Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.


354 15. CALCULATIONS OF WOOD, PAPER, AND OTHER MATERIALS
T - tensile strength in MPa
spgrx9.81MPa-km-^
(15-7)
The tensile strength of wood and many other
materials is often reported in units of Ib/in.^ (psi).
The following equation can be used to determine
the breaking length in kilometers.
Kn
tensile strength in psi
spgrx 1422 psi-km "^
(15-8)
By using the conversion factors given in the
three previous equations, we can compare the
breaking length of a variety of materials. Table
15-1 is a comparison of many materials to give an
overall picture of the strength of available materi-
als.
Specific gravity and tensile strength values of
small, knot-fi:ee, wood samples at 12% MCQD
were obtained from the Wood Handbook. See
Section 2.7 for the maximum theoretical tensile
strength of cellulose fibers, which is about 100
km. Table 15-1 shows that the tensile strength of
pulp fibers is quite high in comparison with many
materials.
EXAMPLE 4. The tensile strength of black
willow is about 15,800 psi parallel to the
grain. It has a specific gravity of 0.41.
What is its breaking length?
SOLUTION. Using Eq. 15-8 the solution is:
ASmm
.27.1km
L
=
15.4 PAPER PROPERTIES
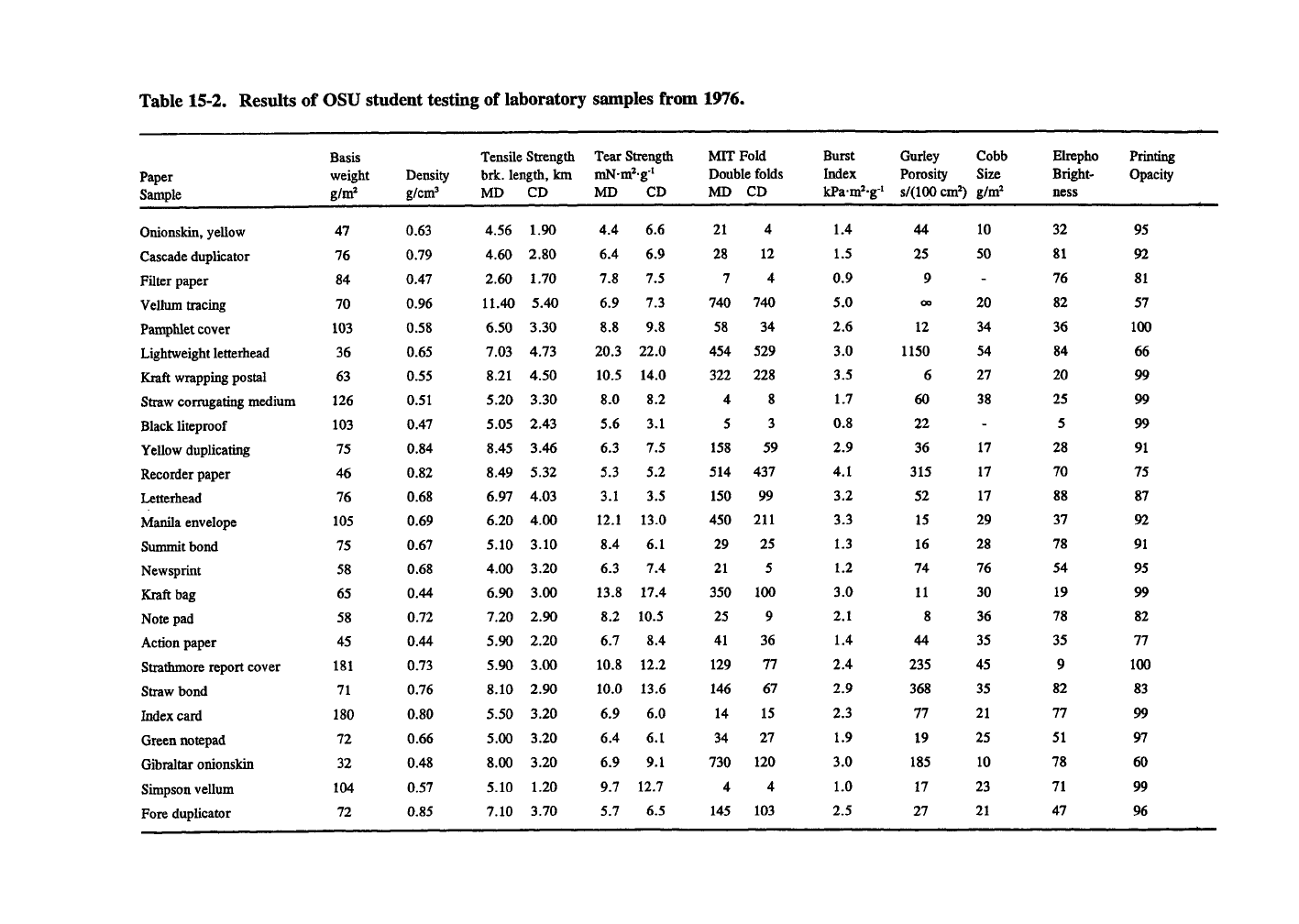
Table 15-2 shows some properties of paper
samples tested by OSU students in a 1976 labora-
tory exercise. These results can be used for com-
parison between grades of paper. They also
supply data for example exercises. Table 12-8
gives some conversion factors of paper properties
from English to metric units. Definitions of some
paper properties are given in Chapter 7.
EXAMPLE 5. The breaking length of the filter
paper listed in Table 15-2 in the machine
direction is given as 2.60 km. What was the
original force in pounds to break a 15 mm
wide specimen?
SOLUTION. Using Eq. 15.8, and a specific
gravity of 0.47 for this paper, one obtains a
tensile strength of 1737 psi. The width of
the paper is 15 mm or 0.591 inches. The
caliper of the paper is determined by the re-
lationship: density = mass/volume. For 1
m^ of paper, the mass is the basis weight (in
g/w?) and the volume (in cm^) = (100 cm)^
X caliper (cm). Therefore,
0.47
glow?
= 84 g/(10,000 cm^ x caliper)
caliper = 0.0178 cm
= 0.178 mm
= 0.00704 in.
0.41
X
1422psi-km
-1
So,
1737 psi X .591 in.
= 7.22 lb.
X 0.00704 in.
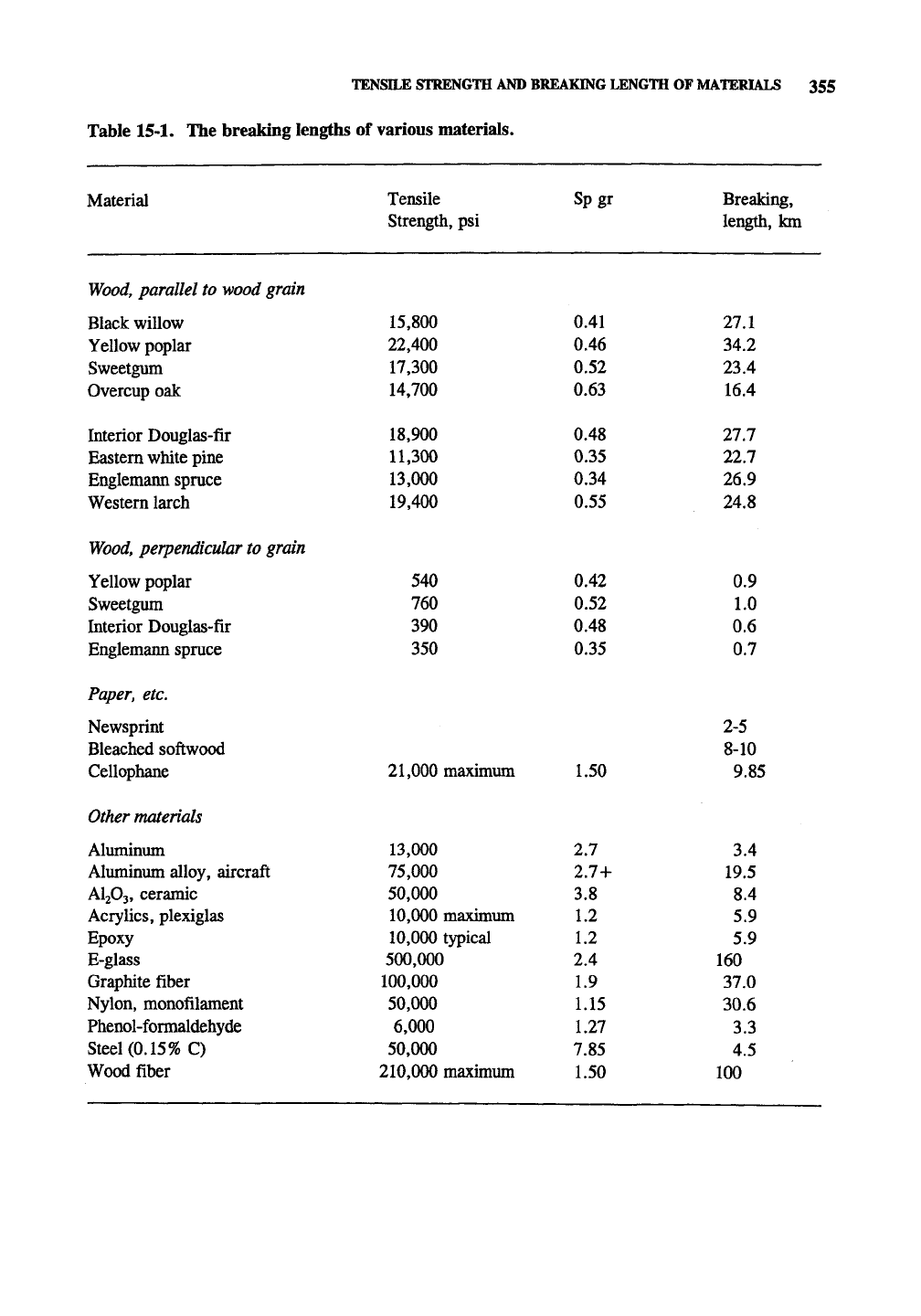
TENSILE STRENGTH AND BREAKING LENGTH OF MATERIALS 355
Table 15-1. The breaking lengths of various materials.
Material
Wood,
parallel to wood grain
Black willow
Yellow poplar
Sweetgum
Overcup oak
Interior Douglas-fir
Eastern white pine
Englemann spruce
Western larch
Wood,
perpendicular to grain
Yellow poplar
Sweetgum
Interior Douglas-fir
Englemann spruce
Pcper,
etc.
Newsprint
Bleached softwood
Cellophane
Other materials
Aluminum
Aluminum alloy, aircraft
AI2O3,
ceramic
Acrylics, plexiglas
Epoxy
E-glass
Graphite fiber
Nylon, monofilament
Phenol-ft)rmaldehyde
Steel (0.15% C)
Wood fiber
Tensile
Strength, psi
15,800
22,400
17,300
14,700
18,900
11,300
13,000
19,400
540
760
390
350
21,000 maximum
13,000
75,000
50,000
10,000 maximimi
10,000 typical
500,000
100,000
50,000
6,000
50,000
210,000 maximum
Spgr
0.41
0.46
0.52
0.63
0.48
0.35
0.34
0.55
0.42
0.52
0.48
0.35
1.50
2.7
2.7+
3.8
1.2
1.2
2.4
1.9
1.15
1.27
7.85
1.50
Breaking,
length, km
27.1
34.2
23.4
16.4
27.7
22.7
26.9
24.8
0.9
1.0
0.6
0.7
2-5
8-10
9.85
3.4
19.5
8.4
5.9
5.9
160
37.0
30.6
3.3
4.5
100
i
CM
O
(31)
f
I
O
1
S-
U CO ^
o £
"^s
III
2 ^
Q eto
e3 « ^
04 CO
ONO\00«o2^®^^^^^°®^^^^^^^°O^ONVOO\ON
2:
fs s c^
«n c>i en
s
«s
«n vo Tj- "-< 00
to ^ ^ r>. 1^
5
m 00
en vo
n en
-* m ON
^ ^ d
^ 2 ^ ?
?
vq ON «o en
vd vd r^ r-^
\q
o
*n
ri en en
r* 00 ON ^^
^ G CA -^
es en en es o "-J "*, "^^ ON en ON O O
rnen^^'enri^'escses^en^
00 00 en
g^r*ON^'^inS5oN^t:r?;
^?50N'-<<^O^ent^**C>
«0 00 TT O O
ir> ^ •n «n
^ tn y-* Tf
?i
^ ^
^ ON NO ^ ^ O
^ ri 2 ^ ^ C
r^'-H«nes«oo»-^TfTf^Tf<SNoo^^iv.«o
ooent^»oenenNdr^r*^doo<^^'^'^ONpJvd
TT
"*
00 ON
t«^*
VO
en «o o NO en
O O oc) »n vd
en ^ '^. Tj- en ^ es t-> 00 O OS Tt o^ 1^
inen^ooNO^ooNd^S^^'^^
O O O 2 O en o
ON 00 i> "^ en r* "o
^' (vj ^' to en Tf Tl-
O en NO es
en (S en «o
Tf en
o ^ es o ^ es es
en r4 <s en r«i rn en
lovo^^.
w^oc^esO'^'*ONes^poNesoNON'-<«opp^^
TfTfeNi;:;^vdt^od»o«r)oc>odNdNd«nTfNdr-'u->ioodvS«no6»nc^
ooooooooooooo
^es^envoONOoo
GO<DO<6cD<6<6<6c>
en vo en ’^O SO
o ^ S
f:2
2
NO JQ «o 00 <o 00
t^ S
«o NO «n
«o ’^ ^ o
:$ 00 f^ 00
«s ^ r<
en 2 ^
O O
S,
TENSILE STRENGTH AND BREAKING LENGTH OF MATERIALS 357
EXAMPLE 6. The burst index is given in Table 15-2 for kraft bag as 3.0 kPa-m^-g
^
What is the burst
strength in psi?
SOLUTION. The burst index is obtained by dividing the burst strength by the basis weight. To solve
for the burst strength it is necessary to take the burst index and multiply by the basis
weight. The burst strength obtained by this method is in kPa. It is necessary to convert
kPa to psi to obtain the desired units. The conversion factor is available in Table 12-8
under flat crush. Although a flat crush was not performed here, the conversion factor
from kPa to psi is always die same. Therefore,
3 kPa-m^-g-^
X
65 g-m"^ = 195 kPa; 195kPa x
^ ^^^ =
28 psi
^ ^
6.895
kPa ^
This exercise shows that to convert tensile or burst indexes to tensile or burst strength
the basis weight must be known. It is possible to convert a tensile or burst index back
and forth from the English to the metric system without knowing the basis weight.
EXERCISES
Wood
1.
A sawmill is
nominally
rated at 150,000 board feet (a board foot is 1/12 of a
ft^)
of 2 in. x 4 in.
lumber per day. The actual dimensions of a two by four are 1.5 in. x 3.5 in. (The actual board
feet production is only 65.6% of 150,000; why?) Douglas-fir wood is used with a specific gravity
of 0.44 and a moisture content of
120%
on an oven-dry basis. One uses log volume tables to learn
that typically (at this mill) for every 100 lb of solid wood coming in, 50 lb come out as lumber,
33.3 lb come out in the form of chips, and 16.7 lb come out in the form of sawdust. Chips have
a bulk density of 10 Ib/ft^ (oven-dry wood material), and sawdust 8 Ib/ft^. Rail cars have a rated
capacity of 18 units (3600 ft^). Chips are worth $160 per BDU, and sawdust $60 per BDU.
Calculate the following:
Lumber: Volume ^ft^; Wet weight ^tons; Dry weight ^tons.
Chips: O.D. weight ^tons; ^BDU; Green weight ^tons.
Number of rail cars needed ; Chips per car ^tons.
Sawdust: O.D. weight ^tons; ^BDU.
Revenue to mill: $ for chips; $ for sawdust; $ ^total.
2.
Given: a piece of wood with a specific gravity of 0.42 and a moisture content (MCGR) of 45%.
Calculate the following on a green volume basis:
kg/m^ Ib/ft^
Oven-dry weight
Green (wet) weight
Weight of water contained

358 15. CALCULATIONS OF WOOD, PAPER, AND OTHER MATERIALS
3.
Douglas-fir wood with a basic specific gravity of 0.45 is converted into chips that have a bulk
density of 10 Ib/ft^ (oven-dry weight of chips). Assume the moisture content on a green basis is
45%.
Calculate:
Volume of chips from 1
ft^
of solid wood: ft^
Volume of chips from 1
m^
of solid wood: ^m^
Oven-dry weight of wood from 1
ft^
of solid wood: ^Ib
Green weight of wood
firom
1
ft^
of solid wood: ^Ib
Oven-dry weight of wood
firom
1
m^
of solid wood: ^kg
Green weight of wood from 1
m^
of solid wood: ^kg
Breaking
length
4.
Show that 9.81 MPa/km is equal to 981 N-cm'^-km
^
5. A plastic material has a tensile strength of 3000 psi and a density of 0.85. What is its breaking
length? Is it intrinsically stronger than paper?
6. Calculate the breaking force (lb/15 mm) of the newsprint listed in Table 15-2.
Miscellaneous
paper tests
7. Calculate the caliper of the kraft bag listed in Table 15-2.

16
PULPING CALCULATIONS
16.1 GENERAL CHEMICAL PULPING
DEFINITIONS
Introduction
Brief definitions are given for the various
pulp liquor terms in this chapter. The significance
and other information about these terms is found
in Chapter 3. Here the terms are presented as
mathematical variables to be manipulated algebra-
ically.
Chemical concentration
Chemical concentration is a measure of the
concentration of the pulping chemical in the
liquor. For example, in sulfite pulping the liquor
may be 6% SO2, indicating 6 grams of sulfite
chemical
(SO2
basis) per 100 ml of liquor. If the
liquorrwood ratio is 4:1, the percent chemical on
wood is 24% as SOj. The following is an impor-
tant relationship, not the definition of chemical
concentration.
chemical concentration
in
liquor
=
percent chemical
on
wood
liquorrwood ratio
(16-1)
Chemical
charge (to a process), percent
chemical
(on
wood or pulp)
The chemical charge is a measure of the
weight of chemical used to process (i.e., pulp or
bleach) a material. For example, kraft pulping is
carried out with about 25% total alkali on wood.
This would indicate 500 pounds of alkali for 2000
pounds of
dry
wood. Chemicals in sulfite pulping
are expressed on an SO2 basis. When bleaching
mechanical pulp, one might use "0.5% sodium
peroxide on
pulp".
This means that 10 pounds of
sodium peroxide are used per ton of dry pulp.
chemical charge,
%
=
dry weight of chemical used -^yv^^
dry weight of material treated
(16-2)
Liquor to wood ratio
The liquor to wood ratio is normally ex-
pressed as a ratio, not as a percent. It is typically
3:1 or 4:1 in full chemical pulping. The numera-
tor may or may not include the weight of water
coming in with the chips, and it must be specified
to avoid ambiguity.
liquor total weight of pulping liquor
wood dry weight of wood
(16-3)
16.2 KRAFT LIQUOR-CHEMICAL
CALCULATIONS
Total chemical
or total alkali
(TA)
The total alkali is the sum of all of the
sodium salts in the liquors (as NajO) that contrib-
ute to AA or are capable of being converted to
AA in the kraft cycle, specifically NaOH, Na2S,
Na2C03,
and Na2SPy (as Na20). All chemical
amounts may be reported as a concentrations of
g/L or lb/gal or as a percent relative to oven dry
wood. Chemicals are reported on a Na20 basis in
North America.
TA = NaOH -h NajS -h Na2C03 + Na2Sp,
(asNa20) (16-4)
Total
titratable
alkali
(TTA)
TTA is the sum of all of the bases in the
white liquor that can be titrated with strong acid.
Generally, it is considered as NaOH, Na2S, and
Na2C03 (as Na20), although small amounts of
Na2S03 and other acids might be present.
TTA = NaOH + Na2S + Na2C03
(asNazO) (16-5)
Active
Alkali (AA)
The sum of the active ingredients in the
pulping process is known as active alkali.
AA = NaOH + NagS (as NazO) (16-6)
Effective
alkali
(EA)
EA is the sum of sodium chemicals that will
produce OH" during kraft pulping. NaOH is
359
360 16. PULPING CALCULATIONS
completely ionized and for every two sodium
atoms of NaiS, there will be one
OH"
produced.
causticizing efficiency =
NaOH
EA = NaOH + Vi Na2S (as NajO) (16-7) NaOH
+
Na2C03
(16-11)
xlOO%
(Na20 basis)
Often AA and EA are given and one needs to
determine the concentration of individual species.
A very useful relationship to remember is that:
Na2S = 2 (AA - EA); all species are expressed as
Na20 in this formula.
Na2S = 2 (AA - EA) (as NajO) (16-8)
Sulfidity
In the white liquor, sulfidity is the ratio of
NaiS to the active alkali, expressed as a percent.
Typically, a mill runs in the vicinity of 25-30%
sulfidity, depending largely on the wood species
pulped. Sulfidity increases the rate of
delignification, which occurs by nucleophilic
action of
the
hydrosulfide anion (HS) and appears
to protect cellulose against degradation.
sulfidity =
Na2S
NaOH+Na2S
(16-9)
xlOO%
(asNa20)
Causticity
The causticity is the ratio of NaOH to active
alkali, expressed as a percentage; therefore,
causticity + sulfidity = 100%. The term sulfidity
is used much more than the term causticity, and
both give the same information. It will not be
considered further in this text.
causticity
NaOH
NaOH+Na^S
(16-10)
xl00%
(asNa^O)
Causticizing efficiency
The causticizing efficiency is the ratio of
NaOH to NaOH and NajCOa. This is a measure
of how efficient causticizing is; it represents the
percentage of
the
NajCOj from the recovery boiler
that is converted back into useful NaOH cooking
chemical. A value of
77-80%
is typical.
Reduction efficiency
The reduction efficiency is the ratio of Na2S
to the sum of
Na^S
and Na2S04 in green liquor ex-
pressed as a percentage. This is a measure of the
reduction efficiency in the recovery boiler. This
value should be high, is usually 95%, and is not
routinely measured in the mill. In addition to
sodium sulfate, other oxidized forms of sulfur are
present, such
as
sodium sulfite and sodium thiosul-
fate, that should be considered.
reduction efficiency
Na2S
Na^S
+
Na^SO^
(16-12)
xl00%
(Na^O basis)
It is convenient to set up a table of conver-
sion factors of
use
to solve some of
the
values just
given. Table 16-1 gives many of these conversion
factors for kraft cooking and chemical recovery.
The cooking chemicals of the NSSC process,
NajSOa and NajCOj, are often expressed on an
NajO basis as well.
Several examples are presented to demon-
strate kraft liquor calculations. Example 1 shows
the conversion factor for gravimetrically convert-
ing NaOH to Na20. While Na20 is a hypothetical
species in aqueous solutions and does not occur in
aqueous solutions, it is a convenient way of ex-
pressing cooking chemicals on a weight basis, but
at the same time on an equal molar basis. Exam-
ple 1 demonstrates how the conversion factors in
Table 16-1 are derived.
Example 2 shows how to calculate the actual
concentration of chemical species based on cook-
ing liquor parameters. Example 3 is a detailed
pulping problem. Example 4 demonstrates the
calculation and use of causticizing efficiency
values.
There are additional exercises on which to
practice these calculations.
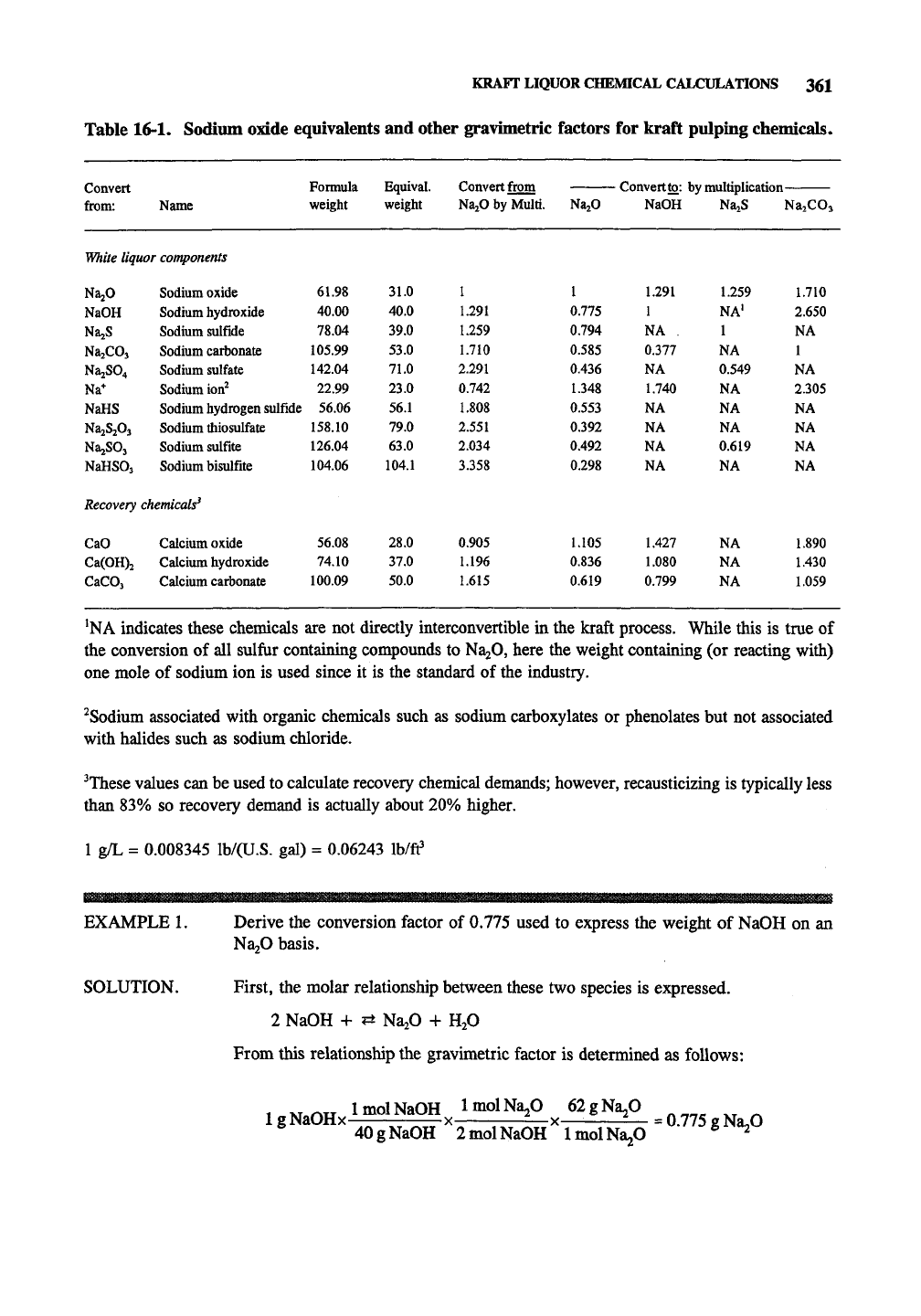
KRAFT LIQUOR CHEMICAL CALCULATIONS 361
Table 16-1. Sodium oxide equivalents and other gravimetric factors for kraft pulping chemicals.
Convert Fonnula Equival. Convert from Convert to: by multiplication-
from: Name weight weight NajO by Multi. N2L2O NaOH NazS NazCOj
White
liquor
components
NajO
NaOH
Na^S
Na^COa
Na2S04
Na^
NaHS
NazSjOj
Na^SOj
NaHSOa
Sodium oxide
Sodium hydroxide
Sodium sulfide
Sodium carbonate
Sodium sulfate
Sodium
ion^
Sodium hydrogen sulfide
Sodium thiosulfate
Sodium sulfite
Sodium bisulfite
Recovery
chemicals^
CaO
Ca(OH)2
CaCOj
Calcium oxide
Calcium hydroxide
Calcium carbonate
61.98
40.00
78.04
105.99
142.04
22.99
56.06
158.10
126.04
104.06
56.08
74.10
100.09
31.0
40.0
39.0
53.0
71.0
23.0
56.1
79.0
63.0
104.1
28.0
37.0
50.0
1
1.291
1.259
1.710
2.291
0.742
1.808
2.551
2.034
3.358
0.905
1.196
1.615
1
0.775
0.794
0.585
0.436
1.348
0.553
0.392
0.492
0.298
1.105
0.836
0.619
1.291
1
NA .
0.377
NA
1.740
NA
NA
NA
NA
1.427
1.080
0.799
1.259
NA'
1
NA
0.549
NA
NA
NA
0.619
NA
NA
NA
NA
1.710
2.650
NA
1
NA
2.305
NA
NA
NA
NA
1.890
1.430
1.059
^NA indicates these chemicals are not directly interconvertible in the kraft process. While this is true of
the conversion of all sulfur containing compounds to NajO, here the weight containing (or reacting with)
one mole of sodium ion is used since it is the standard of the industry.
^Sodium associated with organic chemicals such as sodium carboxylates or phenolates but not associated
with halides such as sodium chloride.
^These values can be used to calculate recovery chemical demands; however, recausticizing is typically less
than 83% so recovery demand is actually about 20% higher.
1 g/L = 0.008345 lb/(U.S. gal) = 0.06243 Ib/ff
EXAMPLE 1. Derive the conversion factor of
0.775
used to express the weight of NaOH on an
NajO basis.
SOLUTION. First, the molar relationship between these two species is expressed.
2 NaOH + ?± NajO + H2O
From this relationship the gravimetric factor is determined as follows:
1 xTriu ImolNaOH ImolNa^O 62gNa20
1 g NaOHx X =—X— =—
=
0.775
e Na»0
40gNaOH 2molNaOH ImolNa^O ^ ^
362 1^. PULPING CALCXJLATIONS
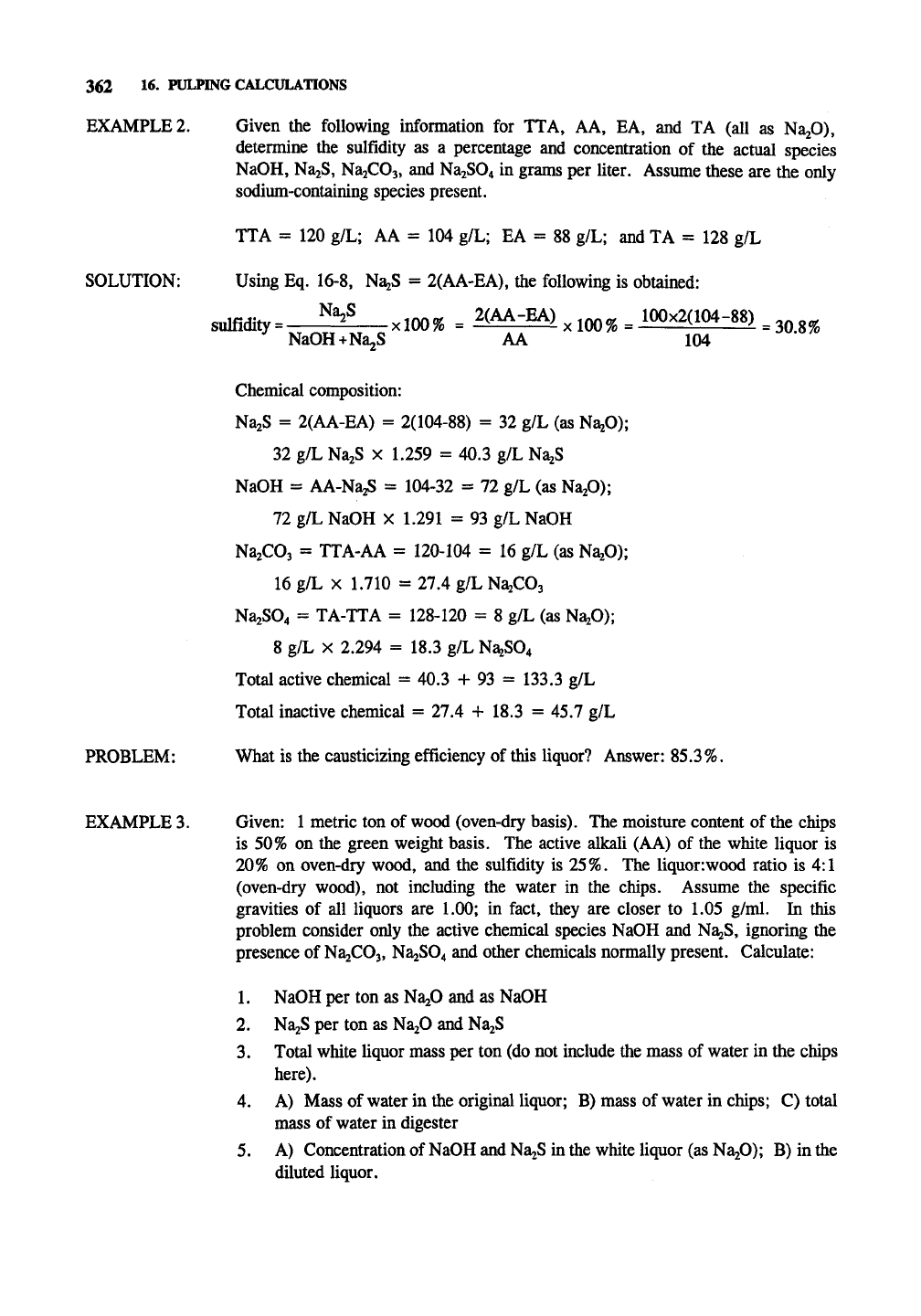
EXAMPLE 2.
SOLUTION:
Given the following information for TTA, AA, EA, and TA (all as NaiO),
determine the sulfidity as a percentage and concentration of the actual species
NaOH, NaiS, NajCOj, and Na2S04 in grams per liter. Assume these are the only
sodium-containing species present.
TTA = 120 g/L; AA = 104 g/L; EA = 88 g/L; and TA = 128 g/L
Using Eq. 16-8, NajS = 2(AA-EA), the following is obtained:
sulfidity
=
Na^S
<100%
= ^(^•^) xlOO% = 100x2(104-88) ^3^^
NaOH+Na^S AA 104
PROBLEM:
Chemical composition:
Na2S = 2(AA-EA) = 2(104-88) = 32 g/L (as Na20);
32 g/L NajS x 1.259 = 40.3 g/L NajS
NaOH = AA-NazS = 104-32 = 72 g/L (as NajO);
72 g/L NaOH x 1.291 = 93 g/L NaOH
NajCOa = TTA-AA = 120-104 = 16 g/L (as N^O);
16 g/L X 1.710 = 27.4 g/L N^COa
Na2S04 = TA-TTA = 128-120 = 8 g/L (as
NB^O);
8 g/L X 2.294 = 18.3 g/L Na2S04
Total active chemical = 40.3 + 93 = 133.3 g/L
Total inactive chemical = 27.4 + 18.3 = 45.7 g/L
What is the causticizing efficiency of this liquor? Answer:
85.3%.
EXAMPLE 3. Given: 1 metric ton of wood (oven-dry basis). The moisture content of the chips
is 50% on the green weight basis. The active alkali (AA) of the white liquor is
20%
on oven-dry wood, and the sulfidity is 25%. The liquor:wood ratio is 4:1
(oven-dry wood), not including the water in the chips. Assume the specific
gravities of all liquors are 1.00; in fact, they are closer to 1.05 g/ml. In this
problem consider only the active chemical species NaOH and NagS, ignoring the
presence of
NajCOj,
Na2S04 and other chemicals normally present. Calculate:
1.
NaOH per ton as Na20 and as NaOH
2.
NajS per ton as NazO and Na2S
3.
Total white liquor mass per ton (do not include the mass of water in the chips
here).
4.
A) Mass of water in the original liquor; B) mass of water in chips; C) total
mass of water in digester
5. A) Concentration of
NaOH
and NaiS in the white liquor (as Na20); B) in the
diluted liquor.
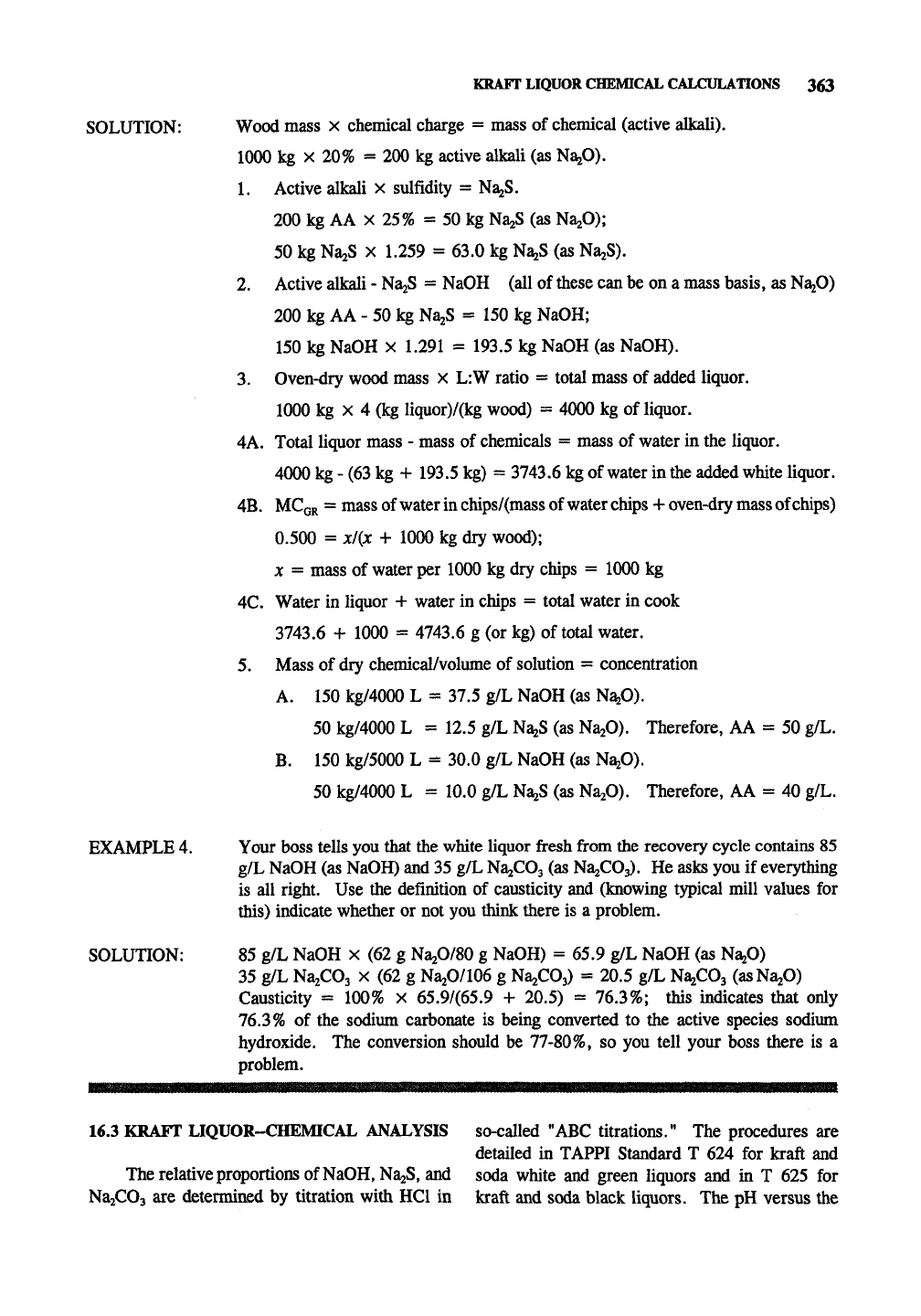
KRAFT LIQUOR CHEMICAL CALCULATIONS 363
SOLUTION: Wood mass x chemical charge = mass of chemical (active alkali).
1000 kg X 20% = 200 kg active alkali (as NaaO).
1.
Active alkali x sulfidity = NajS.
200 kg AA X 25% = 50 kg NaiS (as NajO);
50 kg NajS X 1.259 = 63.0 kg NazS (as NaaS).
2.
Active alkali - NajS = NaOH (all of these can be on a mass basis, as NagO)
200 kg AA - 50 kg NaaS = 150 kg NaOH;
150 kg NaOH X 1.291 = 193.5 kg NaOH (as NaOH).
3.
Oven-dry wood mass x L:W ratio = total mass of added liquor.
1000 kg X 4 (kg liquor)/(kg wood) = 4000 kg of liquor.
4A. Total liquor mass - mass of chemicals = mass of water in the liquor.
4000 kg - (63 kg + 193.5 kg) = 3743.6 kg of water in the added white liquor.
4B.
MCGR = mass of water in chips/(mass of water chips + oven-dry mass of chips)
0.500 = xl{x + 1000 kg dry wood);
X = mass of water per 1000 kg dry chips = 1000 kg
4C.
Water in liquor + water in chips = total water in cook
3743.6 + 1000 = 4743.6 g (or kg) of total water.
5.
Mass of dry chemical/volume of solution = concentration
A. 150 kg/4000 L = 37.5 g/L NaOH (as Na^O).
50 kg/4000 L = 12.5 g/L N^S (as NajO). Therefore, AA = 50 g/L.
B.
150 kg/5000 L = 30.0 g/L NaOH (as Na^O).
50 kg/4000 L = 10.0 g/L N^S (as NaiO). Therefore, AA = 40 g/L.
EXAMPLE 4. Your boss tells you that the white liquor fresh from the recovery cycle contains 85
g/L NaOH (as NaOH) and 35 g/L Na2C03 (as NaaCOj). He asks you if everything
is all right. Use the definition of causticity and (knowing typical mill values for
this) indicate whether or not you think there is a problem.
SOLUTION: 85 g/L NaOH x (62 g Na2O/80 g NaOH) = 65.9 g/L NaOH (as NazO)
35 g/L Na2C03 X (62 g Na2O/106 g Na2C03) = 20.5 g/L Na^COa (asNa20)
Causticity = 100% X 65.9/(65.9 + 20.5) =
76.3%;
this indicates that only
76.3%
of the sodium carbonate is being converted to the active species sodium
hydroxide. The conversion should be 77-80%, so you tell your boss there is a
problem.
16.3 KRAFT LIQUOR-CHEMICAL ANALYSIS so-called "ABC titrations." The procedures are
detailed in TAPPI Standard T 624 for kraft and
TherelativeproportionsofNaOH, NajS, and soda white and green liquors and in T 625 for
NajCOa are determined by titration with HCl in kraft and soda black liquors. The pH versus the
