Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

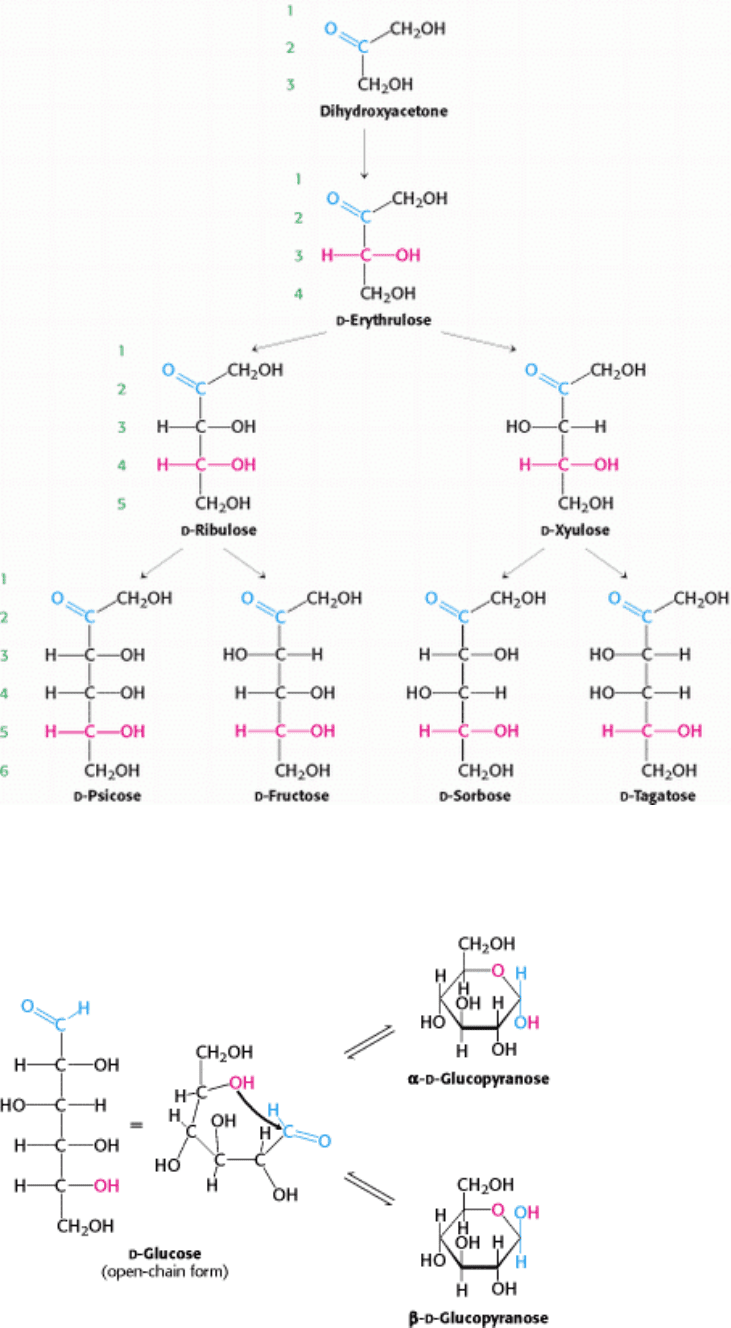
Figure 11.3.
d -Ketoses containing three- four, five, and six carbon atoms. The keto group is shown in blue. The
asymmetric center farthest from the keto group, which determines the d designation, is shown in red.
I. The Molecular Design of Life 11. Carbohydrates 11.1. Monosaccharides Are Aldehydes or Ketones with Multiple Hydroxyl Groups
Figure 11.4. Pyranose Formation. The open-chain form of glucose cyclizes when the C-5 hydroxyl group attacks the
oxygen atom of the C-1 aldehyde group to form an intramolecular hemiacetal. Two anomeric forms, designated α and β ,
can result.
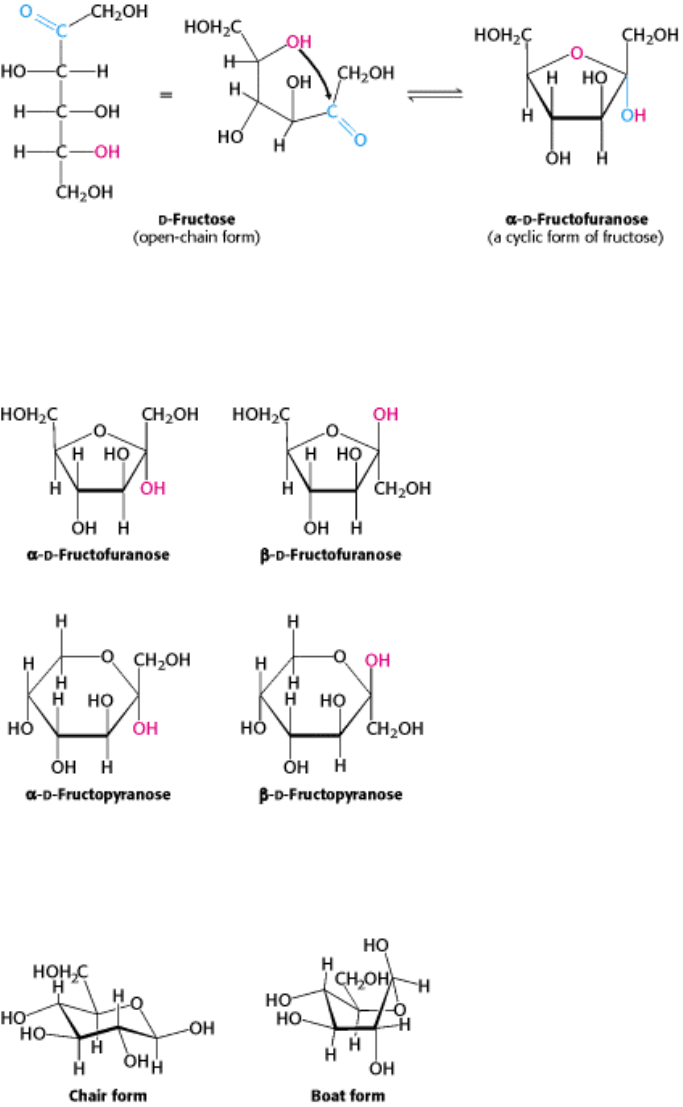
I. The Molecular Design of Life 11. Carbohydrates 11.1. Monosaccharides Are Aldehydes or Ketones with Multiple Hydroxyl Groups
Figure 11.5. Furanose Formation. The open-chain form of fructose cyclizes to a five-membered ring when the C-5
hydroxyl group attacks the C-2 ketone to form an intramolecular hemiketal. Two anomers are possible, but only the α
anomer is shown.
I. The Molecular Design of Life 11. Carbohydrates 11.1. Monosaccharides Are Aldehydes or Ketones with Multiple Hydroxyl Groups
Figure 11.6. Ring Structures of Fructose. Fructose can form both five-membered furanose and six-membered pyranose
rings. In each case, both α and β anomers are possible.
I. The Molecular Design of Life 11. Carbohydrates 11.1. Monosaccharides Are Aldehydes or Ketones with Multiple Hydroxyl Groups
Figure 11.7. Chair and Boat Forms of β -
d -glucopyranose. The chair form is more stable because of less steric
hindrance as the axial positions are occupied by hydrogen atoms.
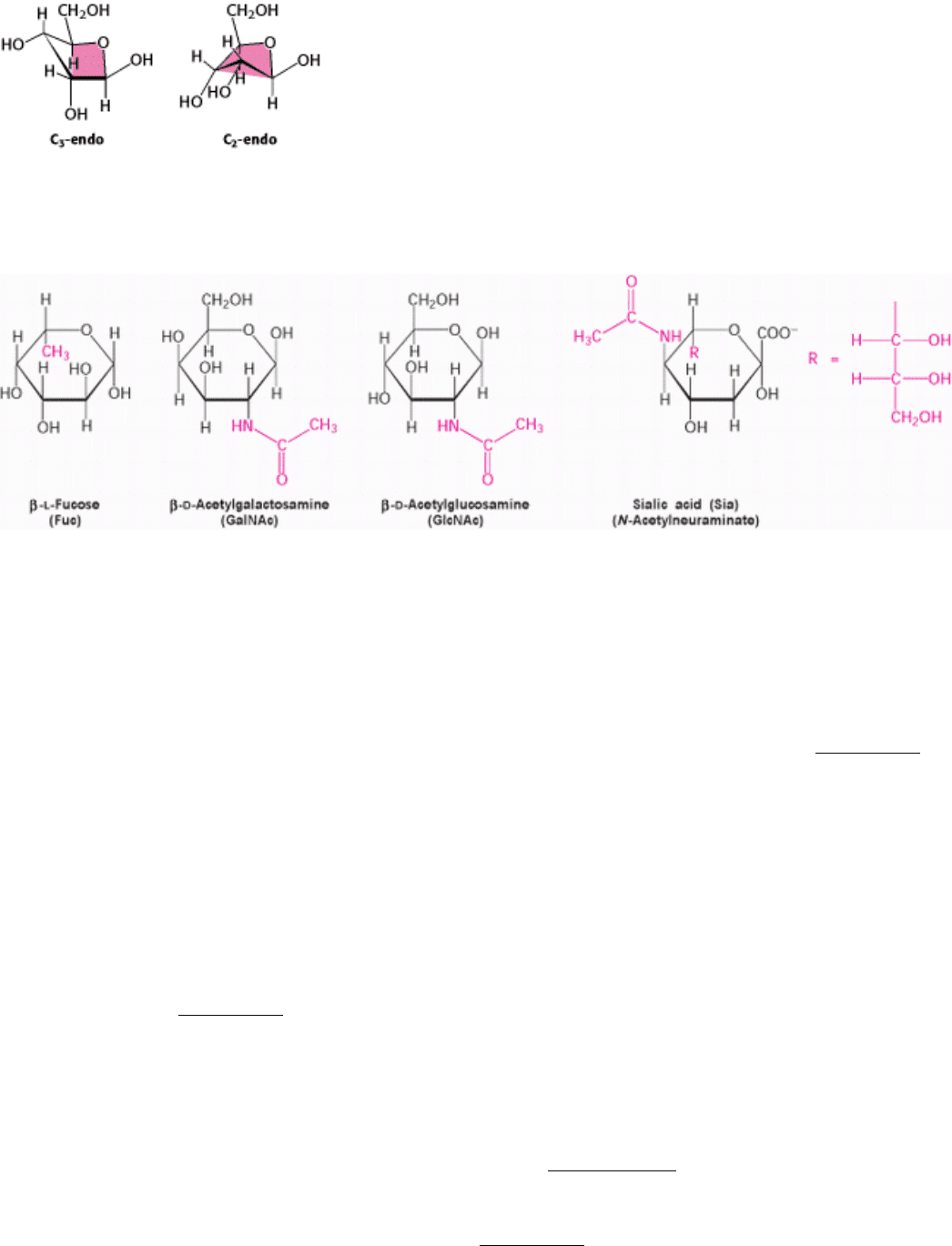
I. The Molecular Design of Life 11. Carbohydrates 11.1. Monosaccharides Are Aldehydes or Ketones with Multiple Hydroxyl Groups
Figure 11.8. Envelope Conformations of β - d -ribose. The C
2
-endo and C
3
-endo forms of β -d-ribose are shown. The
color indicates the four atoms that lie approximately in a plane.
I. The Molecular Design of Life 11. Carbohydrates 11.1. Monosaccharides Are Aldehydes or Ketones with Multiple Hydroxyl Groups
Figure 11.9. Modified Monosaccharides. Carbohydrates can be modified by the addition of substituents (shown in red)
other than hydroxyl groups. Such modified carbohydrates are often expressed on cell surfaces.
I. The Molecular Design of Life 11. Carbohydrates
11.2. Complex Carbohydrates Are Formed by Linkage of Monosaccharides
Because sugars contain many hydroxyl groups, glycosidic bonds can join one monosaccharide to another.
Oligosaccharides are built by the linkage of two or more monosaccharides by O-glycosidic bonds (Figure 11.10). In
maltose, for example, two d-glucose residues are joined by a glycosidic linkage between the α -anomeric form of C-1 on
one sugar and the hydroxyl oxygen atom on C-4 of the adjacent sugar. Such a linkage is called an α -1,4-glycosidic
bond. The fact that monosaccharides have multiple hydroxyl groups means that various glycosidic linkages are possible.
Indeed, the wide array of these linkages in concert with the wide variety of monosaccharides and their many isomeric
forms makes complex carbohydrates information-rich molecules.
11.2.1. Sucrose, Lactose, and Maltose Are the Common Disaccharides
A disaccharide consists of two sugars joined by an O-glycosidic bond. Three abundant disaccharides are sucrose,
lactose, and maltose (Figure 11.11). Sucrose (common table sugar) is obtained commercially from cane or beet. The
anomeric carbon atoms of a glucose unit and a fructose unit are joined in this disaccharide; the configuration of this
glycosidic linkage is α for glucose and β for fructose. Sucrose can be cleaved into its component monosaccharides by the
enzyme sucrase.
Lactose, the disaccharide of milk, consists of galactose joined to glucose by a β-1,4-glycosidic linkage. Lactose is
hydrolyzed to these monosaccharides by lactase in human beings (Section 16.1.12) and by β-galactosidase in bacteria.
In maltose, two glucose units are joined by an α -1,4 glycosidic linkage, as stated earlier. Maltose comes from the
hydrolysis of starch and is in turn hydrolyzed to glucose by maltase. Sucrase, lactase, and maltase are located on the
outer surfaces of epithelial cells lining the small intestine (Figure 11.12).

11.2.2. Glycogen and Starch Are Mobilizable Stores of Glucose
Large polymeric oligosaccharides, formed by the linkage of multiple monosaccharides, are called polysaccharides.
Polysaccharides play vital roles in energy storage and in maintaining the structural integrity of an organism. If all of the
monosaccharides are the same, these polymers are called homopolymers. The most common homopolymer in animal
cells is glycogen, the storage form of glucose. As will be considered in detail in Chapter 21, glycogen is a very large,
branched polymer of glucose residues. Most of the glucose units in glycogen are linked by α -1,4-glycosidic bonds. The
branches are formed by α -1,6-glycosidic bonds, present about once in 10 units (Figure 11.13).
The nutritional reservoir in plants is starch, of which there are two forms. Amylose, the unbranched type of starch,
consists of glucose residues in α -1,4 linkage. Amylopectin, the branched form, has about 1 α -1,6 linkage per 30 α -1,4
linkages, in similar fashion to glycogen except for its lower degree of branching. More than half the carbohydrate
ingested by human beings is starch. Both amylopectin and amylose are rapidly hydrolyzed by α-amylase, an enzyme
secreted by the salivary glands and the pancreas.
11.2.3. Cellulose, the Major Structural Polymer of Plants, Consists of Linear Chains of
Glucose Units
Cellulose, the other major polysaccharide of glucose found in plants, serves a structural rather than a nutritional role.
Cellulose is one of the most abundant organic compounds in the biosphere. Some 10
15
kg of cellulose is synthesized and
degraded on Earth each year. It is an unbranched polymer of glucose residues joined by β-1,4 linkages. The β
configuration allows cellulose to form very long, straight chains. Fibrils are formed by parallel chains that interact with
one another through hydrogen bonds. The α-1,4 linkages in glycogen and starch produce a very different molecular
architecture from that of cellulose. A hollow helix is formed instead of a straight chain (Figure 11.14). These differing
consequences of the α and β linkages are biologically important. The straight chain formed by β linkages is optimal for
the construction of fibers having a high tensile strength. In contrast, the open helix formed by α linkages is well suited to
forming an accessible store of sugar. Mammals lack cellulases and therefore cannot digest wood and vegetable fibers.
11.2.4. Glycosaminoglycans Are Anionic Polysaccharide Chains Made of Repeating
Disaccharide Units
A different kind of repeating polysaccharide is present on the animal cell surface and in the extracellular matrix. Many
glycosaminoglycans are made of disaccharide repeating units containing a derivative of an amino sugar, either
glucosamine or galactosamine (Figure 11.15). At least one of the sugars in the repeating unit has a negatively charged
carboxylate or sulfate group. Chondroitin sulfate, keratan sulfate, heparin, heparan sulfate, dermatan sulfate, and
hyaluronate are the major glycosaminoglycans.
Glycosaminoglycans are usually attached to proteins to form proteoglycans. Heparin is synthesized in a nonsulfated
form, which is then deacet-ylated and sulfated. Incomplete modification leads to a mixture of variously sulfated
sequences. Some of them act as anticoagulants by binding specifically to antithrombin, which accelerates its
sequestration of thrombin (Section 10.5.6). Heparan sulfate is like heparin except that it has fewer N- and O-sulfate
groups and more acetyl groups.
Proteoglycans resemble polysaccharides more than proteins in as much as the carbohydrate makes up as much as 95% of
the biomolecule by weight. Proteoglycans function as lubricants and structural components in connective tissue, mediate
adhesion of cells to the extracellular matrix, and bind factors that stimulate cell proliferation.
11.2.5. Specific Enzymes Are Responsible for Oligosaccharide Assembly
Oligosaccharides are synthesized through the action of specific enzymes, glycosyltransferases, which catalyze the
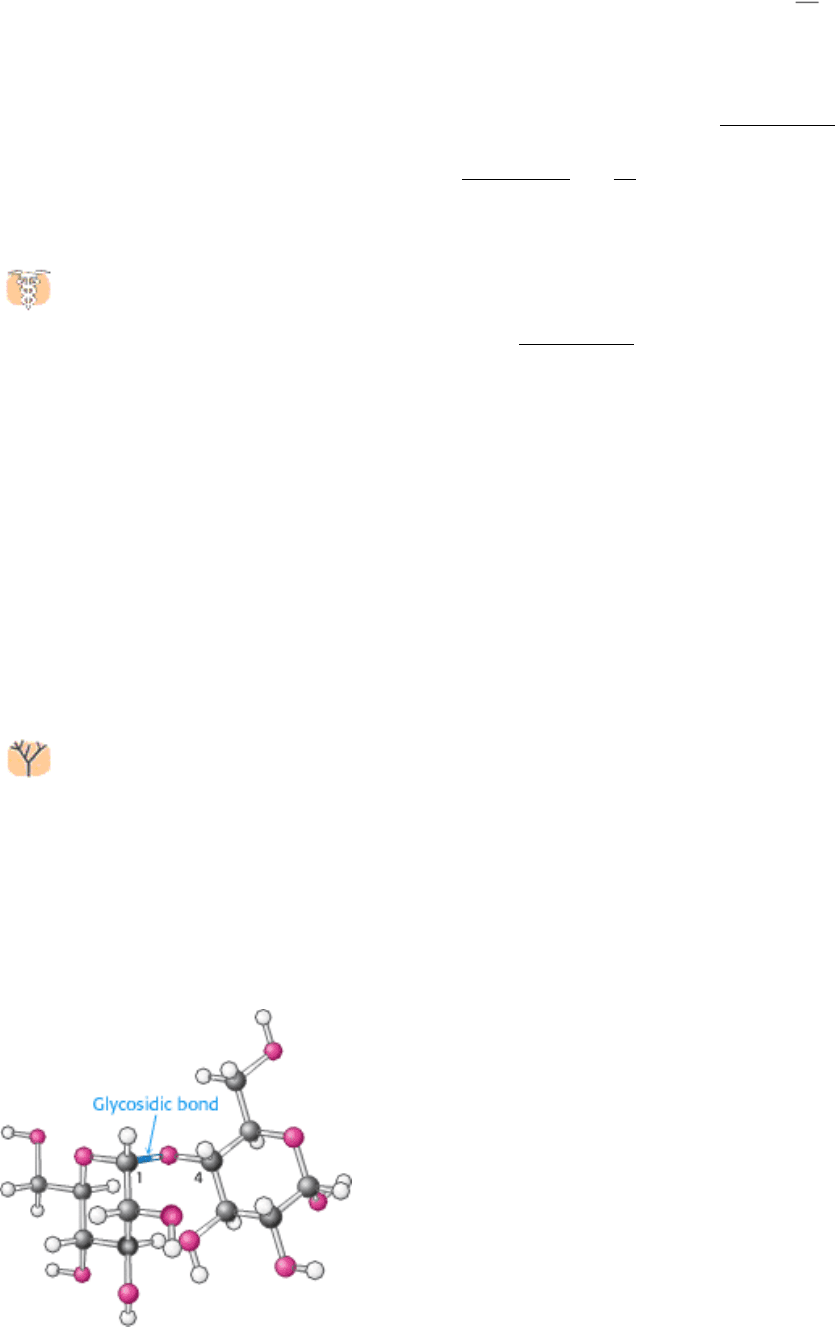
formation of glycosidic bonds. Each enzyme must be specific, to a greater or lesser extent, to the sugars being linked.
Given the diversity of known glycosidic linkages, many different enzymes are required. Note that this mode of assembly
stands in contrast with those used for the other biological polymers heretofore discussed that is, polypeptides and
oligonucleotides. As these polymers are assembled, information about monomer sequence is transferred from a template,
and a single catalytic apparatus is responsible for all bond formation.
The general form of the reaction catalyzed by a glycosyltransferase is shown in Figure 11.16. The sugar to be added
comes in the form of an activated sugar nucleotide. Sugar nucleotides are important intermediates in many processes,
and we will encounter these intermediates again in Chapters 16 and 21. Note that such reactions can proceed with either
retention or inversion of configuration at the glycosidic carbon atom at which the new bond is formed; a given enzyme
proceeds by one stereochemical path or the other.
The human ABO blood groups illustrate the effects of glycosyl- transferases. Carbohydrates are attached to
glycoproteins and glycolipids on the surfaces of red blood cells. For one type of blood group, one of the three
different structures, termed A, B, and O, may be present (Figure 11.17). These structures have in common an
oligosaccharide foundation called the O (or sometimes H) antigen. The A and B antigens differ from the O antigen by
the addition of one extra monosaccharide, either N-acetylgalactosamine (for A) or galactose (for B) through an α -1,3
linkage to a galactose moiety of the O antigen.
Specific glycosyltransferases add the extra monosaccharide to the O antigen. Each person inherits the gene for one
glycosyltransferase of this type from each parent. The type A transferase specifically adds N-acetylgalactosamine,
whereas the type B transferase adds galactose. These enzymes are identical in all but 4 of 354 positions. The O
phenotype is the result of a mutation that leads to premature termination of translation and, hence, to the production of
no active glycosyltransferase.
These structures have important implications for blood transfusions and other transplantation procedures. If an antigen
not normally present in a person is introduced, the person's immune system recognizes it as foreign. Adverse reactions
can ensue, initiated by the intravascular destruction of the incompatible red blood cells.
Why are different blood types present in the human population? Suppose that a pathogenic organism such as a
parasite expresses on its cell surface a carbohydrate antigen similar to one of the blood-group antigens. This
antigen may not be readily detected as foreign in a person with the blood type that matches the parasite antigen, and the
parasite will flourish. However, other people with different blood types will be protected. Hence, there will be selective
pressure on human beings to vary blood type to prevent parasitic mimicry and a corresponding selective pressure on
parasites to enhance mimicry. The constant "arms race" between pathogenic microorganisms and human beings drives
the evolution of diversity of surface antigens within the human population.
I. The Molecular Design of Life 11. Carbohydrates 11.2. Complex Carbohydrates Are Formed by Linkage of Monosaccharides
Figure 11.10. Maltose, a Disaccharide. Two molecules of glucose are linked by an α -1,4-glycosidic bond to form the
disaccharide maltose.
I. The Molecular Design of Life 11. Carbohydrates 11.2. Complex Carbohydrates Are Formed by Linkage of Monosaccharides
Figure 11.11. Common Disaccharides. Sucrose, lactose, and maltose are common dietary components.
I. The Molecular Design of Life 11. Carbohydrates 11.2. Complex Carbohydrates Are Formed by Linkage of Monosaccharides
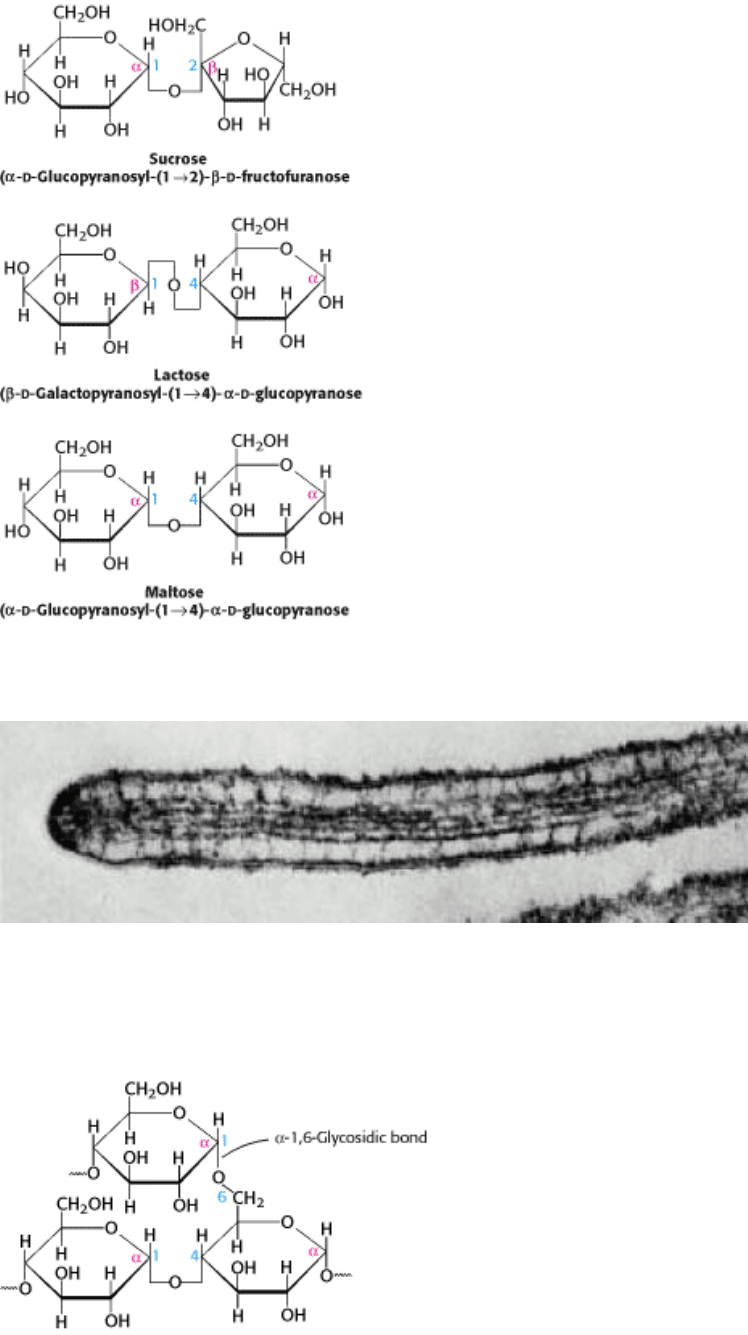
Figure 11.12. Electron Micrograph of a Microvillus. Lactase and other enzymes that hydrolyze carbohydrates are
present on microvilli that project from the outer face of the plasma membrane of intestinal epithelial cells. [From M. S.
Mooseker and L. G. Tilney, J. Cell. Biol. 67(1975):725.]
I. The Molecular Design of Life 11. Carbohydrates 11.2. Complex Carbohydrates Are Formed by Linkage of Monosaccharides
Figure 11.13. Branch Point in Glycogen. Two chains of glucose molecules joined by α -1,4-glycosidic bonds are
linked by an α -1,6-glycosidic bond to create a branch point. Such an α -1,6-glycosidic bond forms at approximately
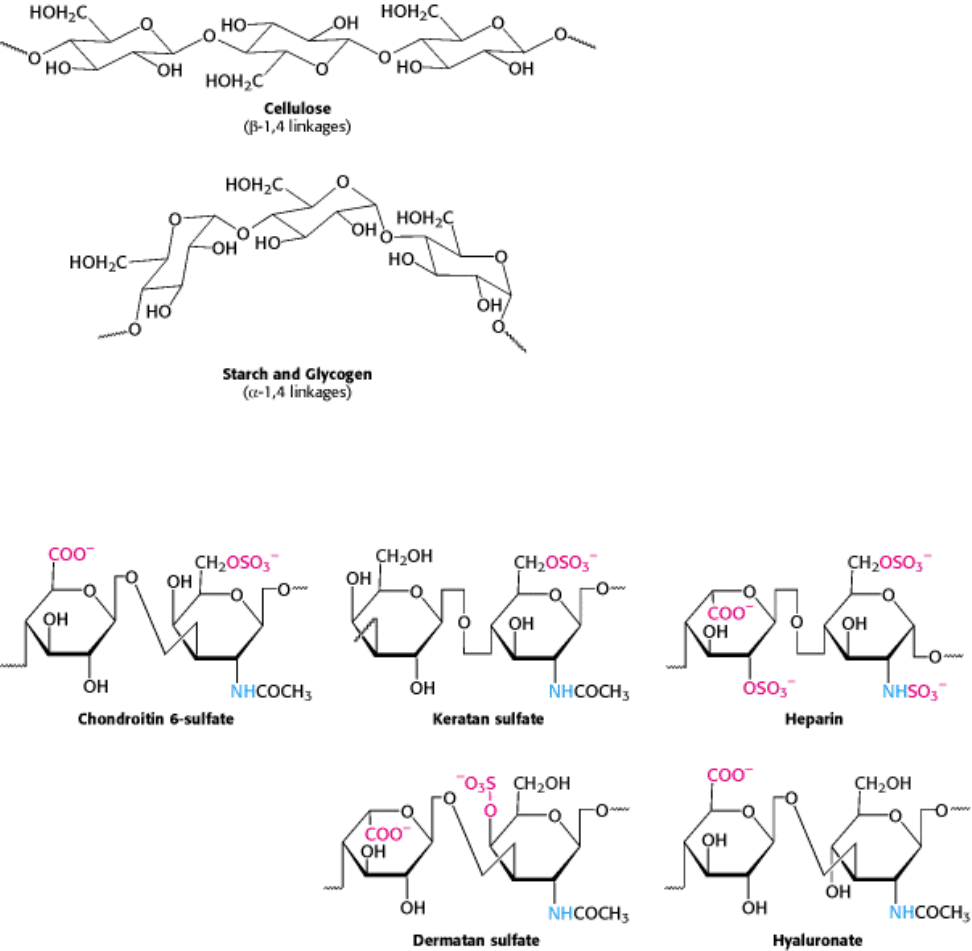
every 10 glucose units, making glycogen a highly branched molecule.
I. The Molecular Design of Life 11. Carbohydrates 11.2. Complex Carbohydrates Are Formed by Linkage of Monosaccharides
Figure 11.14. Glycosidic Bonds Determine Polysaccharide Structure. The β-1,4 linkages favor straight chains, which
are optimal for structural purposes. The α-1,4 linkages favor bent structures, which are more suitable for storage.
I. The Molecular Design of Life 11. Carbohydrates 11.2. Complex Carbohydrates Are Formed by Linkage of Monosaccharides
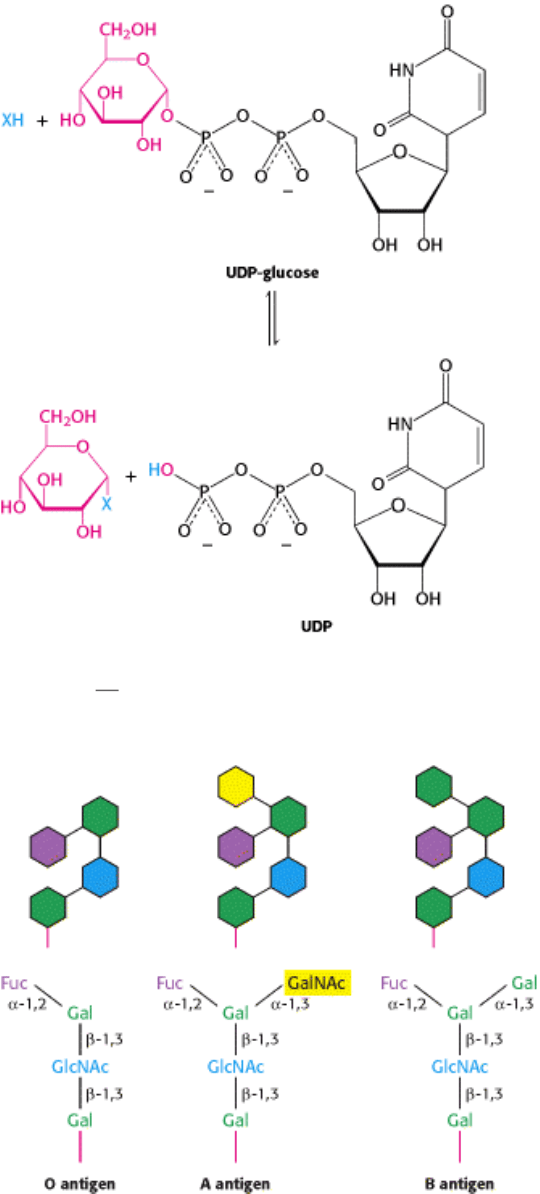
Figure 11.15. Repeating Units in Glycosaminoglycans. Structural formulas for five repeating units of important
glycosaminoglycans illustrate the variety of modifications and linkages that are possible. Amino groups are shown in
blue and negatively charged groups in red. Hydrogens have been omitted for clarity. The right-hand structure is
glucosamine in each case.
I. The Molecular Design of Life 11. Carbohydrates 11.2. Complex Carbohydrates Are Formed by Linkage of Monosaccharides
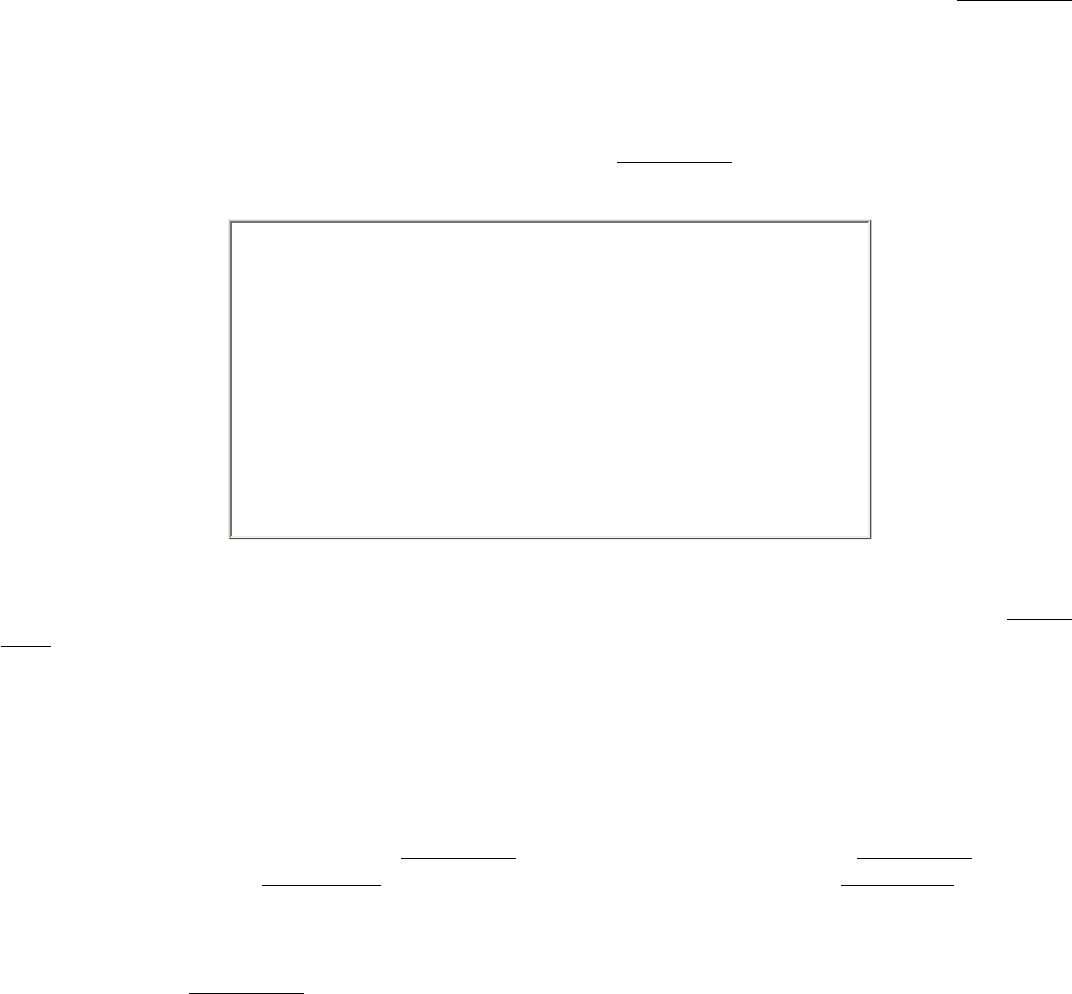
Figure 11.16. General Form of a Glycosyltransferase Reaction. The sugar to be added comes from a sugar
nucleotide
in this case, UDP-glucose.
I. The Molecular Design of Life 11. Carbohydrates 11.2. Complex Carbohydrates Are Formed by Linkage of Monosaccharides
Figure 11.17. Structures of A, B, and O Oligosaccharide Antigens. Abbreviations: Fuc, fucose; Gal, galactose;
GalNAc, N-acetylgalactosamine; GlcNAc, N-acetylglucosamine.
I. The Molecular Design of Life 11. Carbohydrates
11.3. Carbohydrates Can Be Attached to Proteins to Form Glycoproteins
Carbohydrate groups are covalently attached to many different proteins to form glycoproteins. Carbohydrates are a much
smaller percentage of the weight of glycoproteins than of proteoglycans. Many glycoproteins are components of cell
membranes, where they play a variety of roles in processes such as cell adhesion and the binding of sperm to eggs.
11.3.1. Carbohydrates May Be Linked to Proteins Through Asparagine (N-Linked) or
Through Serine or Threonine (O-Linked) Residues
In glycoproteins, sugars are attached either to the amide nitrogen atom in the side chain of asparagine (termed an N-
linkage) or to the oxygen atom in the side chain of serine or threonine (termed an O-linkage), as shown in Figure 11.18.
An asparagine residue can accept an oligosaccharide only if the residue is part of an Asn-X-Ser or Asn-X-Thr sequence,
in which X can be any residue. Thus, potential glycosylation sites can be detected within amino acid sequences.
However, which of these potential sites is actually glycosylated depends on other aspects of the protein structure and on
the cell type in which the protein is expressed. All N-linked oligosaccharides have in common a pentasaccharide core
consisting of three mannose and two N-acetylglucosamine residues. Additional sugars are attached to this core to form
the great variety of oligosaccharide patterns found in glycoproteins (Figure 11.19).
Abbreviations for sugars
Fuc Fucose
Gal Galactose
GalNAc N-Acetylgalactosamine
Glc Glucose
GlcNAc N-Acetylglucosamine
Man Mannose
Sia Sialic acid
NeuNAc N-Acetylneuraminate (sialic acid)
Carbohydrates are linked to some soluble proteins as well as membrane proteins. In particular, many of the proteins
secreted from cells are glycosylated. Most proteins present in the serum component of blood are glycoproteins (Figure
11.20). Furthermore, N-acetylglucosamine residues are O-linked to some intracellular proteins. The role of these
carbohydrates, which are dynamically added and removed, is under active investigation.
11.3.2. Protein Glycosylation Takes Place in the Lumen of the Endoplasmic Reticulum
and in the Golgi Complex
Protein glycosylation takes place inside the lumen of the endoplasmic reticulum (ER) and the Golgi complex, organelles
that play central roles in protein trafficking (Figure 11.21). One such glycoprotein (depicted in Figure 11.20) is the
proteolytic enzyme elastase (Section 9.1.4), which is secreted by the pancreas as a zymogen (Section 10.5). This protein
is synthesized by ribosomes attached to the cytoplasmic face of the ER membrane, and the peptide chain is inserted into
the lumen of the ER as it grows, guided by a signal sequence of 29 amino acids at the amino terminus. This signal
sequence, which directs the protein through a channel in the ER membrane, is cleaved from the protein in the transport
process into the ER (Figure 11.22). After the protein has entered the ER, the glycosylation process begins. The N-linked
glycosylation begins in the ER and continues in the Golgi complex, whereas the O-linked glycosylation takes place
exclusively in the Golgi complex.
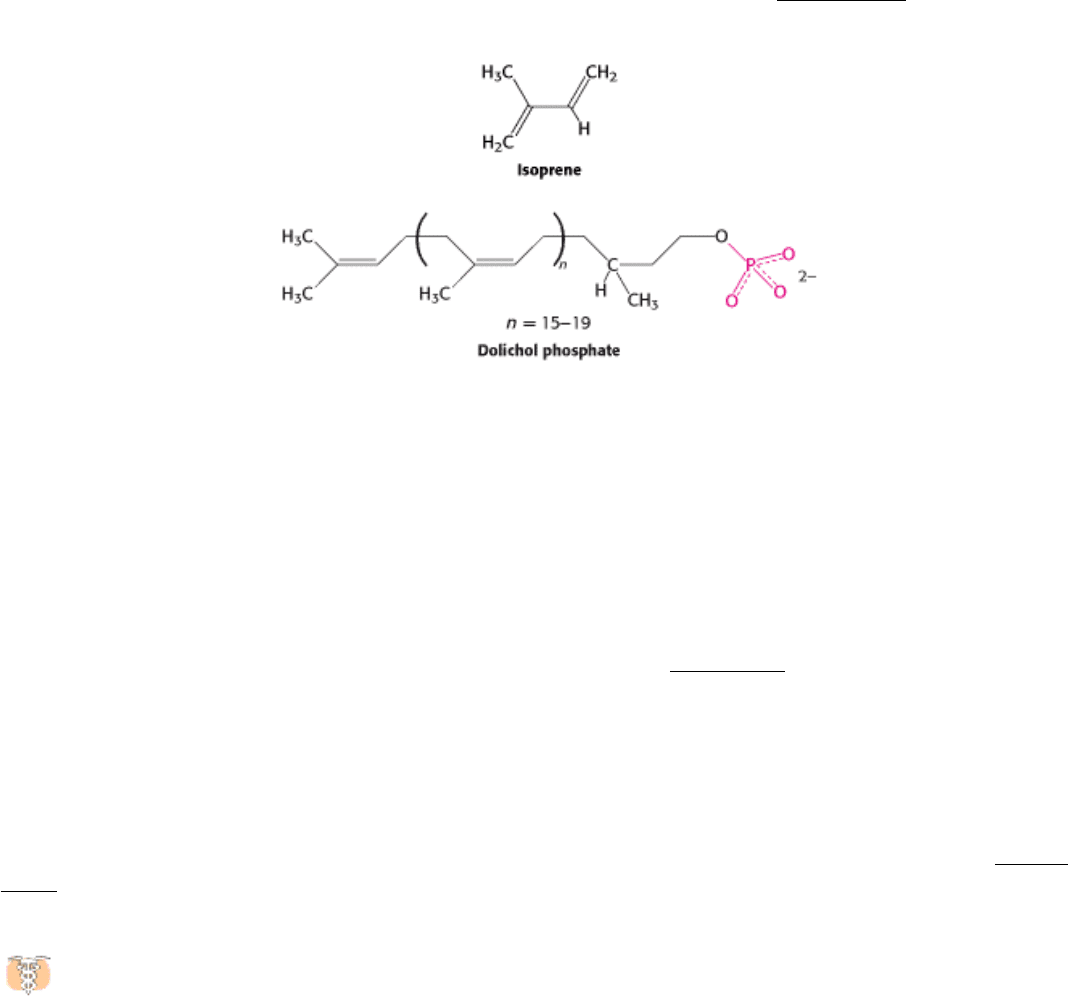
11.3.3. N-Linked Glycoproteins Acquire Their Initial Sugars from Dolichol Donors in
the Endoplasmic Reticulum
A large oligosaccharide destined for attachment to the asparagine residue of a protein is assembled attached to dolichol
phosphate, a specialized lipid molecule containing as many as 20 isoprene (C
5
) units (Section 26.4.8).
The terminal phosphate group is the site of attachment of the activated oligosaccharide, which is subsequently
transferred to the protein acceptor. Dolichol phosphate resides in the ER membrane with its phosphate terminus on the
cytoplasmic face.
The assembly process proceeds in three stages. First, 2 N-acetylglucosamine residues and 5 mannose residues are added
to the dolichol phosphate through the action of a number of cytoplasmic enzymes that catalyze monosaccharide transfer
from sugar nucleotides. Then, in a remarkable (and, as yet, not well understood) process, this large structure is "flipped"
through the ER membrane into the lumen of the ER. Finally, additional sugars are added by enzymes in the ER lumen,
this time with the use of monosaccharides activated by attachment to dolichol phosphate. This process ends with the
formation of a 14-residue oligosaccharide attached to dolichol phosphate (Figure 11.23).
The 14-sugar-residue precursor attached to this dolichol phosphate intermediate is then transferred en bloc to a specific
asparagine residue of the growing polypeptide chain. In regard to elastase, oligosaccharides are linked to the asparagine
residues in the recognition sequences Asn 109-Gly-Ser and Asn 159-Val-Thr. Both the activated sugars and the complex
enzyme that is responsible for transferring the oligosaccharide to the protein are located on the lumenal side of the ER,
accounting for the fact that proteins in the cytosol are not glycosylated by this pathway. Before the glycoprotein leaves
the lumen of the ER, 3 glucose molecules are removed from the 14-residue oligosaccharide. As we will see in Section
11.3.6, the sequential removal of these glucose molecules is a quality-control step that ensures that only properly folded
glycoproteins are further processed.
Dolichol pyrophosphate released in the transfer of the oligosaccharide to the protein is recycled to dolichol
phosphate by the action of a phosphatase. This hydrolysis is blocked by bacitracin, an antibiotic. Another
interesting antibiotic inhibitor of N-glycosylation is tunicamycin, a hydrophobic analog of the sugar nucleotide uridine
diphosphate-N-acetylglucosamine (UDP-GlcNAc), the activated form of N-acetylglucosamine used as a substrate for the
enzymes that synthesize the oligosaccharide unit on dolichol phosphate. Tunicamycin blocks the addition of N-
acetylglucosamine to dolichol phosphate, the first step in the formation of the core oligosaccharide.
11.3.4. Transport Vesicles Carry Proteins from the Endoplasmic Reticulum to the
Golgi Complex for Further Glycosylation and Sorting
Proteins in the lumen of the ER and in the ER membrane are transported to the Golgi complex, which is a stack of
flattened membranous sacs. The Golgi complex has two principal roles. First, carbohydrate units of glycoproteins are
