Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.

374 16.
PULPING
CALCULATIONS
19-,
IH-
lU
o
% 6-
4"
L
0-
0
20'
40 60
Bisulfite ion, %
80 100 80
K
^^
60
40
20
0
fi
100 80 60 40
Sulfurous acid, %
20
20 40 60 80 100
Sulfite ion, %
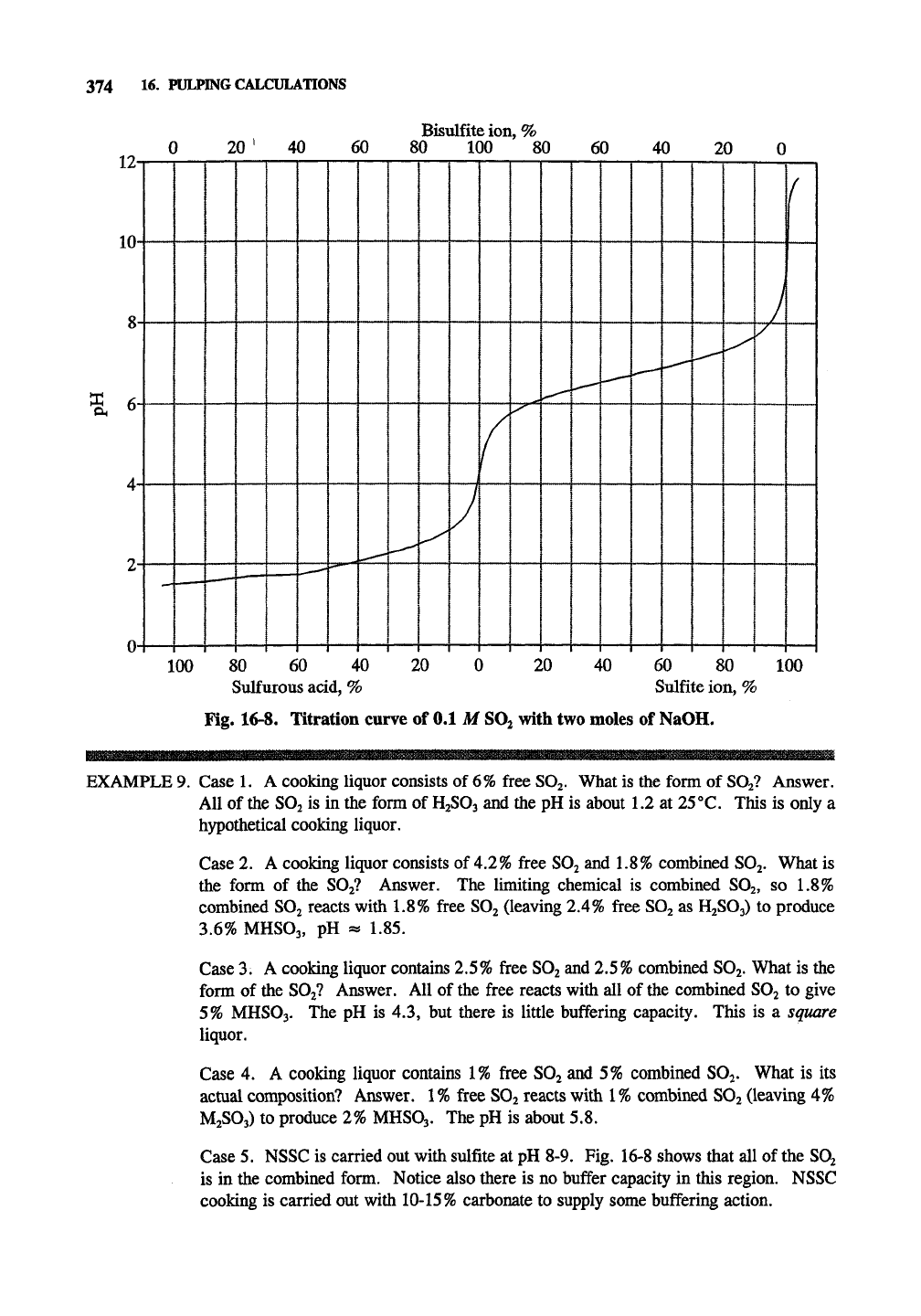
Fig. 16-8. Titration curve of 0.1 M
SO2
with two moles of NaOH.
EXAMPLE 9. Case 1. A cooking liquor consists of 6% free SOj. What is the form of
SO2?
Answer.
All of the SO2 is in the form of
H2SO3
and the pH is about 1.2 at 25°C. This is only a
hypothetical cooking liquor.
Case 2. A cooking liquor consists of 4.2% free SO2 and 1.8% combined SO2. What is
the form of the SO2? Answer. The limiting chemical is combined SO2, so 1.8%
combined SO2 reacts with 1.8% free SO2 (leaving 2.4% free SO2 as H2SO3) to produce
3.6% MHSO3, pH « 1.85.
Case 3. A cooking liquor contains 2.5% free SO2 and 2.5% combined SO2. What is the
form of the SO2? Answer. All of the free reacts with all of the combined SO2 to give
5%
MHSO3. The pH is 4.3, but there is little buffering capacity. This is a square
liquor.
Case 4. A cooking liquor contains 1% free SO2 and 5% combined SOj. What is its
actual composition? Answer. 1% free SO2 reacts with 1% combined SO2 (leaving 4%
M2SO3) to produce 2% MHSO3. The pH is about 5.8.
Case 5. NSSC is carried out with sulfite at pH 8-9. Fig. 16-8 shows that all of the SO2
is in the combined form. Notice also there is no buffer capacity in this region. NSSC
cooking is carried out with 10-15% carbonate to supply some buffering action.
SULFITE LIQUOR CALCULATIONS
375
EXAMPLE 10. A solution is
3%
NajSOa (as SO2) and
2%
NaHSOj (as SO2). What is the free and
combined SO2? What is the actual concentration of NaHSQj?
SOLUTION. 2% NaHSOj -* 1% H2SO3 + l%Na2S03. Therefore, this corresponds to 4%
combined SO2 and
1 %
free
SO2. From Table 16-4 the gravimetric factor of 1.624
is obtained to convert
SO2
to
NaHSOj.
2% NaHSOj
(SO2
basis) x 1.624 = 3.25%
NaHSOa = 32.5 g/L NaHSOj.
EXAMPLE 11. Given: A digest charge with 1 kg of dry wood, a calcium liquor containing 5%
free
SO2
and
1 %
combined
SOj
and a liquorrwood ratio of
4:1
(including the water
in the wood).
Calculate: the concentrations and amounts of
the
actual chemical species. Assume
the specific gravity of the liquor is 1.00.
SOLUTION. The total SO2 is equal to the concentration in the liquor times the liquor to wood
ratio,
or
24%
on wood, which is 240 grams
SO2
for the charge. The
1 %
combined
reacts with
1 %
free to give 2% (60 g on wood) as bisulfite leaving 4% (160 g on
wood) as sulfiirous acid. From Table 16-4 80 grams bisulfite (SO2 basis)
corresponds to 80 x 1.578 = 126.4 g Ca(HS03)2 on wood or 31.6 g/L. In a
similar fashion 160 g free
SO2
corresponds to 205.2 g
H2SO3
on wood or 51.3 g/L.
PROBLEM. For this problem, what would the concentrations be for Mg based cooking?
Answer: 29.1 g/L Mg(HS03)2.
16.8 SULFITE LIQUOR ANALYSIS
Sulfite pulping liquors could be titrated with
NaOH to each endpoint to determine the free SO2
and combined SO2. However the first endpoint is
not very sharp, and even the second endpoint may
not be very sharp in sulfite liquors. Palmrose
(1935) developed a method where, under acidic
conditions, all of the SO2 is converted to
804"^
by
periodate ion as shown in the following two
chemical equations. Periodate thus measures the
total
SO2.
KIO3 + 3H2SO3 ^ KI + 3H2SO4
2KIO3 + 3(HS03)2 -* 2KI + 3H2SO4 + 35042-
I of KIO3 is reduced from +5 to -1 while each
sulfur of
SOs^'
is oxidized from +4 to +6. The
equivalent weight of KIO3 is 1/6 the molecular
weight, and the equivalent weight of
H2SO3
is one-
half the molecular weight. These reactions are
actually fairly slow and the endpoint would easily
be overrun. Small amounts of KI (from the
indicator solution), however, allow the following
two reactions, which are rapid, to occur:
IO3-
+ 3H2SO3 +
51-
-* 3804^- + 3I2 + 3H2O
3SO42-
+ 3I2 + 3H2O +
3H2SO3
-*
61-
+ 6H2SO4
The titration is carried out with 0.1
iV
potas-
sium iodate to blue endpoint using Kl/starch
indicator. The excess
I2
at the end of
the
reaction
reacts with starch to give a characteristic blue
color, a well known reaction used as an indicator
for starch. The slight excess of
KIO3
at the end of
the reaction is reacted with thiosulfate.
The liberated acid is then titrated with 0.1 M
NaOH to a methyl red endpoint and represents the
free SO2, Notice that
the
latter titration is a strong
376
16.
PULPING CALCULATIONS
acid-Strong base titration with a well-resolved
endpoint. When this method was first developed
calcium was the only base used for sulfite pulping
and the liquors were necessarily acidic. With
other bases where the cooking liquor is above pH
of 4-5, a known amount of sulfuric acid is added
before the iodate titration. The liberated acid is
then titrated but the additional amount of sulfuric
acid added is subtracted from the free SO2 value
obtained in the second titration. This procedure is
described in TAPPI Standard T 604.
EXAMPLE 12. A sulfite liquor was diluted 1:10.
A 10.00 ml aliquot of the diluted solution
was titrated by the Palmrose method. Calcu-
late the total and combined SO2 in the origi-
nal solution based on the following amounts
of titrants:
10.78 ml of 0.0946 iVNaOH
Cyclooctasulfur
Catenasulfur
12.18 ml of 0.2060 NKIO^ and
SOLUTION: The total SO2 'mN= 10 x (12.18
ml X 0.2060 N)nO ml = 2.51 N,
The total SO2 = 2.51 N x 32 g/eq SO2 =
80.32 g/L total SO2 or 8.03% total SO2.
The free SO2 = 10 x (10.78 ml x 0.0946
iV)/10ml = 1.020 JV.
The free SO2 = 1.02
AT
x 32 g/eq SO2 =
32.64 g/L free SO2 or 3.26% free SOj.
16.9 THE CHEMISTRY OF SULFUR
Elemental sulfur
Elemental sulfur occurs in many complex
forms.
The chemistry of elemental sulfur present-
ed here is a simplification of
its
complex chemistry
but will be useful to explain its properties. Crys-
talline sulfur contains sulfur rings with 6 to 20
sulfur atoms or chains of sulfur atoms called
catenasulfur, S«,. The most common form is
cyclooctasulfur, Sg, which has two common allo-
tropes: orthorhombic sulfur, S„, and monoclinic
sulfur, S^. S„ is, thermodynamically, the most
stable of the Sg forms. The structures are:
Above 95 °C S^ slowly converts to S^. With
rapid heating, the melting point of S« (113°C) is
obtained. S^ melts at 119°C. S^ crystallizes
from sulfur melts and over the course of months
converts to S„. Liquid Sg sulfur becomes increas-
ing viscous above 160°C as it is converted to
catenasulfur. Above 200°C the viscosity of the
catenasulfur decreases as its maximum degree of
polymerization is at 200°, where the formula
weight is above 200,000. The boiling point of
sulfur is 445
°C.
If catenasulfur of high viscosity
is quenched by pouring into ice water, a plastic
solid results. The solid catenasulfur slowly reverts
to Sg over time. Sg is soluble in CS2, S« is not
soluble.
Sulfur is recovered in large amounts from
H2S in natural gas by the reaction:
2H2S + SO2 -^ 3S 4- H2O
In Europe large amoimts of sulfur are used
from iron pyrite, FeS (the substance called fool's
gold because of its similarity to real gold), which
is a solid. Sulfur is also a by-product of copper
production from CuS. Sulfur is also mined in
large amounts in its elemental form from volcanic
deposits by the Frasch process, where steam is
injected into the ground to heat the sulfur and the
molten sulfur is pumped from the ground.
Sulfur
combustion to
produce
SO2
Elemental sulfur is burned to produce sulfur
dioxide.
S +
O2
^ SO2 (gas)
Above 1000°C (1830°F) no sulfur trioxide is
produced; however, some might be produced in
the process of cooling the gases. Sulfur trioxide,
which produces sulfuric acid upon reaction with
water, is very undesirable in pulping reactions and
is removed during the cooling/scrubbing process
(by counter current flow of water and sulfur
dioxide).

THE
CHEMISTRY
OF SULFUR
377
SO3 + H2O -^ H2SO4
The SO2 forms H2SO3 in water which in turn
is reacted with metal bases to produce sulfite pulp-
ing liquors, as described in other sections. Be-
cause H2SO3 is much more acidic than H2CO3,
salts of carbonate can be used to form salts of
sulfite. For example, wet SO2 is traditionally
formed into calcium bisulfite by calcium carbonate
(limestone) by the following equation:
CaC03 + 2H2SO3 -* Ca(HS03)2 + CO2 + H2O
Total sulfur by gravimetric analysis
Often the total content of organically bound
and inorganic sulfur is desired. This is accom-
plished by treating the sample with a strong
oxidant under alkaline conditions to convert the
sulfiir to sulfate. For example, when sodium
peroxide is used, sodium sulfate is formed and the
organic chemicals are largely converted to carbon
dioxide and water. After the sample is acidified
with HCl, the SO/" is precipitated with excess
BaCl2.
The precipitate is washed with small
amoxmts of water and then dried in a muffle
furnace. The precipitate is weighed and converted
to a sulfiir equivalent with the appropriate gravi-
metric factor.
16.10 CALCINING EQUATIONS
Two equations are used to characterize
calcining of lime mud to produce fresh lime. The
specific energy consumption is an indication of
how much fiiel is required to process the lime mud
and is often reported as Btu per ton of lime.
16.10 ANNOTATED BIBLIOGRAPHY
H'factor and process control equations
1.
Edwards, L. and S.-E. Norberg, Alkaline
delignification kinetics, A general model
applied to oxygen bleaching and kraft pulp-
ing,
TappiJ.
56(11):
108-111(1973).
2.
Kubes, G.J., B.I. Fleming, J.M. MacLeod,
and H.I. Bolker, Viscosities of unbleached
alkaline pulps. 11. The G-factor, /. Wood
Chem,
Tech, 3(3):313-333(1983).
3.
Hatton, J.V., Development of yield predic-
tion equations in kraft pulping, Tappi J.
56(7):97-100(1973).
4.
Hatton, J.V., The potential of process control
in kraft pulping of hardwoods relative to
softwoods, Tappi /. 59(8):48-50(1973).
5.
Lin, C.P., W.Y. Mao, and Jane, C.Y.,
Development of a kappa number predictive
equation in kraft pulping for all types of
hardwood, Tappi /. 61(2)72(1978).
6. Paulonis, M.A. and A. Krishnagopalan,
Adaptive inferential control of kraft batch
digesters as based on pulping liquor analysis,
TappiJ.
74(6):
169-175(1991).
7.
Tasman, J.E., Kraft delignification models,
TappiJ.
64(3)175-176(1981).
specific energy consumption =
fuel to kiln
CaO output
The lime availability is an indication of the
purity of the lime in terms of available CaO
divided by the amount of lime product.
lime availability
=
CaO
lime
8. Vroom, K.E., The "H" factor: A means of
expressing cooking times and temperatures as
a single variable. Pulp Paper Mag. Can.
58(3):228-231(1957).
Sulfite liquor analysis
9. Palmrose, G.V., A mill test for the exact
determination of combined sulphur dioxide.
Tech.
Assn. Papers, XVin:309-310(1935);
the same article is reprinted as
ibid.
Paper
Trade
J. 100(3)38-39(1935).
Chemistry
of sulfur
10.
Cotton, F.A. and G. Wilkinson, Advanced
as mass ratio Inorganic Chemistry, 4th ed., Wiley and
Sons,
New York, 1980. 1396 p.
378 16. PULPING CALCULATIONS
EXERCISES
Kraft
liquor-chemical calculations
1.
Derive the gravimetric conversion factor to
express NajCOj as NajO.
2.
White liquor has the following composition
of the actual chemical species. Calculate
TTA, AA, EA, TA, causticizing efficiency
and sulfidity.
NaOH = 145 g/L Na^S = 55 g/L
Na^COs = 17 g/L Na2S04 = 14 g/L
Kraft
liquor-chemical analysis
3.
The effective alkali concentration of a white
liquor is 93.7 g NasO/L. The sulfidity is
37.5%
and the causticizing efficiency is
77.9%.
Calculate the following.
a) The A, B, C values in ml for a 10 ml
aliquot of liquor titrated with 1.017 N
HCl.
b) The molar concentrations of the chemi-
cal species NaOH, NajS, and Na2C03 in
this liquor.
c) The active and effective alkali charges
on wood (as a percent) if 200 ml of this
liquor is used to pulp 200 g Douglas-fir
chips at
50%
MCgr
(with some addition-
al dilution water).
H-factor
4.
What is the H-factor for a cook which is held
at 165 °C for L43 hours? If this H-factor is
satisfactory, how long should one cook if the
temperature is increased to 170°C for a
dif-
ferent cook?
Sulfite
liquor
calculations
5. By comparing sulfite cooking chemicals all
on a
SO2
basis, one is really comparing them
on an equal (molar or weight) basis. Circle
the correct choice.
6. Analysis of a sulfite cooking liquor indicates
a total SO2 of
7%
and
4%
free SO2.
The combined SO-, is %.
The SO2 as sulfiirous acid is
The SO2 in the form of the bisulfite ion
(HSO3) is %, and the SO2 in the form
of sulfite ion (SOj^) is %.
7. Analysis of a sulfite cooking liquor indicates
a total SO2 of 8%, and a combined SO2 of
2%.
Solve for unknown free SOj, as well as
the concentration of
the
sulfiirous acid, bisul-
fite
ion,
and sulfite ion species (all as
%
SO2
which equals g/100 ml), and the pH of the
liquor.
8. A solution is made by weighing 1.1 grams
sulfiirous acid (as sulfiirous acid) and 10.3
grams sodium sulfite (as sodium sulfite) to
make 0.193 liters of solution. Solve for
unknown free SO2, combined SO2, total SO2,
as well as the concentration of the sulfiirous
acid, bisulfite ion, and sulfite ion species (all
as % SO2 which equals g/100 ml), and the
pH of the liquor.
Sulfite liquor analysis
9. Analysis of a pulping liquor with iodometric
titration gives a
SO2
value of 10%. Titration
with NaOH gives 6.5% SOj. Solve for un-
known
free
SO2, combined SO2, total
SO2,
as
well as the concentration of the sulfiirous
acid, bisulfite ion, and sulfite ion species (all
as % SO2 which equals g/100 ml), and the
pH of
the
liquor. If sodium is the base, how
many grams of sulfiirous acid, sodium bisul-
fite, and/or sodium sulfite are needed to
make one liter of cooking liquor?
17
BLEACHING AND PULP PROPERTIES CALCULATIONS
17.1 DILUTION WATER CALCULATIONS
The water per ton of pulp ratio (V) is solved
from the consistency (c) as V = (100-c)/c.
EXAMPLE 1. After the brown stock washers,
the consistency of unbleached pulp is 11.2%.
Calculate the volume of water (in m^/t oven-
dry pulp) required to dilute the slurry to 3%
consistency for chlorination.
SOLUTION. At 11% consistency there are 89 t
water/(ll t pulp) = 8.09 t water/t pulp. At
3%
consistency there are 97 t water/(3 t
pulp) = 32.33 t/t. Therefore 32.33 - 8.09 =
24.24 t water/t pulp to be added. Since 1 t
= 1000 kg = 1 m^ of water, this is 24.24 m^
of water per metric ton of pulp.
17.2 CHLORINE BLEACHING
The pH is of utmost importance in chlorine
bleaching for several reasons. First the form of
chlorine in solution is dependent on the pH ac-
cording to the following equilibrium reactions.
CI2 + H2O •=i H^ + CI + HOCl
K^ = 3.9 X 10-^at25°C (17-1)
HOCl
4=^
H^ + ocr
K^ = 3.5 X 10-^ at 18°C
(17-2)
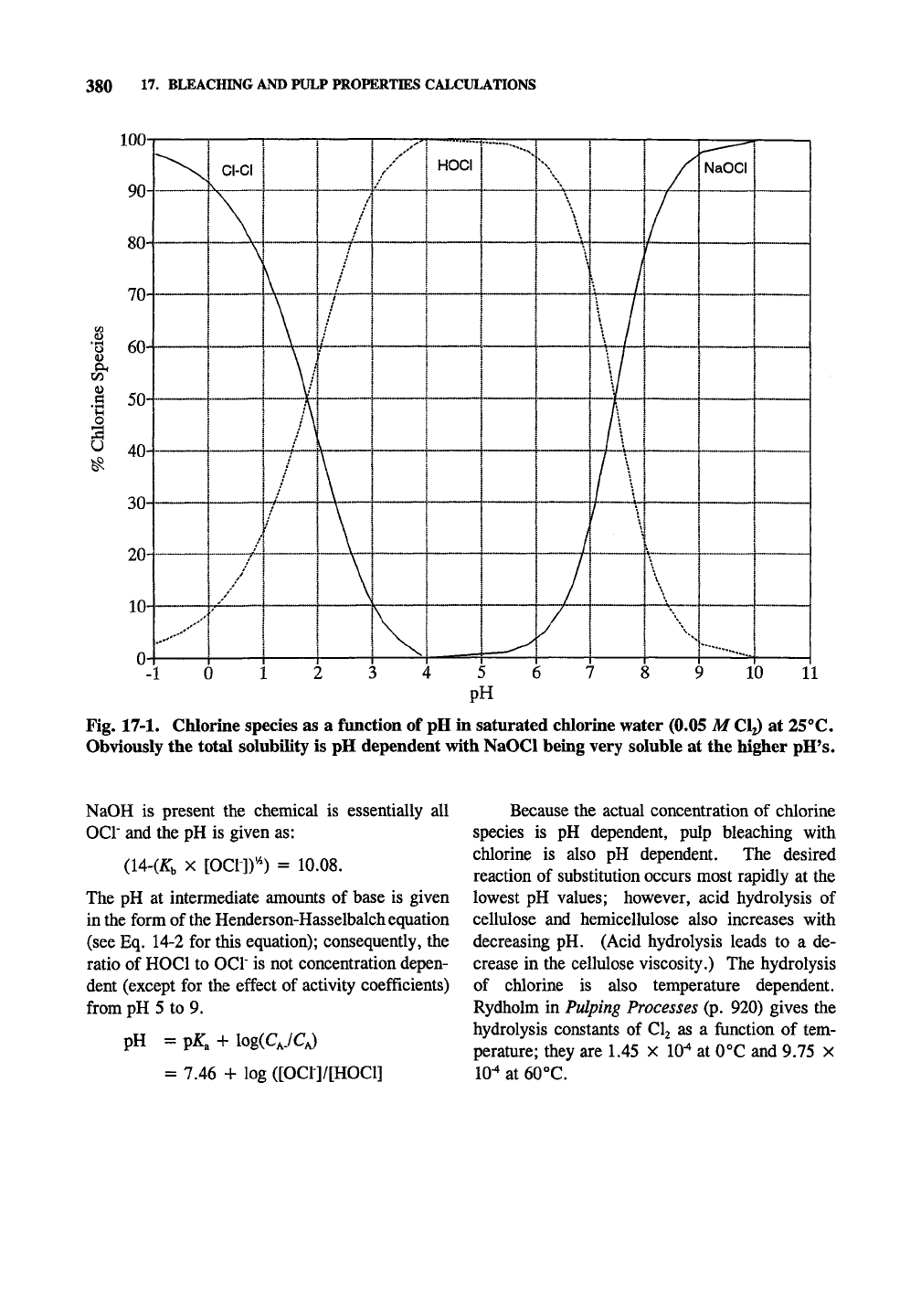
From these equilibria. Fig. 17-1 can be construct-
ed in the manner of Giertz (1951). This is very
similar to other diprotic acids plotted in Chapter
16,
except the three species are plotted together.
Determining the concentration of the actual species
at low pH is tricky because chlorine is a moderate-
ly strong acid (and so its ionization alters the pH
significantly) and three species are formed by the
ionization instead of two.
First, the concentration of chlorine in water
at 25°C is 0.091 M at saturation (i.e., one atmo-
sphere pressure of CI2). (Like the solubility of
CO2,
the solubility of
CI2
is highly pH dependent.)
The pH of saturated chlorine water is calculated as
follows:
ir,
=
[Hoci][Hi[cr]/[cy
[H1
n3
(0.091 -[HI)
Solving for [H+] gives [H"'] = 0.029 M. There-
fore,
a saturated chlorine solution in water, with-
out addition of acid or base, has a pH of -log
0.029 or 1.54, an actual chlorine concentration,
[Cy, of 0.062 M, a hydrochloric acid concentra-
tion of 0.029 M, and a hypochlorous acid concen-
tration of 0.029 Af.
The concentration of chlorine species will be
considered using 0.05 M CI2 initially, since this
will not exceed the saturated pressure of chlorine
at any pH, as a fimction of pH. Acid or base can
be hypothetically added without dilution to achieve
the pH's shown in Fig. 17-1. Initially [Cy =
0.028, [H+] = 0.022, and the pH is 1.66.
The ratio of
CI2
to HOCl as a function of pH
(where the source of the acid is immaterial) is
solved in the following manner, where x =
[HOCl] = [CI]:
K, = [HT A:2/(0.05 - x)
[H-^]
:^
•\-
K,X' 0.05 ^, = 0
This equation is easily solved at various pH's by
use of the quadratic equation (see page 322). (At
pH = 0, X = 0.00423 M. At pH 1.66, x =
0.0222 in agreement with the calculation above.)
When 0.05 M NaOH is added, all of the CI2
is converted to HOCl and the pH is of the weak
acid system
HOCl.
HOCl is easily handled as any
other weak acid. The pH is then calculated from
Eq. 13-5 as4og(^, x Q'^ = 4.38. When 0.10 M
379
380 17. BLEACHING AND PULP PROPERTIES CALCULATIONS
100
90-
80-
3 60-
(0
OH
•g
o
70-
50-
40-
30-
20-
10
""""''
Cl-C!
/
/
\/
/
/
1
>•"
«.i....,
HOCI
'**"*»
^^\
\
\
1 /
\/
/\
/ \
\
\
\^
NaOCI
5
PH
10 11
Fig. 17-1. Chlorine species as a function of pH in saturated chlorine water (0.05 M
CI2)
at 25°C.
Obviously the total solubility is pH dependent with NaOCl being very soluble at the higher pH's.
NaOH is present the chemical is essentially all
OCl' and the pH is given as:
(14-(^b X [OCl])''') = 10.08.
The pH at intermediate amounts of base is given
in the form of
the
Henderson-Hasselbalch equation
(see Eq. 14-2 for this equation); consequently, the
ratio of HOCI to OCl' is not concentration depen-
dent (except for the effect of activity coefficients)
from pH 5 to 9.
pH = p^, + log(C;,./Q)
= 7.46 + log ([OCl-]/[HOCl]
Because the actual concentration of chlorine
species is pH dependent, pulp bleaching with
chlorine is also pH dependent. The desired
reaction of substitution occurs most rapidly at the
lowest pH values; however, acid hydrolysis of
cellulose and hemicellulose also increases with
decreasing pH. (Acid hydrolysis leads to a de-
crease in the cellulose viscosity.) The hydrolysis
of chlorine is also temperature dependent.
Rydholm in Pulping Processes (p. 920) gives the
hydrolysis constants of CI2 as a function of tem-
perature; they are 1.45 x
10"^
at 0°C and 9.75 x
10-^
at 60°C.
CHLORINE DIOXIDE 381
17.3 CHLORINE DIOXroE
The action of
CIO2
has been studied in detail
by Schmidt (1923). He found CIO2 to be highly
reactive with aromatics and phenolics but not
reactive with carbohydrates. Today CIO2 is used
in the later bleaching stages since it is very selec-
tive for lignin removal. It is also being used in
early stages for environmental reasons. Chlorine
dioxide can be produced from chlorite by oxida-
tion with hypochlorite or reduction with suitable
reducing agents (Giertz, 1951). Chlorine dioxide
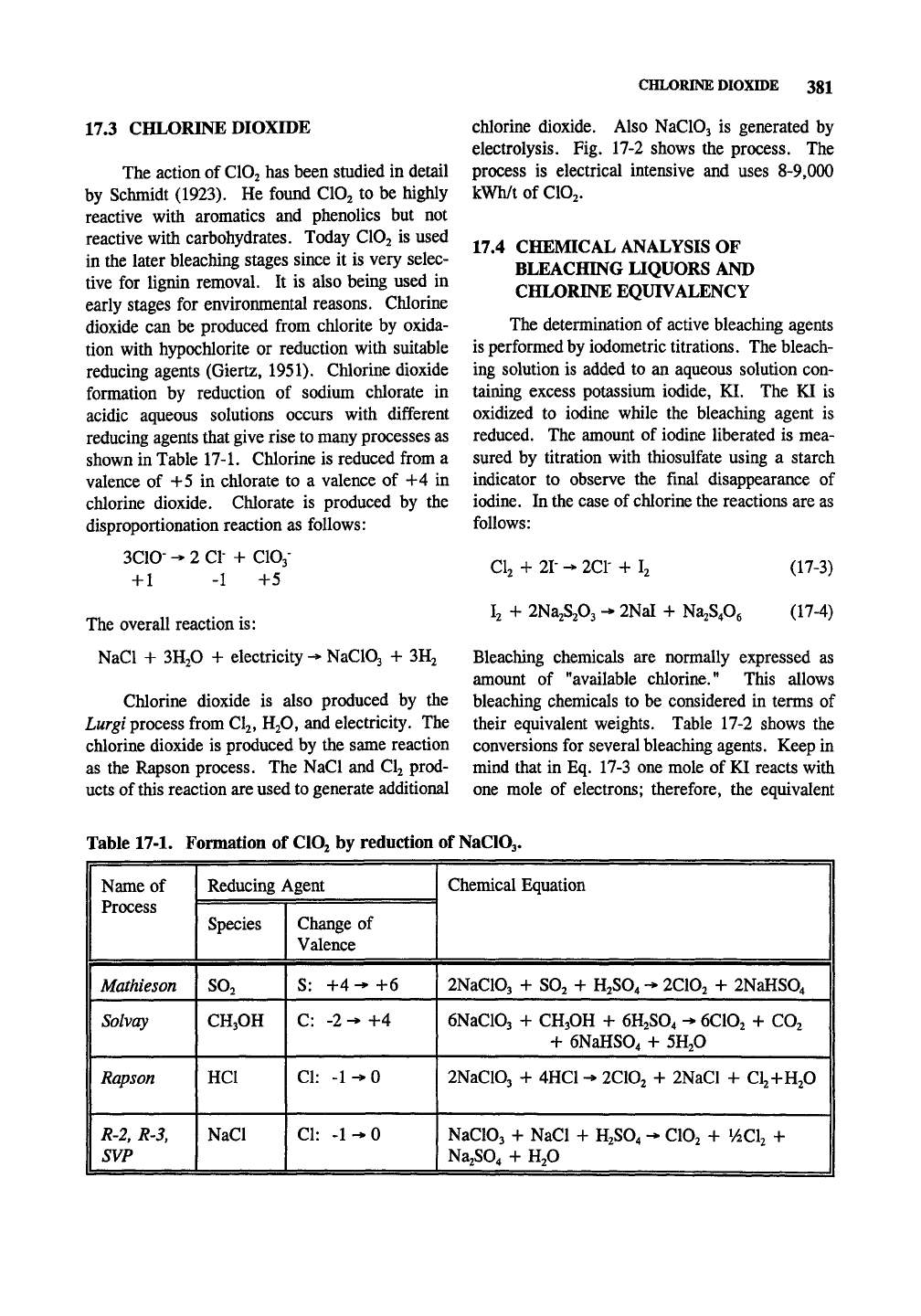
formation by reduction of sodium chlorate in
acidic aqueous solutions occurs with different
reducing agents that give rise to many processes as
shown in Table 17-1. Chlorine is reduced from a
valence of +5 in chlorate to a valence of +4 in
chlorine dioxide. Chlorate is produced by the
disproportionation reaction as follows:
3C10-
+
1
2 CI- +
CIO3-
-1 +5
The overall reaction is:
NaCl -h 3H2O + electricity-* NaClOs + 3H2
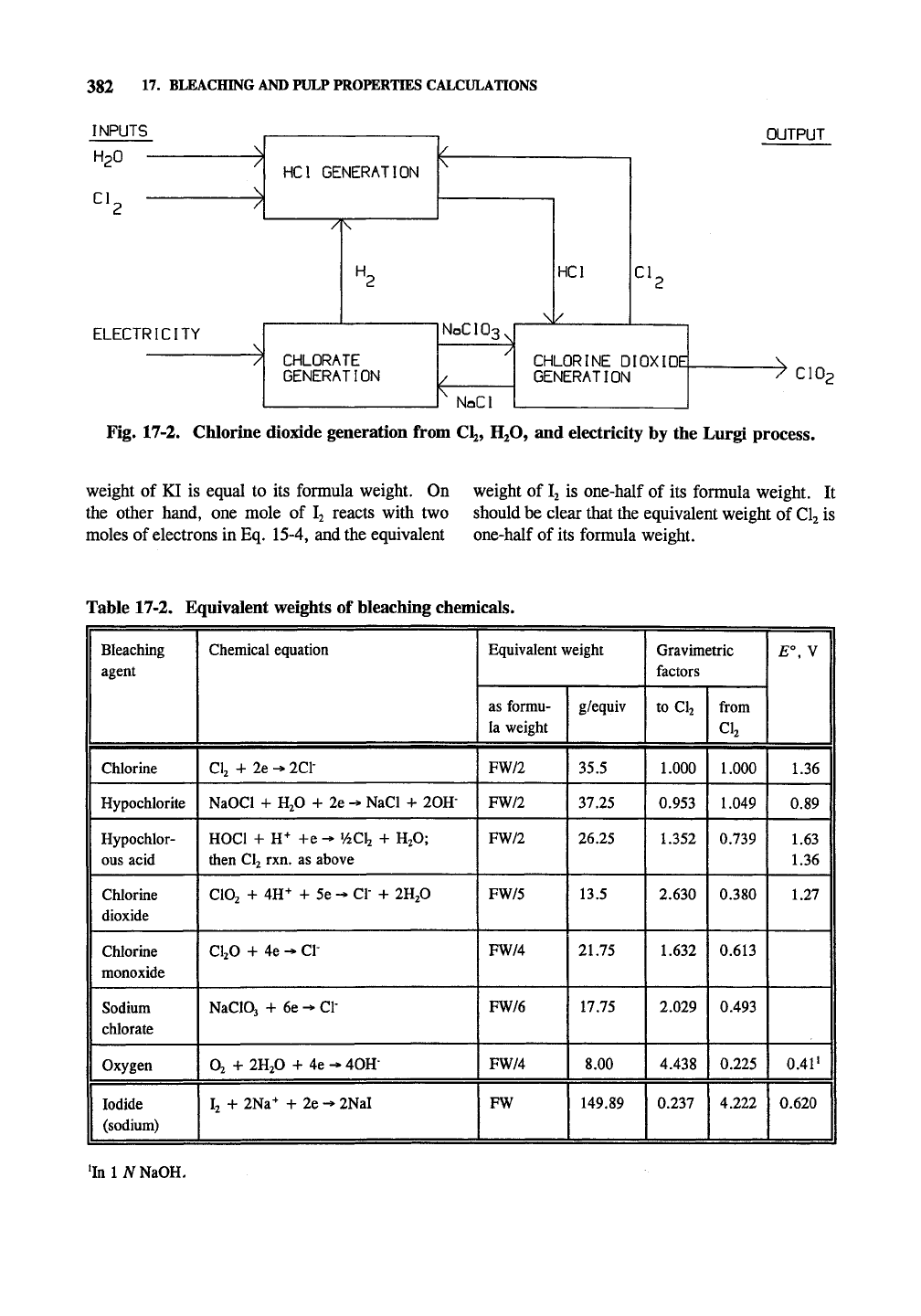
Chlorine dioxide is also produced by the
Lurgi process from
CI2,
H2O, and electricity. The
chlorine dioxide is produced by the same reaction
as the Rapson process. The NaCl and CI2 prod-
ucts of
this
reaction are used to generate additional
chlorine dioxide. Also NaClOj is generated by
electrolysis. Fig. 17-2 shows the process. The
process is electrical intensive and uses
8-9,000
kWh/tofC102.
17.4 CHEMICAL ANALYSIS OF
BLEACHING LIQUORS AND
CHLORINE EQUIVALENCY
The determination of active bleaching agents
is performed by iodometric titrations. The bleach-
ing solution is added to an aqueous solution con-
taining excess potassium iodide, KI. The KI is
oxidized to iodine while the bleaching agent is
reduced. The amount of iodine liberated is mea-
sured by titration with thiosulfate using a starch
indicator to observe the final disappearance of
iodine. In the case of chlorine the reactions are as
follows:
CI2 +
21-
-*
2C1-
+ I2 (17-3)
I2 + 2Na2S203 -> 2NaI + Na2SA (17-4)
Bleaching chemicals are normally expressed as
amount of "available chlorine." This allows
bleaching chemicals to be considered in terms of
their equivalent weights. Table 17-2 shows the
conversions for several bleaching agents. Keep in
mind that in Eq. 17-3 one mole of KI reacts with
one mole of electrons; therefore, the equivalent
Table 17-1. Formation of CIO2 by reduction of NaClOj.
Name of
Process
Mathieson
Solvay
Rapson
R-2,
R-3,
1 SVP
Reducing Agent
Species
SO2
CH3OH
HCl
NaCl
Change of
Valence
S: +4-+6
C: -2 - +4
CI:
-1-0
CI:
-1-0
Chemical Equation
2NaC103 + SO2 + H2SO4 - 2CIO2
-1-
2NaHS04 |
eNaClOj + CH3OH + 6H2SO4 - 6CIO2 + CO2
+ 6NaHS04
-1-
SHP |
2NaC103 +
4HC1
- 2C10j
-1-
2NaCl + Clj+H^ol
NaC103
-1-
NaCl + H2SO4 - CIO2 + ^ACk +
Na;S04 + H;0
382 17. BLEACHING
AND
PULP PROPERTIES CALCULATIONS
INPUTS
H2O
cu
HCl
GENERATION
"7^
^
ELECTRICITY
-^
OUTPUT
CHLORATE
GENERATION
N0CIO3
^
^
NoCl
HCl
CL
CHLORINE DI
OX
I DEI
GENERATION
->
CIO2
Fig. 17-2. Chlorine dioxide generation from CI2, H2O, and electricity by the Lurgi process.
weight of KI is equal to its formula weight. On weight of
I2
is one-half of its formula weight. It
the other hand, one mole of I2 reacts with two should be clear that the equivalent weight of
CI2
is
moles of electrons in Eq. 15-4, and the equivalent one-half of its formula weight.
Table 17-2. Equivalent weights of bleaching chemicals.
Bleaching
agent
Chlorine
Hypochlorite
Hypochlor-
ous acid
Chlorine
dioxide
Chlorine
monoxide
Sodium
chlorate
Oxygen
Iodide
(sodium)
Chemical equation
CI2 + 2e -*
2C1-
NaOCl 4- H2O + 2e -* NaCl + 20H-
HOCl + H+ +e ^
ViClj
+ H2O;
then CI2 rxn. as above
CIO2 + 4H+ + 5e -* Cr + 2H2O
CI2O + 4e -* Cl-
NaClOj + 6e -* CI"
O2 + 2H2O + 4e ^ 40H-
I2 + 2Na-' + 2e -» 2NaI
Equivalent weight
as formu-
la weight
FW/2
FW/2
FW/2
FW/5
FW/4
FW/6
FW/4
FW
g/equiv
35.5
37.25
26.25
13.5
21.75
17.75
8.00
149.89
Gravimetric
factors
to CI2
1.000
0.953
1.352
2.630
1.632
2.029
4.438
0.237
from
CI2
1.000
1.049
0.739
0.380
0.613
0.493
0.225
4.222
E\W
1.36
II
0.89
1.63
1.36
1.27
0.41^
1
0.620
•inliVNaOH.
CHEMICAL ANALYSIS OF BLEACHING LIQUORS 383
EXAMPLE 2. Write the balanced reaction of chlorine dioxide with iodide.
SOLUTION: 2CIO2 + 10 T + 8 H^ -*
201"
+ 5I2 4- 4H2O
EXAMPLE 3. Suppose a chemical loading of
2%
NaClO on pulp (as chlorine) is desired. What is the
actual amount of NaClO that should be used?
SOLUTION: 2% as CI2 x (37.25 g NaC10)/(35.5 g Cy = 2.1% NaClO as NaClO.
PROBLEM: Suppose 2% CIO2 as chlorine is desired. What should the actual concentration be?
Answer: 0.76% CIO2.
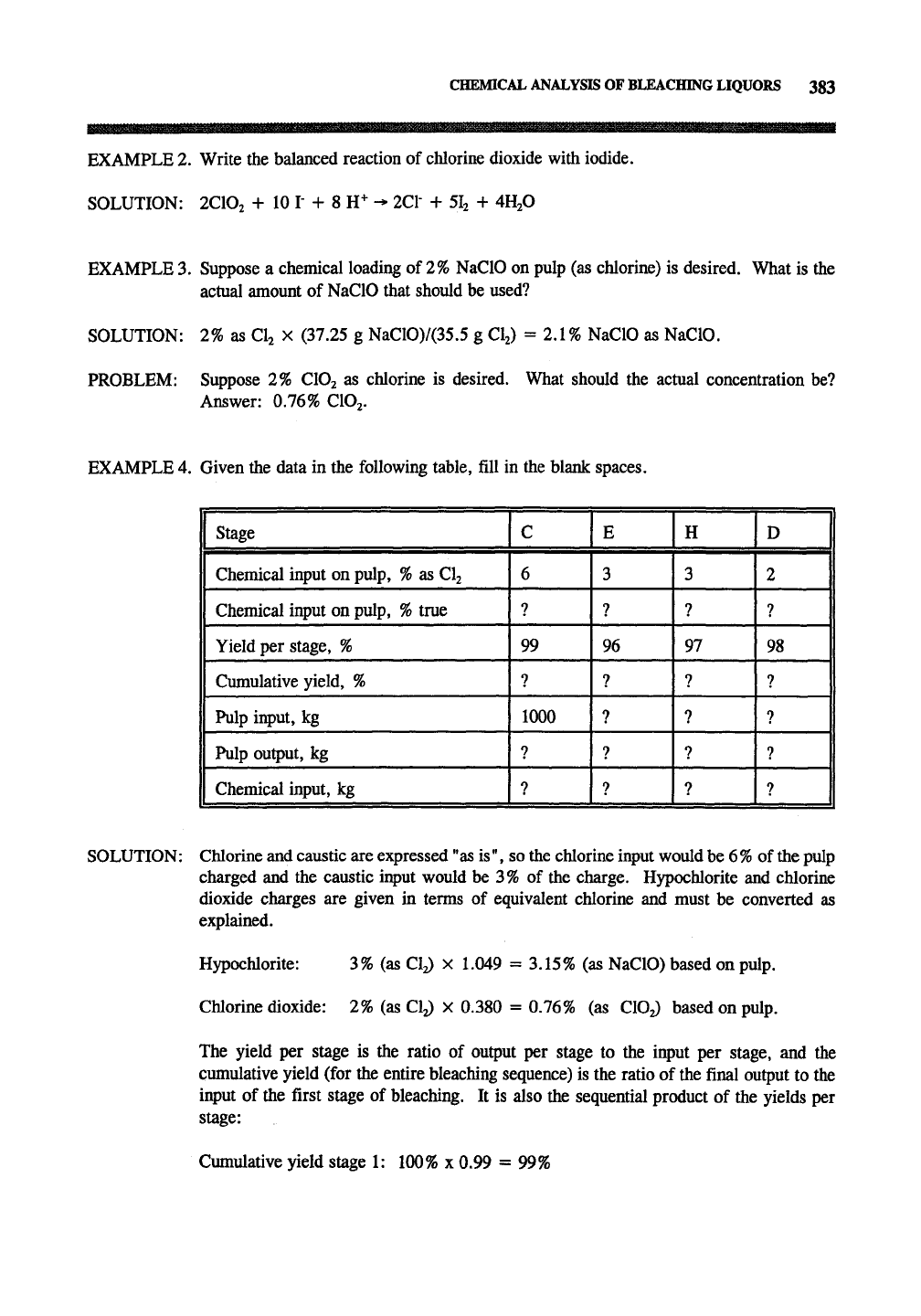
EXAMPLE 4. Given the data in the following table, fill in the blank spaces.
1 Stage
1 Chemical input on pulp,
%
as CI2
1 Chemical input on pulp,
%
true
1 Yield per stage, %
1 Cumulative yield, %
1 Pulp input, kg
1 Pulp output, kg
Chemical input, kg
C
6
?
99
?
1000
?
?
E
3
7
96
7
7
7
7
H
3
7
97
7
7
7
7
D
1
2
1
7
98
7
7
7
7
SOLUTION: Chlorine and caustic are expressed
"as
is", so the chlorine input would be
6%
of
the
pulp
charged and the caustic input would be 3% of the charge. Hypochlorite and chlorine
dioxide charges are given in terms of equivalent chlorine and must be converted as
explained.
Hypochlorite: 3% (as Cy X 1.049 = 3.15% (as NaClO) based on pulp.
Chlorine dioxide: 2% (as Cy x
0.380
= 0.76% (as CIO2) based on pulp.
The yield per stage is the ratio of output per stage to the input per stage, and the
cumulative yield (for the entire bleaching sequence) is the ratio of the final output to the
input of the first stage of bleaching. It is also the sequential product of the yields per
stage:
Cumulative yield stage 1: 100% x 0.99 = 99%
