom at the distal end of the chain is called the ω-
carbon atom. The position of a double bond is represented by the symbol ∆ followed by a superscript number. For
example, cis-∆
9
means that there is a cis double bond between carbon atoms 9 and 10; trans-∆
2
means that there is a
trans double bond between carbon atoms 2 and 3. Alternatively, the position of a double bond can be denoted by
counting from the distal end, with the ω-carbon atom (the methyl carbon) as number 1. An ω-3 fatty acid, for example,
has the structure shown in the margin. Fatty acids are ionized at physiological pH, and so it is appropriate to refer to
them according to their carboxylate form: for example, palmitate or hexadecanoate.
12.2.2. Fatty Acids Vary in Chain Length and Degree of Unsaturation
Fatty acids in biological systems usually contain an even number of carbon atoms, typically between 14 and 24 (Table
12.1). The 16- and 18-carbon fatty acids are most common. The hydrocarbon chain is almost invariably unbranched in
animal fatty acids. The alkyl chain may be saturated or it may contain one or more double bonds. The configuration of
the double bonds in most unsaturated fatty acids is cis. The double bonds in polyunsaturated fatty acids are separated by
at least one methylene group.
The properties of fatty acids and of lipids derived from them are markedly dependent on chain length and degree of
saturation. Unsaturated fatty acids have lower melting points than saturated fatty acids of the same length. For example,
the melting point of stearic acid is 69.6°C, whereas that of oleic acid (which contains one cis double bond) is 13.4°C.
The melting points of polyunsaturated fatty acids of the C
18
series are even lower. Chain length also affects the melting
point, as illustrated by the fact that the melting temperature of palmitic acid (C
16
) is 6.5 degrees lower than that of stearic
acid (C
18
). Thus, short chain length and unsaturation enhance the fluidity of fatty acids and of their derivatives.
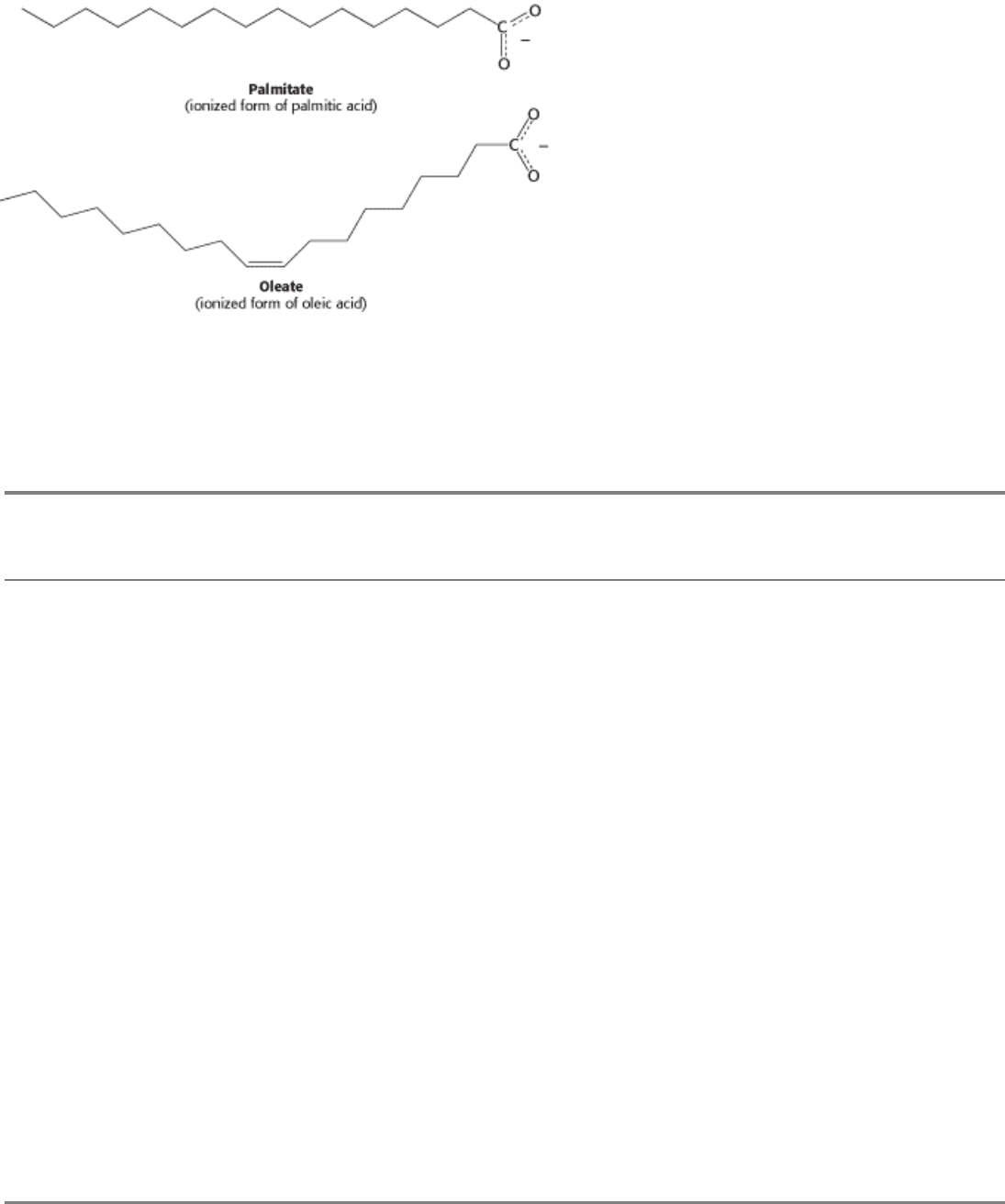
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.2. Fatty Acids Are Key Constituents of Lipids
Figure 12.2. Structures of Two Fatty Acids. Palmitate is a 16-carbon, saturated fatty acid, and oleate is an 18-carbon
fatty acid with a single cis double bond.
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.2. Fatty Acids Are Key Constituents of Lipids
Table 12.1. Some naturally occurring fatty acids in animals
Number of
carbons
Number of
double bonds
Common name Systematic name Formula
12 0 Laurate n-Dodecanoate
CH
3
(CH
2
)
10
COO
-
14 0 Myristate n-Tetradecanoate
CH
3
(CH
2
)
12
COO
-
16 0 Palmitate n-Hexadecanoate
CH
3
(CH
2
)
14
COO
-
18 0 Stearate n-Octadecanoate
CH
3
(CH
2
)
16
COO
-
20 0 Arachidate n-Eicosanoate
CH
3
(CH
2
)
18
COO
-
22 0 Behenate n-Docosanoate
CH
3
(CH
2
)
20
COO
-
24 0 Lignocerate n-Tetracosanoate
CH
3
(CH
2
)
22
COO
-
16 1 Palmitoleate
cis-∆
9
-Hexadecenoate
CH
3
(CH
2
)
5
CH=CH(CH
2
)
7
COO
-
18 1 Oleate
cis-∆
9
-Octadecenoate
CH
3
(CH
2
)
7
CH=CH(CH
2
)
7
COO
-
18 2 Linoleate
cis,cis-∆
9
,∆
12
-Octadecadienoate
CH
3
(CH
2
)
4
(CH=CHCH
2
)
2
(CH
2
)
6
COO
-
18 3 Linolenate
all-cis-∆
9
,∆
12
,∆
15
-
Octadecatrienoate
CH
3
CH
2
(CH=CHCH
2
)
3
(CH
2
)
6
COO
-
20 4 Arachidonate
all-cis-∆
5
,∆
8
,∆
11
,∆
14
-
Eicosatetraenoate
CH
3
(CH
2
)
4
(CH=CHCH
2
)
4
(CH
2
)
2
COO
-
I. The Molecular Design of Life 12. Lipids and Cell Membranes
12.3. There Are Three Common Types of Membrane Lipids
Lipids differ markedly from the other groups of biomolecules considered thus far. By definition, lipids are water-
insoluble biomolecules that are highly soluble in organic solvents such as chloroform. Lipids have a variety of biological
roles: they serve as fuel molecules, highly concentrated energy stores, signal molecules, and components of membranes.
The first three roles of lipids will be discussed in later chapters. Here, our focus is on lipids as membrane constituents.
The three major kinds of membrane lipids are phospho-lipids, glycolipids, and cholesterol. We begin with lipids found in
eukaryotes and bacteria. The lipids in archaea are distinct, although they have many features related to their membrane-
forming function in common with lipids of other organisms.
12.3.1. Phospholipids Are the Major Class of Membrane Lipids
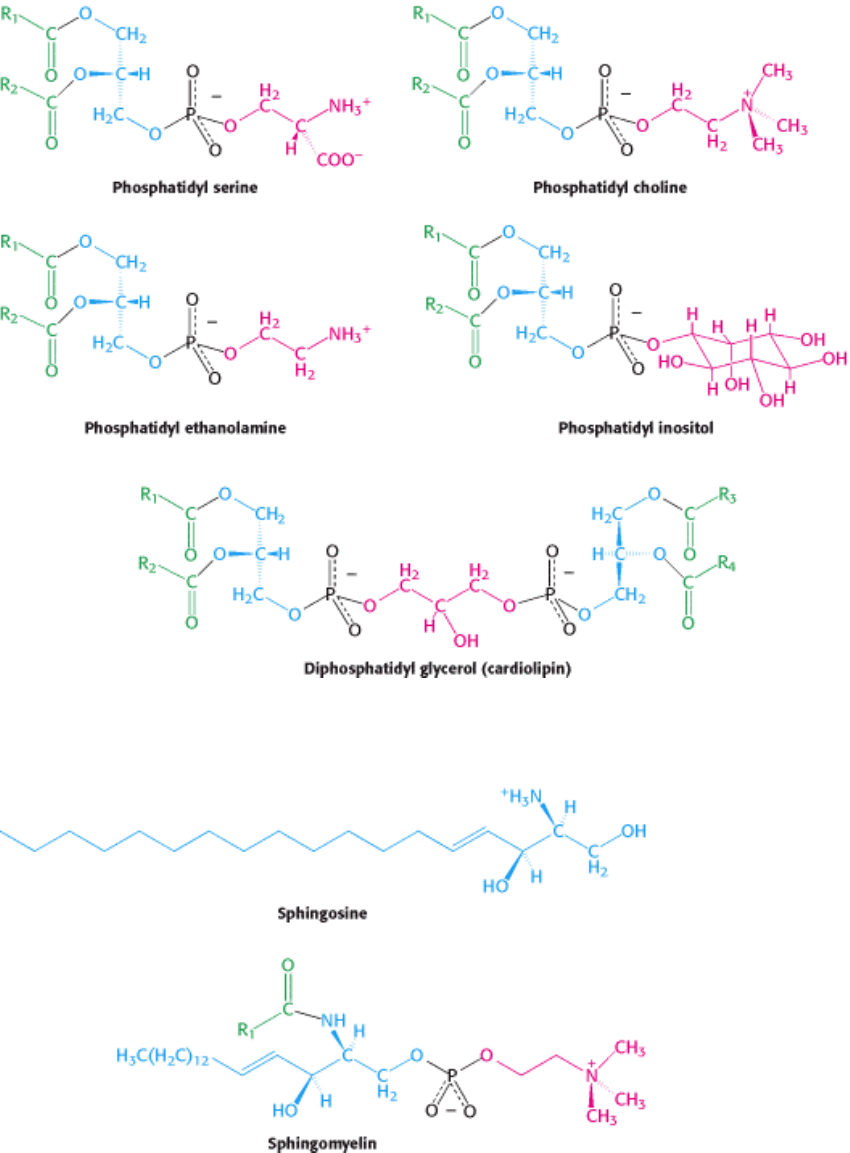
Phospholipids are abundant in all biological membranes. A phospholipid molecule is constructed from four components:
fatty acids, a platform to which the fatty acids are attached, a phosphate, and an alcohol attached to the phosphate (Figure
12.3). The fatty acid components provide a hydrophobic barrier, whereas the remainder of the molecule has hydrophilic
properties to enable interaction with the environment.
The platform on which phospholipids are built may be glycerol, a 3- carbon alcohol, or sphingosine, a more complex
alcohol. Phospholipids derived from glycerol are called phosphoglycerides. A phosphoglyceride consists of a glycerol
backbone to which two fatty acid chains (whose characteristics were described in Section 12.2.2) and a phosphorylated
alcohol are attached.
In phosphoglycerides, the hydroxyl groups at C-1 and C-2 of glycerol are esterified to the carboxyl groups of the two
fatty acid chains. The C-3 hydroxyl group of the glycerol backbone is esterified to phosphoric acid. When no further
additions are made, the resulting compound is phosphati-date (diacylglycerol 3-phosphate), the simplest
phosphoglyceride. Only small amounts of phosphatidate are present in membranes. However, the molecule is a key
intermediate in the biosynthesis of the other phosphoglycerides (Section 26.1). The absolute configuration of the glycerol
3-phosphate moiety of membrane lipids is shown in Figure 12.4.
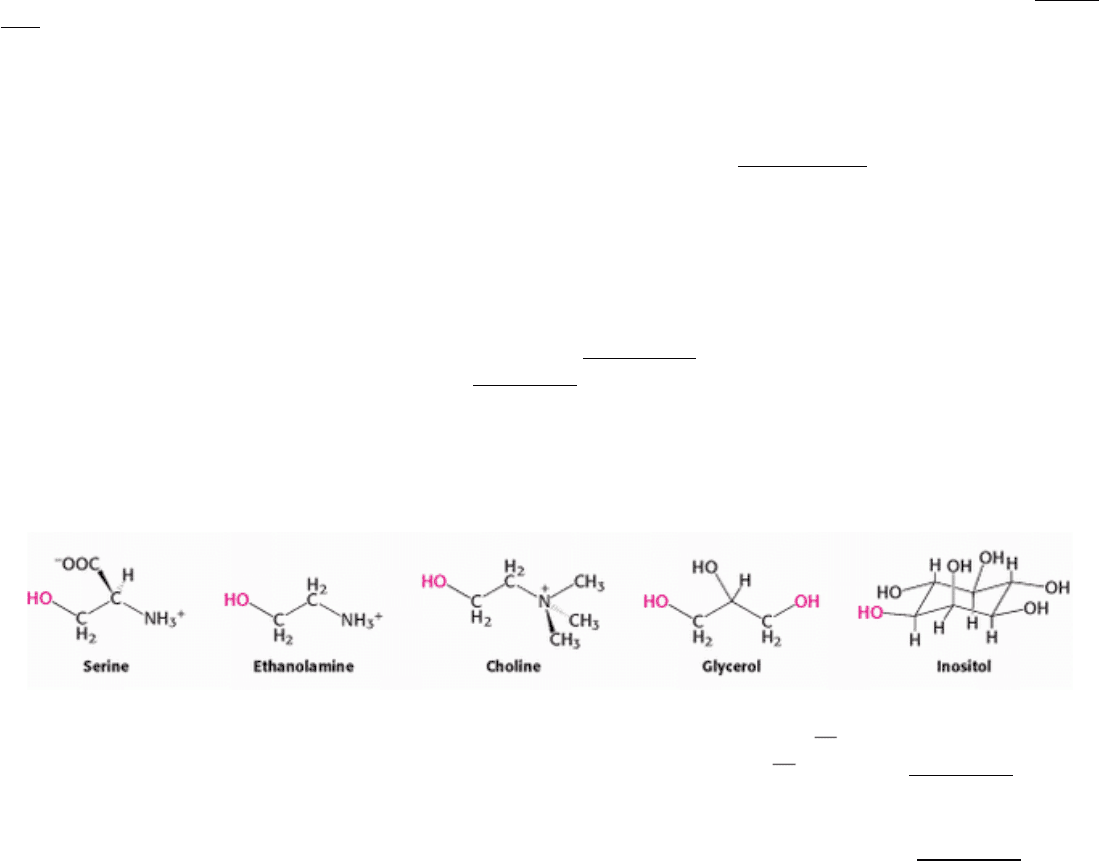
The major phosphoglycerides are derived from phosphatidate by the formation of an ester bond between the phosphate
group of phosphatidate and the hydroxyl group of one of several alcohols. The common alcohol moieties of
phosphoglycerides are the amino acid serine, ethanolamine, choline, glycerol, and the inositol.
The structural formulas of phosphatidyl choline and the other principal phosphoglycerides
namely, phosphatidyl
ethanolamine, phosphatidyl serine, phosphatidyl inositol, and diphosphatidyl glycerol are given in Figure 12.5.
Sphingomyelin is a phospholipid found in membranes that is not derived from glycerol. Instead, the backbone in
sphingomyelin is sphingosine, an amino alcohol that contains a long, unsaturated hydrocarbon chain (Figure 12.6). In
sphingomyelin, the amino group of the sphingosine backbone is linked to a fatty acid by an amide bond. In addition, the
primary hydroxyl group of sphingosine is esterified to phosphoryl choline.
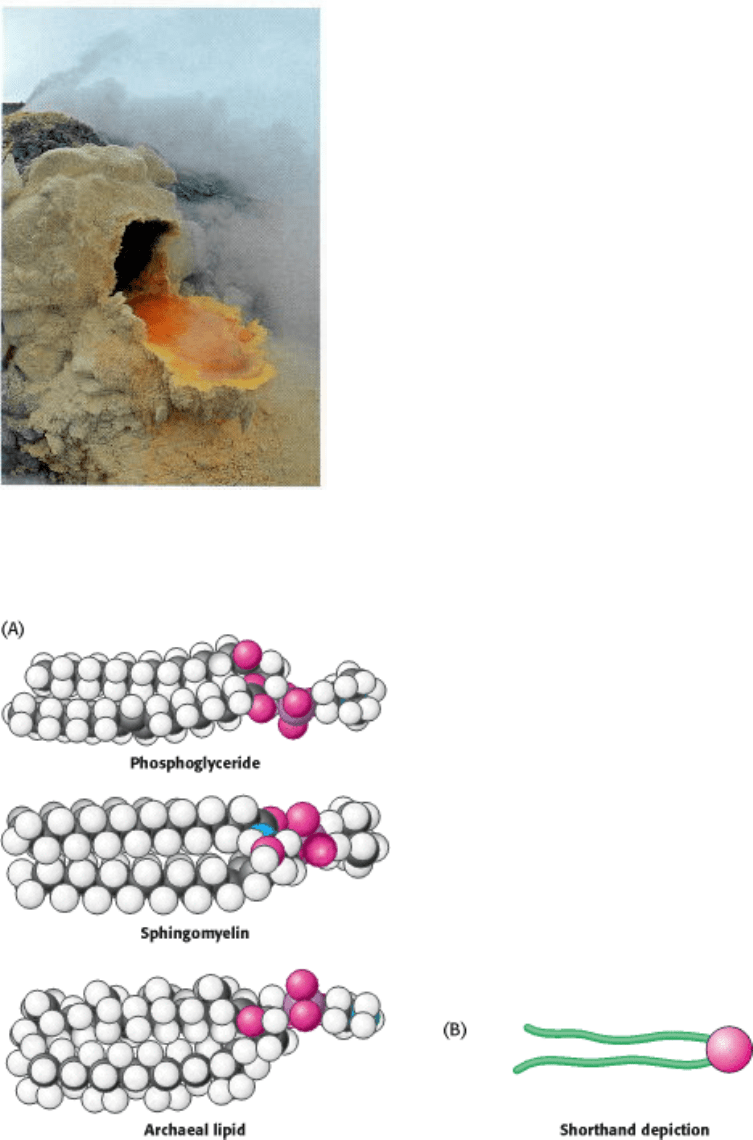
12.3.2. Archaeal Membranes Are Built from Ether Lipids with Branched Chains
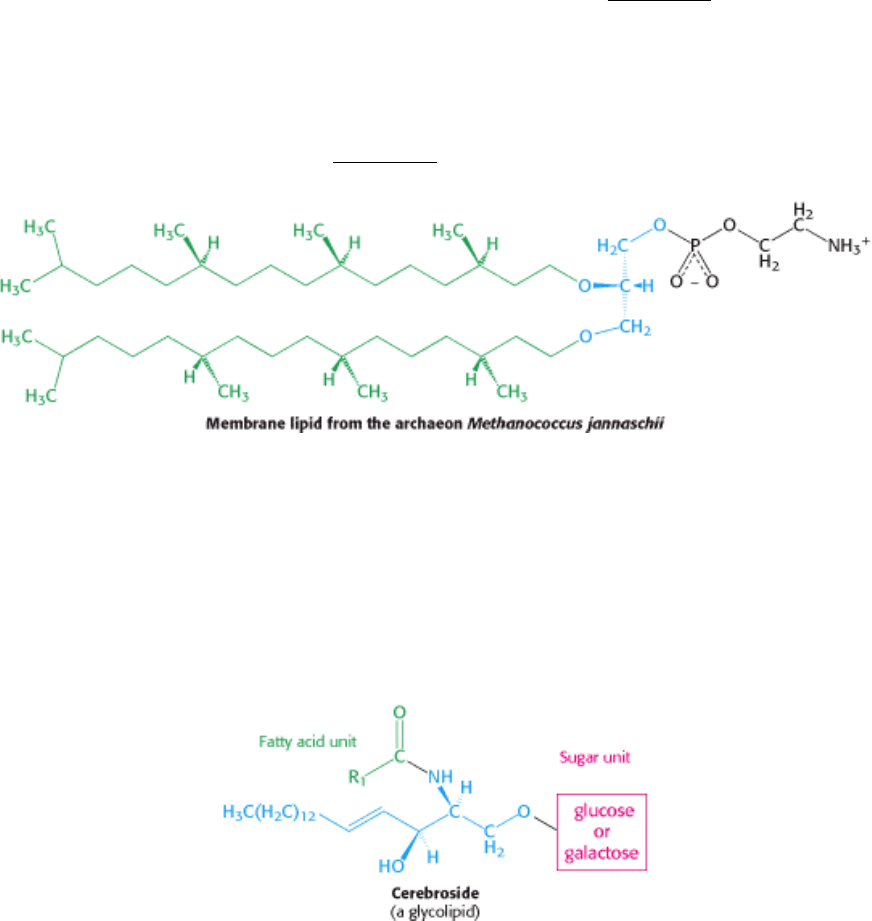
The membranes of archaea differ in composition from those of eukaryotes or bacteria in three important ways. Two of
these differences clearly relate to the hostile living conditions of many archaea (Figure 12.7). First, the nonpolar chains
are joined to a glycerol backbone by ether rather than ester linkages. The ether linkage is more resistant to hydrolysis.
Second, the alkyl chains are branched rather than linear. They are built up from repeats of a fully saturated five-carbon
fragment. These branched, saturated hydrocarbons are more resistant to oxidation. The ability of archaeal lipids to resist
hydrolysis and oxidation may help these organisms to withstand the extreme conditions, such as high temperature, low
pH, or high salt concentration, under which some of these archaea grow. Finally, the stereochemistry of the central
glycerol is inverted compared with that shown in Figure 12.4.
12.3.3. Membrane Lipids Can Also Include Carbohydrate Moieties
Glycolipids, as their name implies, are sugar-containing lipids. Like sphingomyelin, the glycolipids in animal cells are
derived from sphingosine. The amino group of the sphingosine backbone is acylated by a fatty acid, as in sphingomyelin.
Glycolipids differ from sphingomyelin in the identity of the unit that is linked to the primary hydroxyl group of the
sphingosine backbone. In glycolipids, one or more sugars (rather than phosphoryl choline) are attached to this group.
The simplest glycolipid, called a cerebroside, contains a single sugar residue, either glucose or galactose.
More complex glycolipids, such as gangliosides, contain a branched chain of as many as seven sugar residues.
Glycolipids are oriented in a completely asymmetric fashion with the sugar residues always on the extracellular side of
the membrane.
12.3.4. Cholesterol Is a Lipid Based on a Steroid Nucleus
Cholesterol is a lipid with a structure quite different from that of phospholipids. It is a steroid, built from four linked
hydrocarbon rings.

A hydrocarbon tail is linked to the steroid at one end, and a hydroxyl group is attached at the other end. In membranes,
the molecule is oriented parallel to the fatty acid chains of the phospholipids, and the hydroxyl group interacts with the
nearby phospholipid head groups. Cholesterol is absent from prokaryotes but is found to varying degrees in virtually all
animal membranes. It constitutes almost 25% of the membrane lipids in certain nerve cells but is essentially absent from
some intracellular membranes.
12.3.5. A Membrane Lipid Is an Amphipathic Molecule Containing a Hydrophilic and
a Hydrophobic Moiety
The repertoire of membrane lipids is extensive, perhaps even bewildering, at first sight. However, they possess a critical
common structural theme: membrane lipids are amphipathic molecules (amphiphilic molecules). A membrane lipid
contains both a hydrophilic and a hydrophobic moiety.
Let us look at a model of a phosphoglyceride, such as phosphatidyl choline. Its overall shape is roughly rectangular
(Figure 12.8A). The two hydrophobic fatty acid chains are approximately parallel to each other, whereas the hydrophilic
phosphoryl choline moiety points in the opposite direction. Sphingomyelin has a similar conformation, as does the
archaeal lipid depicted. Therefore, the following shorthand has been adopted to represent these membrane lipids: the
hydrophilic unit, also called the polar head group, is represented by a circle, whereas the hydrocarbon tails are depicted
by straight or wavy lines (Figure 12.8B).
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.3. There Are Three Common Types of Membrane Lipids
Figure 12.3. Schematic Structure of a Phospholipid.
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.3. There Are Three Common Types of Membrane Lipids
Figure 12.4. Structure of Phosphatidate (Diacylglycerol 3-Phosphate). The absolute configuration of the center
carbon (C-2) is shown.
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.3. There Are Three Common Types of Membrane Lipids
Figure 12.5. Some Common Phosphoglycerides Found in Membranes.
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.3. There Are Three Common Types of Membrane Lipids
Figure 12.6. Structures of Sphingosine and Sphingomyelin. The sphingosine moiety of sphingomyelin is highlighted
in blue.
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.3. There Are Three Common Types of Membrane Lipids
Figure 12.7. An Archaeon and Its Environment. Archaea can thrive in habitats as harsh as a volcanic vent. Here, the
archaea form an orange mat surrounded by yellow sulfurous deposits. [Krafft-Explorer/Photo Researchers.]
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.3. There Are Three Common Types of Membrane Lipids
Figure 12.8. Representations of Membrane Lipids. (A) Space-filling models of a phosphoglyceride, sphingomyelin,
and an archaeal lipid show their shapes and distribution of hydrophilic and hydrophobic moieties. (B) A shorthand
depiction of a membrane lipid.
I. The Molecular Design of Life 12. Lipids and Cell Membranes
12.4. Phospholipids and Glycolipids Readily Form Bimolecular Sheets in Aqueous
Media
What properties enable phospholipids to form membranes? Membrane formation is a consequence of the amphipathic
nature of the molecules. Their polar head groups favor contact with water, whereas their hydrocarbon tails interact with
one another, in preference to water. How can molecules with these preferences arrange themselves in aqueous solutions?
One way is to form a micelle, a globular structure in which polar head groups are surrounded by water and hydrocarbon
tails are sequestered inside, interacting with one another (Figure 12.9).
Alternatively, the strongly opposed preferences of the hydrophilic and hydrophobic moieties of membrane lipids can be
satisfied by forming a lipid bilayer, composed of two lipid sheets (Figure 12.10). A lipid bilayer is also called a
bimolecular sheet. The hydrophobic tails of each individual sheet interact with one another, forming a hydrophobic
interior that acts as a permeability barrier. The hydrophilic head groups interact with the aqueous medium on each side
of the bilayer. The two opposing sheets are called leaflets.
The favored structure for most phospholipids and glycolipids in aqueous media is a bimolecular sheet rather than a
micelle. The reason is that the two fatty acyl chains of a phospholipid or a glycolipid are too bulky to fit into the interior
of a micelle. In contrast, salts of fatty acids (such as sodium palmitate, a constituent of soap), which contain only one
chain, readily form micelles. The formation of bilayers instead of micelles by phospholipids is of critical biological
importance. A micelle is a limited structure, usually less than 20 nm (200 Å) in diameter. In contrast, a bimolecular sheet
can have macroscopic dimensions, such as a millimeter (10
6
nm, or 10
7
Å). Phospholipids and related molecules are
important membrane constituents because they readily form extensive bimolecular sheets (Figure 12.11).
The formation of lipid bilayers is a self-assembly process. In other words, the structure of a bimolecular sheet is inherent
in the structure of the constituent lipid molecules. The growth of lipid bilayers from phospholipids is a rapid and
spontaneous process in water. Hydrophobic interactions are the major driving force for the formation of lipid bilayers.
Recall that hydrophobic interactions also play a dominant role in the folding of proteins (Sections 1.3.4 and 3.4) and in
the stacking of bases in nucleic acids (Section 5.2.1). Water molecules are released from the hydrocarbon tails of
membrane lipids as these tails become sequestered in the nonpolar interior of the bilayer. Furthermore, van der Waals
attractive forces between the hydrocarbon tails favor close packing of the tails. Finally, there are electrostatic and
hydrogen-bonding attractions between the polar head groups and water molecules. Thus, lipid bilayers are stabilized by
the full array of forces that mediate molecular interactions in biological systems.
Because lipid bilayers are held together by many reinforcing, noncovalent interactions (predominantly hydrophobic),
they are cooperative structures. These hydrophobic interactions have three significant biological consequences: (1) lipid
bilayers have an inherent tendency to be extensive; (2) lipid bilayers will tend to close on themselves so that there are no
edges with exposed hydrocarbon chains, and so they form compartments; and (3) lipid bilayers are self-sealing because a
hole in a bilayer is energetically unfavorable.
12.4.1. Lipid Vesicles Can Be Formed from Phospholipids
The propensity of phospholipids to form membranes has been used to create an important experimental and clinical tool.
Lipid vesicles, or liposomes, aqueous compartments enclosed by a lipid bilayer (Figure 12.12), can be used to study
membrane permeability or to deliver chemicals to cells. Liposomes are formed by suspending a suitable lipid, such as
phosphatidyl choline, in an aqueous medium, and then sonicating (i.e., agitating by high-frequency sound waves) to give
a dispersion of closed vesicles that are quite uniform in size. Vesicles formed by these methods are nearly spherical in
shape and have a diameter of about 50 nm (500 Å). Larger vesicles (of the order of 1 µm, or 10
4
Å, in diameter) can be
prepared by slowly evaporating the organic solvent from a suspension of phospholipid in a mixed solvent system.
Ions or molecules can be trapped in the aqueous compartments of lipid vesicles by forming the vesicles in the presence
of these substances (Figure 12.13). For example, 50-nm-diameter vesicles formed in a 0.1 M glycine solution will trap
about 2000 molecules of glycine in each inner aqueous compartment. These glycine-containing vesicles can be separated
from the surrounding solution of glycine by dialysis or by gel-filtration chromatography (Section 4.1.3). The
permeability of the bilayer membrane to glycine can then be determined by measuring the rate of efflux of glycine from
the inner compartment of the vesicle to the ambient solution. Specific membrane proteins can be solubilized in the
presence of detergents and then added to the phospholipids from which liposomes will be formed. Protein-liposome
complexes provide valuable experimental tools for examining a range of membrane-protein functions.
Experiments are underway to develop clinical uses for liposomes. For example, liposomes containing drugs or
DNA for gene-therapy experiments can be injected into patients. These liposomes fuse with the plasma membrane
of many kinds of cells, introducing into the cells the molecules that they contain. Drug delivery with liposomes also
alters the distribution of a drug within the body and often lessens its toxicity. For instance, less of the drug is distributed
to normal tissues because longcirculating liposomes have been shown to concentrate in regions of increased blood
circulation, such as solid tumors and sites of inflammation. Moreover, the selective fusion of lipid vesicles with
particular kinds of cells is a promising means of controlling the delivery of drugs to target cells.
Another well-defined synthetic membrane is a planar bilayer membrane. This structure can be formed across a 1-mm
hole in a partition between two aqueous compartments by dipping a fine paintbrush into a membrane-forming solution,
such as phosphatidyl choline in decane. The tip of the brush is then stroked across a hole (1 mm in diameter) in a
partition between two aqueous media. The lipid film across the hole thins spontaneously into a lipid bilayer. The
electrical conduction properties of this macroscopic bilayer membrane are readily studied by inserting electrodes into
each aqueous compartment (Figure 12.14). For example, its permeability to ions is determined by measuring the current
across the membrane as a function of the applied voltage.
12.4.2. Lipid Bilayers Are Highly Impermeable to Ions and Most Polar Molecules
The results of permeability studies of lipid vesicles and electrical-conductance measurements of planar bilayers have
shown that lipid bilayer membranes have a very low permeability for ions and most polar molecules. Water is a
conspicuous exception to this generalization; it readily traverses such membranes because of its small size, high
concentration, and lack of a complete charge. The range of measured permeability coefficients is very wide (Figure
12.15). For example, Na
+
and K
+
traverse these membranes 10
9
times as slowly as does H
2
O. Tryptophan, a zwitterion
at pH 7, crosses the membrane 10
3
times as slowly as does indole, a structurally related molecule that lacks ionic groups.
In fact, the permeability coefficients of small molecules are correlated with their solubility in a nonpolar solvent relative
to their solubility in water. This relation suggests that a small molecule might traverse a lipid bilayer membrane in the
following way: first, it sheds its solvation shell of water; then, it becomes dissolved in the hydrocarbon core of the
membrane; finally, it diffuses through this core to the other side of the membrane, where it becomes resolvated by water.
An ion such as Na
+
tra-verses membranes very slowly because the replacement of its coordination shell of polar water
molecules by nonpolar interactions with the membrane interior is highly unfavorable energetically.
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.4. Phospholipids and Glycolipids Readily Form Bimolecular Sheets in Aqueous Media
Figure 12.9. Diagram of a Section of a Micelle. Ionized fatty acids readily form such structures, but most
phospholipids do not.
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.4. Phospholipids and Glycolipids Readily Form Bimolecular Sheets in Aqueous Media
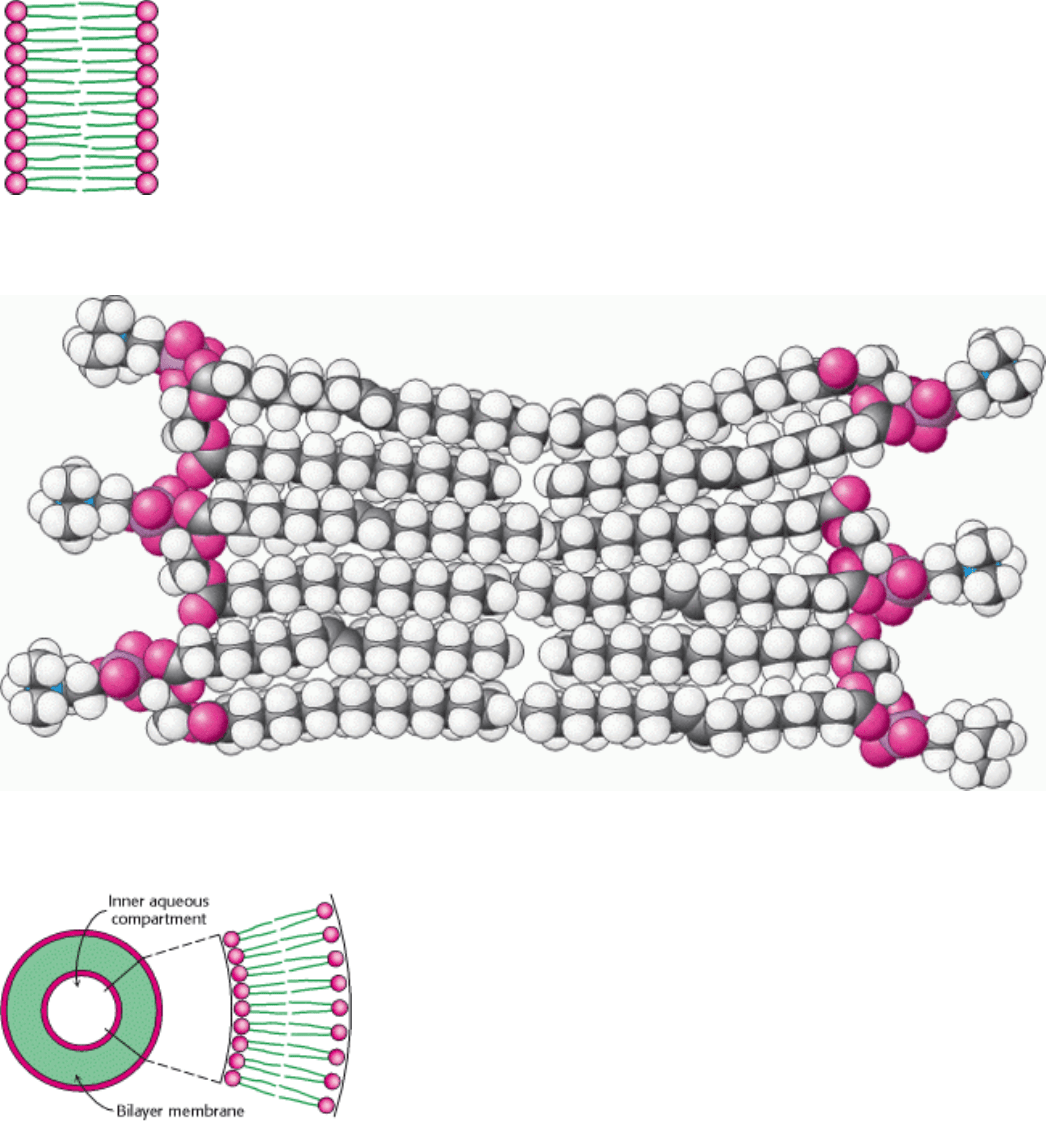
Figure 12.10. Diagram of a Section of a Bilayer Membrane.
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.4. Phospholipids and Glycolipids Readily Form Bimolecular Sheets in Aqueous Media
Figure 12.11. Space-Filling Model of a Section of Phospholipid Bilayer Membrane.
I. The Molecular Design of Life 12. Lipids and Cell Membranes 12.4. Phospholipids and Glycolipids Readily Form Bimolecular Sheets in Aqueous Media
Figure 12.12. Liposome. A liposome, or lipid vesicle, is a small aqueous compartment surrounded by a lipid bilayer.
