Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

of galactose joined to glucose by a β -1,4 linkage. Maltose (from starch) consists of two glucoses joined by an α -1,4
linkage. Starch is a polymeric form of glucose in plants, and glycogen serves a similar role in animals. Most of the
glucose units in starch and glycogen are in α -1,4 linkage. Glycogen has more branch points formed by α -1,6 linkages
than does starch, which makes glycogen more soluble. Cellulose, the major structural polymer of plant cell walls,
consists of glucose units joined by β -1,4 linkages. These β linkages give rise to long straight chains that form fi-brils
with high tensile strength. In contrast, the α linkages in starch and glycogen lead to open helices, in keeping with their
roles as mobilizable energy stores. Cell surfaces and extracellular matrices of animals contain polymers of repeating
disaccharides called glycosaminoglycans. One of the units in each repeat is a derivative of glucosamine or
galactosamine. These highly anionic carbohydrates have a high density of carboxylate or sulfate groups. Proteins bearing
covalently linked glycosaminoglycans are termed proteoglycans.
Carbohydrates Can Attach to Proteins to Form Glycoproteins
Specific enzymes link the oligosaccharide units on proteins either to the side-chain oxygen atom of a serine or threonine
residue or to the side-chain amide nitrogen atom of an asparagine residue. Protein glycosylation takes place in the lumen
of the endoplasmic reticulum. The N-linked oligosaccharides are synthesized on dolichol phosphate and subsequently
transferred to the protein acceptor. Additional sugars are attached in the Golgi complex to form diverse patterns.
Lectins Are Specific Carbohydrate-Binding Proteins
Carbohydrates are recognized by proteins called lectins, which are found in animals, plants, and microorganisms. In
animals, the interplay of lectins and their sugar targets guides cell-cell contact. The viral protein hemagglutinin on the
surface of the influenza virus recognizes sialic acid residues on the surfaces of the cells invaded by the virus. A small
number of carbohydrate residues can be joined in many different ways to form highly diverse patterns that can be
distinguished by the lectin domains of protein receptors.
Key Terms
monosaccharide
triose
ketose
aldose
enantiomer
tetrose
pentose
hexose
heptose
diastereoisomer
epimer
hemiacetal
pyranose
hemiketal
furanose
anomer
glycosidic bond
reducing sugar
nonreducing sugar
oligosaccharide
disaccharide
polysaccharide
glycogen
starch
cellulose
glycosaminoglycan
proteoglycan
glycosyltransferase
glycoprotein
endoplasmic reticulum
Golgi complex
dolichol phosphate
lectin
selectin

I. The Molecular Design of Life 11. Carbohydrates
Problems
1.
Word origin. Account for the origin of the term
carbohydrate.
See answer
2.
Diversity. How many different oligosaccharides can be made by linking one glucose, one mannose, and one
galactose? Assume that each sugar is in its pyranose form. Compare this number with the number of tripeptides that
can be made from three different amino acids.
See answer
3.
Couples. Indicate whether each of the following pairs of sugars consists of anomers, epimers, or an aldose-ketose
pair:
See answer
(a)
d-glyceraldehyde and dihydroxyacetone
(b)
d-glucose and d-mannose
(c)
d-glucose and d-fructose
(d) α-
d-glucose and β-d-glucose
(e)
d-ribose and d-ribulose
(f)
d-galactose and d-glucose
4.
Tollen's test. Glucose and other aldoses are oxidized by an aqueous solution of a silver-ammonia complex. What are
the reaction products?
See answer
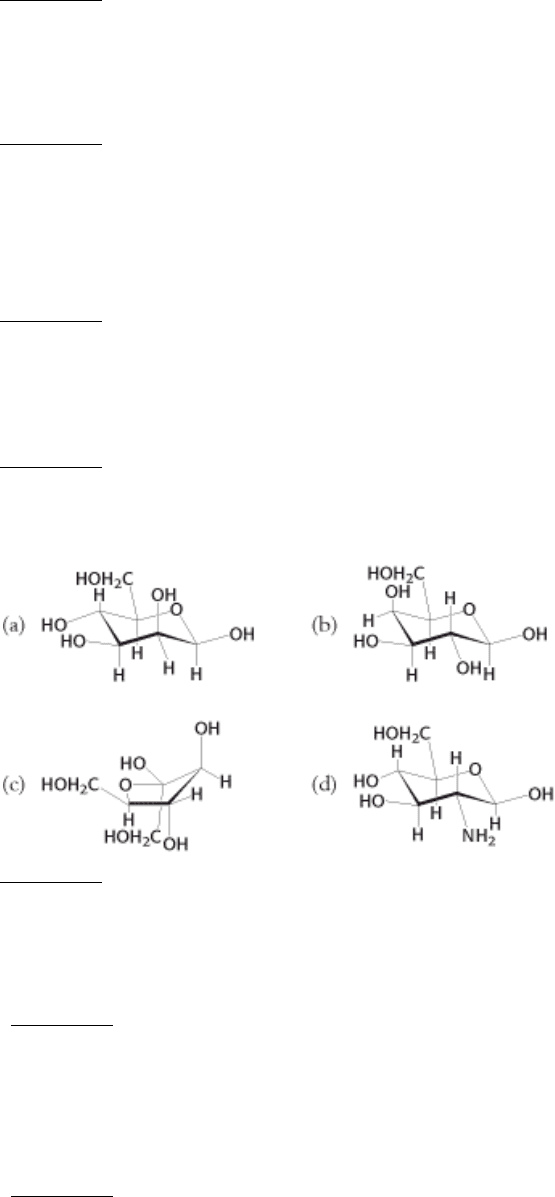
5.
Mutarotation. The specific rotations of the α and β anomers of d-glucose are +112 degrees and +18.7 degrees,
respectively. Specific rotation, [ α ]
d
, is defined as the observed rotation of light of wavelength 589 nm (the d line
of a sodium lamp) passing through 10 cm of a 1 g ml
-1
solution of a sample. When a crystalline sample of α-d-
glucopyranose is dissolved in water, the specific rotation decreases from 112 degrees to an equilibrium value of 52.7
degrees. On the basis of this result, what are the proportions of the α and β anomers at equilibrium? Assume that the
concentration of the open-chain form is negligible.
See answer
6.
Telltale adduct. Glucose reacts slowly with hemoglobin and other proteins to form covalent compounds. Why is
glucose reactive? What is the nature of the adduct formed?
See answer
7.
Periodate cleavage. Compounds containing hydroxyl groups on adjacent carbon atoms undergo carbon-carbon bond
cleavage when treated with periodate ion (IO
4
-
). How can this reaction be used to distinguish between pyranosides
and furanosides?
See answer
8.
Oxygen source. Does the oxygen atom attached to C-1 in methyl α-d-glucopyranoside come from glucose or
methanol?
See answer
9.
Sugar lineup. Identify the following four sugars.
See answer
10.
Cellular glue. A trisaccharide unit of a cell-surface glycoprotein is postulated to play a critical role in mediating
cell-cell adhesion in a particular tissue. Design a simple experiment to test this hypothesis.
See answer
11.
Mapping the molecule. Each of the hydroxyl groups of glucose can be methylated with reagents such as
dimethylsulfate under basic conditions. Explain how exhaustive methylation followed by compete digestion of a
known amount of glycogen would enable you to determine the number of branch points and reducing ends.
See answer
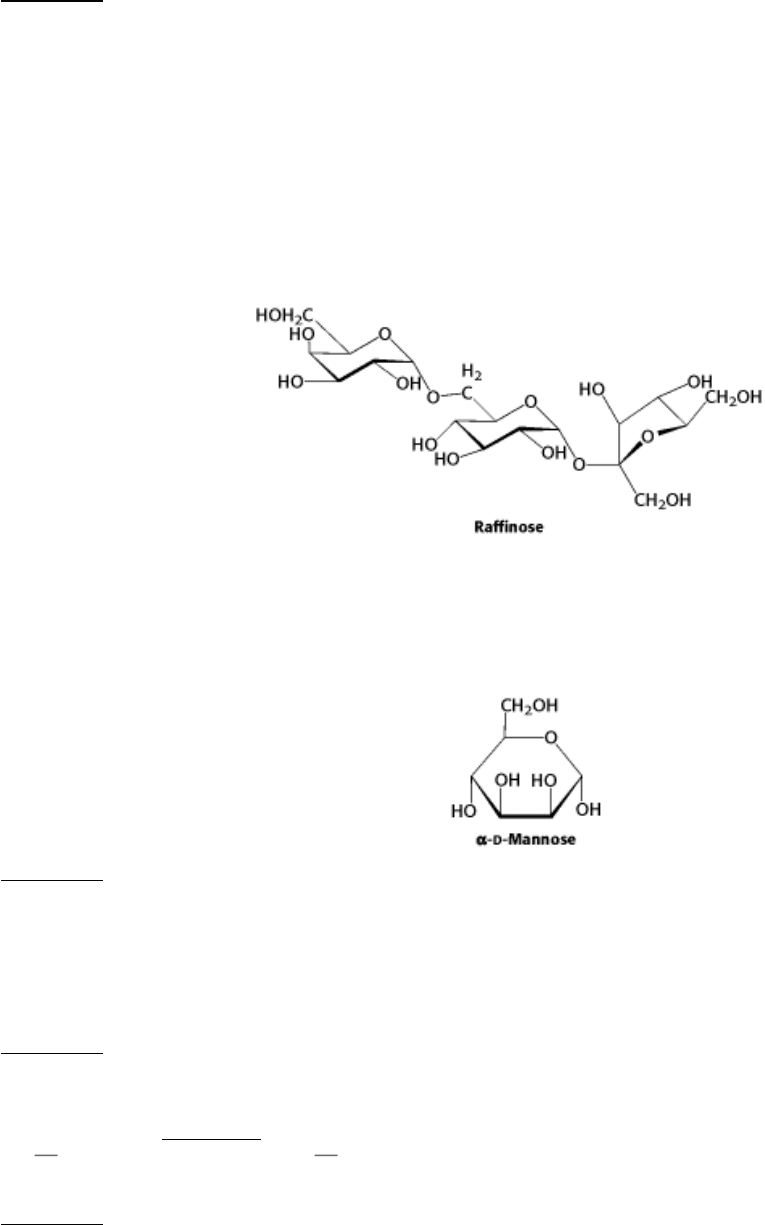
12.
Component parts. Raffinose is a trisaccharide and a minor constituent in sugar beets.
See answer
(a) Is raffinose a reducing sugar? Explain.
(b) What are the monosaccharides that compose raffinose?
(c) β-Galactosidase is an enzyme that will remove galactose residues from an oligosaccharide. What are the products
of β-galactosidase treatment of raffinose?
13.
Anomeric differences. α -d-Mannose is a sweet-tasting sugar. β-d-Mannose, on the other hand, tastes bitter. A pure
solution of α-d-mannose loses its sweet taste with time as it is converted into the β anomer. Draw the β anomer and
explain how it is formed from the α anomer.
See answer
14.
A taste of honey. Fructose in its β-d-pyranose form accounts for the powerful sweetness of honey. The β-d-furanose
form, although sweet, is not as sweet as the pyranose form. The furanose form is the more stable form. Draw the
two forms and explain why it may not always be wise to cook with honey.
See answer
15.
Making ends meet. (a) Compare the number of reducing ends to nonreducing ends in a molecule of glycogen. (b)
As we will see in Chapter 21, glycogen is an important fuel storage form that is rapidly mobilized. At which
end the reducing or nonreducing would you expect most metabolism to take place?
See answer
16.
Carbohydrates and proteomics. Suppose that a protein contains six potential N-linked glycosylation sites. How
many possible proteins can be generated, depending on which of these sites is actually glycosylated? Do not
include the effects of diversity within the carbohydrate added.
See answer
Chapter Integration Problem
17.
Stereospecificity. Sucrose, a major product of photosynthesis in green leaves, is synthesized by a battery of
enzymes. The substrates for sucrose synthesis, d-glucose and d-fructose, are a mixture of α and β anomers as well
as acyclic compounds in solution. Nonetheless, sucrose consists of α-d-glucose linked by its carbon-1 atom to the
carbon-2 atom of β-d-fructose. How can the specificity of sucrose be explained in light of the potential substrates?
See answer
I. The Molecular Design of Life 11. Carbohydrates
Selected Readings
Where to start
N. Sharon and H. Lis. 1993. Carbohydrates in cell recognition Sci. Am. 268: (1) 82-89. (PubMed)
L.A. Lasky. 1992. Selectins: Interpreters of cell-specific carbohydrate information during inflammation Science 258:
964-969. (PubMed)
P. Weiss and G. Ashwell. 1989. The asialoglycoprotein receptor: Properties and modulation by ligand Prog. Clin. Biol.
Res. 300: 169-184. (PubMed)
N. Sharon. 1980. Carbohydrates Sci. Am. 245: (5) 90-116. (PubMed)
J.C. Paulson. 1989. Glycoproteins: What are the sugar side chains for? Trends Biochem. Sci. 14: 272-276. (PubMed)
R.J. Woods. 1995. Three-dimensional structures of oligosaccharides Curr. Opin. Struct. Biol. 5: 591-598. (PubMed)
Books
Varki, A., Cummings, R., Esko, J., Freeze, H., Hart, G., and Marth, J., 1999. Essentials of Glycobiology. Cold Spring
Harbor Laboratory Press.
Fukuda, M., and Hindsgaul, O., 2000. Molecular Glycobiology. IRL Press at Oxford University Press.
El Khadem, H. S., 1988. Carbohydrate Chemistry. Academic Press.
Ginsburg, V., and Robbins, P. W. (Eds.), 1981. Biology of Carbohydrates (vols. 1
3). Wiley.
Fukuda, M. (Ed.), 1992. Cell Surface Carbohydrates and Cell Development. CRC Press.
Preiss, J. (Ed.), 1988. The Biochemistry of Plants: A Comprehensive Treatise: Carbohydrates. Academic Press.
Structure of carbohydrate-binding proteins
U. Ünligil and J.M. Rini. 2000. Glycosyltransferase structure and mechanism Curr. Opin. Struct. Biol. 10: 510-517.
(PubMed)
J. Bouckaert, T. Hamelryck, L. Wyns, and R. Loris. 1999. Novel structures of plant lectins and their complexes with
carbohydrates Curr. Opin. Struct. Biol. 9: 572-577. (PubMed)
W.I. Weis and K. Drickamer. 1996. Structural basis of lectincarbohydrate recognition Annu. Rev. Biochem. 65: 441-473.
(PubMed)
N.K. Vyas. 1991. Atomic features of protein-carbohydrate interactions Curr. Opin. Struct. Biol. 1: 732-740.
W.I. Weis, K. Drickamer, and W.A. Hendrickson. 1992. Structure of a C-type mannose-binding protein complexed with
an oligosaccharide Nature 360: 127-134. (PubMed)
C.S. Wright. 1992. Crystal structure of a wheat germ agglutinin/ glycophorin-sialoglycopeptide receptor complex:
Structural basis for cooperative lectin-cell binding J. Biol. Chem. 267: 14345-14352. (PubMed)
B. Shaanan, H. Lis, and N. Sharon. 1991. Structure of a legume lectin with an ordered N-linked carbohydrate in complex
with lactose Science 254: 862-866. (PubMed)
Glycoproteins
R.G. Spiro. 2000. Glucose residues as key determinants in the biosynthesis and quality control of glycoproteins with N-
linked oligosaccharides J. Biol. Chem. 275: 35657-35660. (PubMed)
M. Bernfield, M. Götte, P.W. Park, O. Reizes, M.L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell
surface heparan sulfate proteoglycans Annu. Rev. Biochem. 68: 729-777. (PubMed)
R.V. Iozzo. 1998. Matrix proteoglycans: From molecular design to cellular function Annu. Rev. Biochem. 67: 609-652.
(PubMed)
E.S. Trombetta and A. Helenius. 1998. Lectins as chaperones in glycoprotein folding Curr. Opin. Struct. Biol. 8: 587-
592. (PubMed)
M. Yanagishita and V.C. Hascall. 1992. Cell surface heparan sulfate proteoglycans J. Biol. Chem. 267: 9451-9454.
(PubMed)
R.V. Iozzo. 1999. The biology of small leucine-rich proteoglycans: Functional network of interactive proteins J. Biol.
Chem. 274: 18843-18846. (PubMed)
Carbohydrates in recognition processes
W.I. Weis. 1997. Cell-surface carbohydrate recognition by animal and viral lectins Curr. Opin. Struct. Biol. 7: 624-630.
(PubMed)
N. Sharon and H. Lis. 1989. Lectins as cell recognition molecules Science 246: 227-234. (PubMed)
M.L. Turner. 1992. Cell adhesion molecules: A unifying approach to topographic biology Biol. Rev. Camb. Philos. Soc.
67: 359-377. (PubMed)
T. Feizi. 1992. Blood group-related oligosaccharides are ligands in cell-adhesion events Biochem. Soc. Trans. 20: 274-
278. (PubMed)
T.M. Jessell, M.A. Hynes, and J. Dodd. 1990. Carbohydrates and carbohydrate-binding proteins in the nervous system
Annu. Rev. Neurosci. 13: 227-255. (PubMed)
C. Clothia and E.V. Jones. 1997. The molecular structure of cell adhesion molecules Annu. Rev. Biochem. 66: 823-862.
(PubMed)
Carbohydrate sequencing
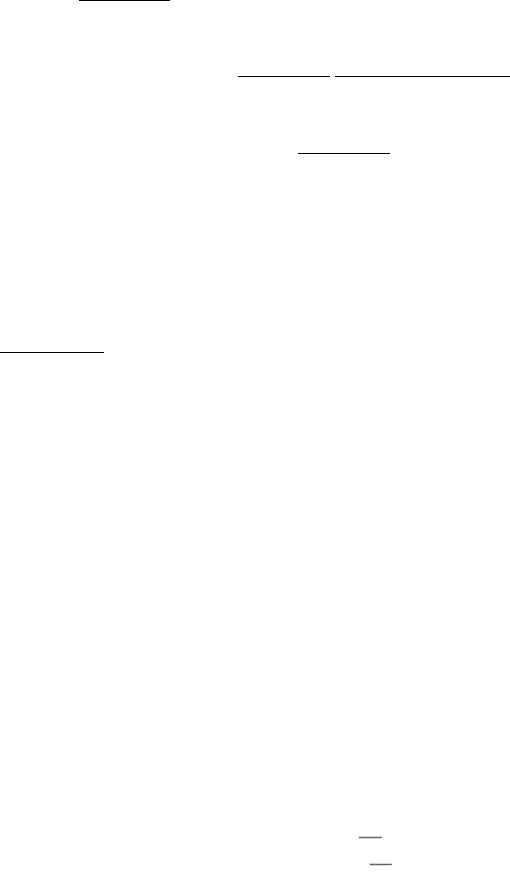
G. Venkataraman, Z. Shriver, R. Raman, and R. Sasisekharan. 1999. Sequencing complex polysaccharides Science 286:
537-542. (PubMed)
Y. Zhao, S.B.H. Kent, and B.T. Chait. 1997. Rapid, sensitive structure analysis of oligosaccharides Proc. Natl. Acad.
Sci. U.S.A. 94: 1629-1633. (PubMed) (Full Text in PMC)
P.M. Rudd, G.R. Guile, B. Küster, D.J. Harvey, G. Opdenakker, and R.A. Dwek. 1997. Oligosaccharide sequencing
technology Nature 388: 205-207. (PubMed)
I. The Molecular Design of Life
12. Lipids and Cell Membranes
The boundaries of cells are formed by biological membranes, the barriers that define the inside and the outside of a cell
(Figure 12.1). These barriers prevent molecules generated inside the cell from leaking out and unwanted molecules from
diffusing in; yet they also contain transport systems that allow specific molecules to be taken up and unwanted
compounds to be removed from the cell. Such transport systems confer on membranes the important property of
selective permeability.
Membranes are dynamic structures in which proteins float in a sea of lipids. The lipid components of the membrane form
the permeability barrier, and protein components act as a transport system of pumps and channels that endow the
membrane with selective permeability.
In addition to an external cell membrane (called the plasma membrane), eukaryotic cells also contain internal membranes
that form the boundaries of organelles such as mitochondria, chloroplasts, peroxisomes, and lysosomes. Functional
specialization in the course of evolution has been closely linked to the formation of such compartments. Specific systems
have evolved to allow targeting of selected proteins into or through particular internal membranes and, hence, into
specific organelles. External and internal membranes have essential features in common, and these essential features are
the subject of this chapter.
Biological membranes serve several additional important functions indispensable for life, such as energy storage and
information transduction, that are dictated by the proteins associated with them. In this chapter, we will examine the
general properties of membrane proteins
how they can exist in the hydrophobic environment of the membrane while
connecting two hydrophilic environments and delay a discussion of the functions of these proteins to the next and later
chapters.
I. The Molecular Design of Life 12. Lipids and Cell Membranes
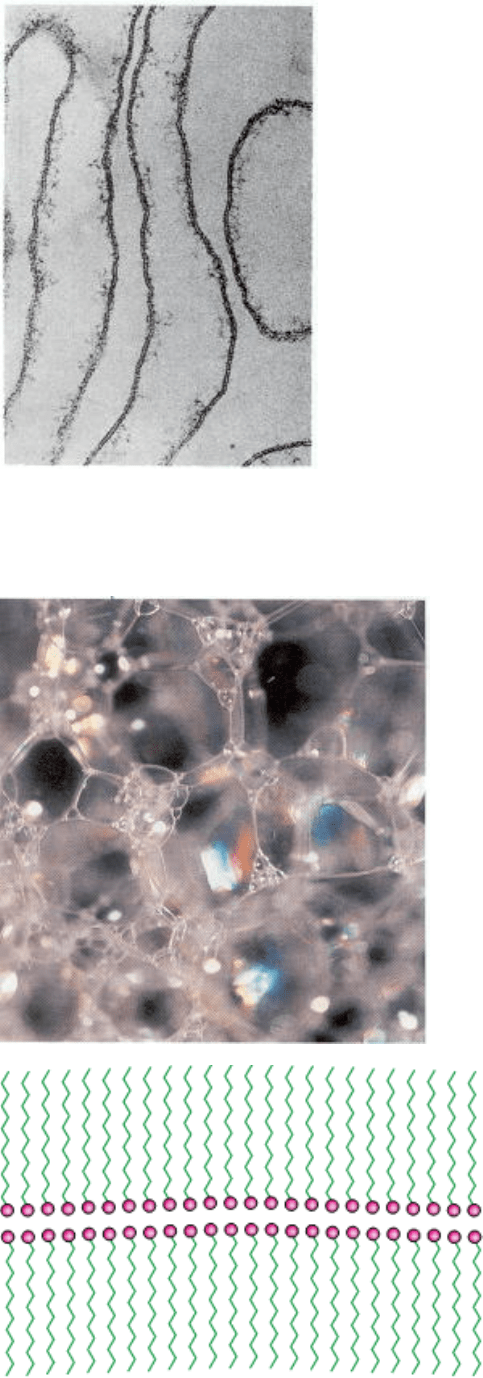
Figure 12.1. Red-Blood-Cell Plasma Membrane. An electron micrograph of a preparation of plasma membranes from
red blood cells showing the membranes as seen "on edge," in cross section. [Courtesy of Dr. Vincent Marchesi.]
I. The Molecular Design of Life 12. Lipids and Cell Membranes
The surface of a soap bubble is a bilayer formed by detergent molecules. The polar heads (red) pack together leaving
the hydrophobic groups (green) in contact with air on the inside and outside of the bubble. Other bilayer structures define

the boundary of a cell. [(Left) Photonica.]
I. The Molecular Design of Life 12. Lipids and Cell Membranes
12.1. Many Common Features Underlie the Diversity of Biological Membranes
Membranes are as diverse in structure as they are in function. However, they do have in common a number of important
attributes:
1. Membranes are sheetlike structures, only two molecules thick, that form closed boundaries between different
compartments. The thickness of most membranes is between 60 Å (6 nm) and 100 Å (10 nm).
2. Membranes consist mainly of lipids and proteins. Their mass ratio ranges from 1:4 to 4:1. Membranes also contain
carbohydrates that are linked to lipids and proteins.
3. Membrane lipids are relatively small molecules that have both hydrophilic and hydrophobic moieties. These lipids
spontaneously form closed bimolecular sheets in aqueous media. These lipid bilayers are barriers to the flow of polar
molecules.
4. Specific proteins mediate distinctive functions of membranes. Proteins serve as pumps, channels, receptors, energy
transducers, and enzymes. Membrane proteins are embedded in lipid bilayers, which create suitable environments for
their action.
5. Membranes are noncovalent assemblies. The constituent protein and lipid molecules are held together by many
noncovalent interactions, which are cooperative.
6. Membranes are asymmetric. The two faces of biological membranes always differ from each other.
7. Membranes are fluid structures. Lipid molecules diffuse rapidly in the plane of the membrane, as do proteins, unless
they are anchored by specific interactions. In contrast, lipid molecules and proteins do not readily rotate across the
membrane. Membranes can be regarded as two-dimensional solutions of oriented proteins and lipids.
8. Most cell membranes are electrically polarized, such that the inside is negative [typically - 60 millivolts (mV)].
Membrane potential plays a key role in transport, energy conversion, and excitability (Chapter 13).
I. The Molecular Design of Life 12. Lipids and Cell Membranes
12.2. Fatty Acids Are Key Constituents of Lipids
Among the most biologically significant properties of lipids are their hydrophobic properties. These properties are
mainly due to a particular component of lipids: fatty acids, or simply fats. Fatty acids also play important roles in signal-
transduction pathways (Sections 15.2 and 22.6.2).
12.2.1. The Naming of Fatty Acids
Fatty acids are hydrocarbon chains of various lengths and degrees of unsaturation that terminate with carboxylic acid
groups. The systematic name for a fatty acid is derived from the name of its parent hydrocarbon by the substitution of oic
for the final e. For example, the C
18
saturated fatty acid is called octadecanoic acid because the parent hydrocarbon is
octadecane. A C
18
fatty acid with one double bond is called octadecenoic acid; with two double bonds, octadecadienoic
acid; and with three double bonds, octadecatrienoic acid. The notation 18:0 denotes a C
18
fatty acid with no double
bonds, whereas 18:2 signifies that there are two double bonds. The structures of the ionized forms of two common fatty
acids
palmitic acid (C
16
, saturated) and oleic acid (C
18
, monounsaturated) are shown in Figure 12.2.
