Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.

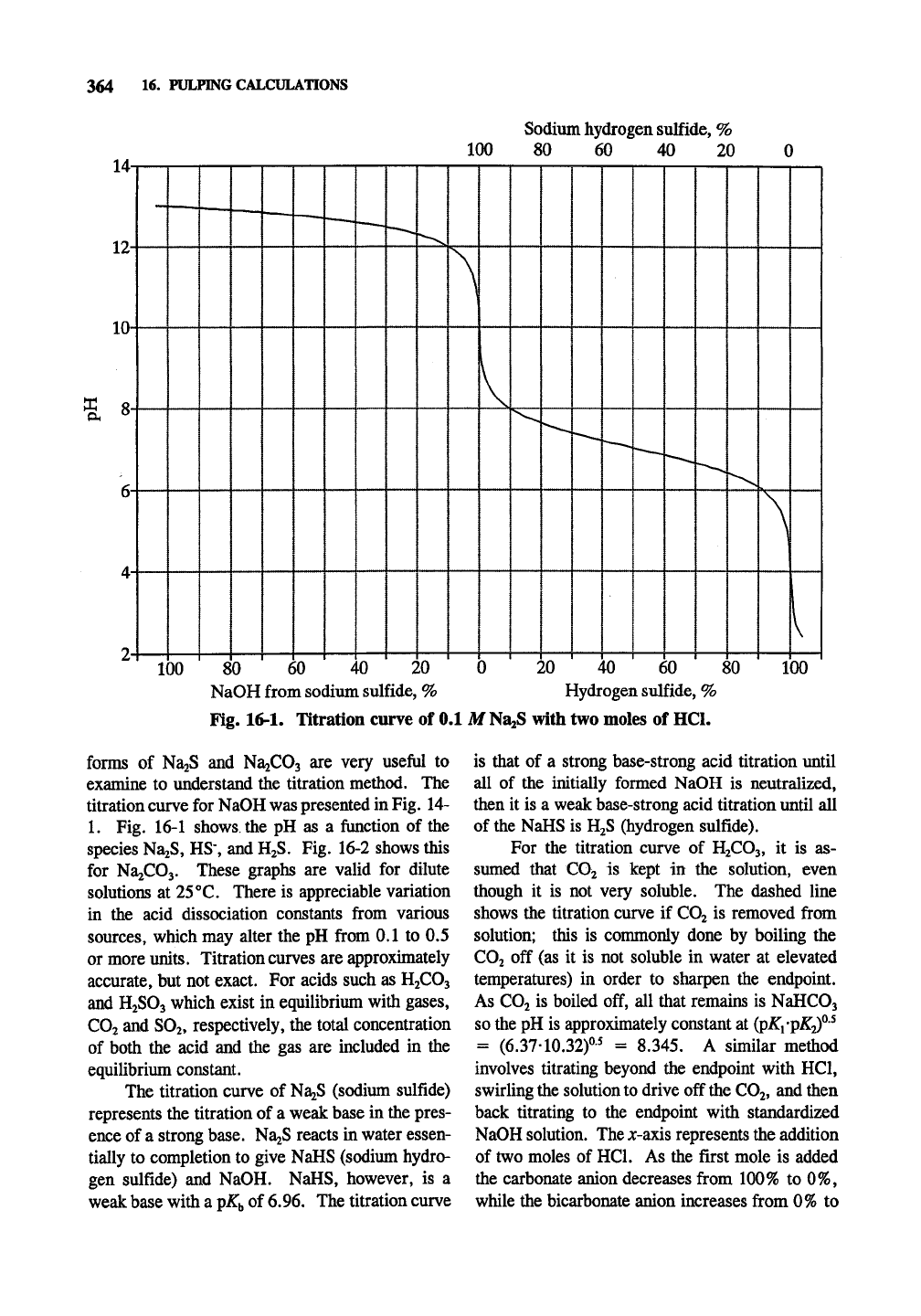
364 W- PULPING CALCULATIONS
14-
100
Sodium hydrogen sulfide, %
80 60 40 20
12-
K
DO 8 0
i
»
^
K)
to
^
0 10
i
w
60 80
'^
I
100
la
^ 8-
NaOH from sodium sulfide, % Hydrogen sulfide, %
Fig. 16-1. Titration curve of 0.1 M
NaaS
with two moles of HCl.
forms of NajS and NajCOa are very useful to
examine to understand the titration method. The
titration curve for NaOH was presented in
Fig.
14-
1.
Fig. 16-1 shows the pH as a function of the
species NaiS, HS, and
H2S.
Fig. 16-2 shows this
for NajCOj. These graphs are valid for dilute
solutions at 25
^'C.
There is appreciable variation
in the acid dissociation constants from various
sources, which may alter the pH from 0.1 to 0.5
or more units. Titration curves are approximately
accurate, but not exact. For acids such as H2CO3
and H2SO3 which exist in equilibrium with gases,
CO2 and SO2, respectively, the total concentration
of both the acid and the gas are included in the
equilibrium constant.
The titration curve of NajS (sodium sulfide)
represents the titration of a weak base in the pres-
ence of a strong base. Na2S reacts in water essen-
tially to completion to give NaHS (sodium hydro-
gen sulfide) and NaOH. NaHS, however, is a
weak base with a ^K^ of 6.96. The titration curve
is that of a strong base-strong acid titration until
all of the initially formed NaOH is neutralized,
then it is a weak base-strong acid titration until all
of the NaHS is H2S (hydrogen sulfide).
For the titration curve of H2CO3, it is as-
sumed that CO2 is kept in the solution, even
though it is not very soluble. The dashed line
shows the titration curve if CO2 is removed from
solution; this is commonly done by boiling the
CO2 off (as it is not soluble in water at elevated
temperatures) in order to sharpen the endpoint.
As CO2 is boiled off, all that remains is NaHC03
so the pH is approximately constant at (pJ^fpjQ®-^
= (6.37-10.32)«-^ = 8.345. A similar method
involves titrating beyond the endpoint with HCl,
swirling the solution to drive off
the
CO2, and then
back titrating to the endpoint with standardized
NaOH solution. The x-axis represents the addition
of two moles of HCl. As the first mole is added
the carbonate anion decreases from 100% to 0%,
while the bicarbonate anion increases from 0% to
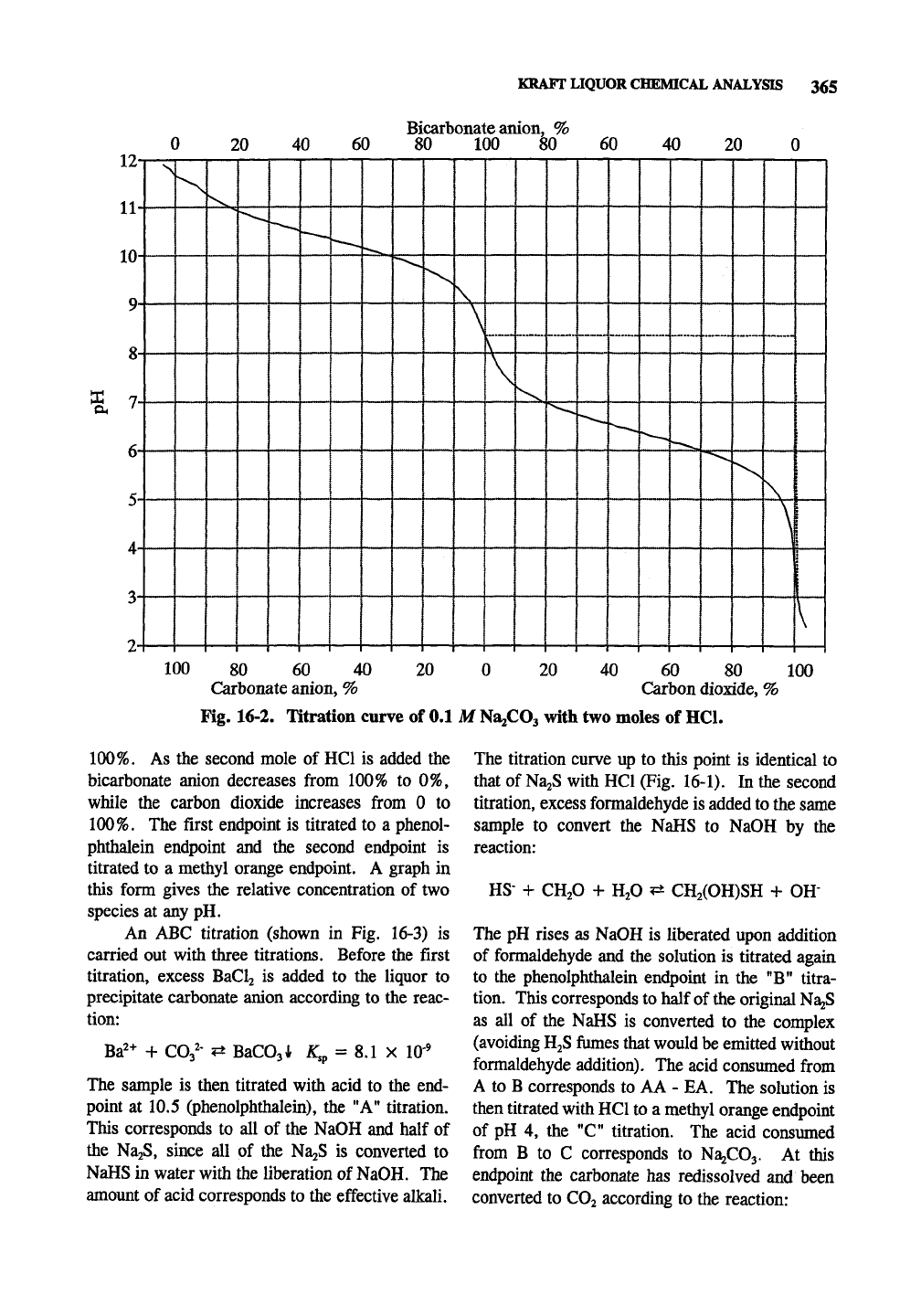
12-
11-
10-
9
Bicarbonate anion, %
20 40 60 80 100 80 60 40
KRAFT LIQUOR CHENflCAL ANALYSIS 365
20 0
\.
1
1
l\
X 1.
100 80 60 40
Carbonate anion, %
20 0 20 40
60 80 100
Carbon dioxide, %
Fig. 16-2. Titration curve of 0.1 M
1^2iflO^
with two moles of HCl.
100%.
As the second mole of HCl is added the
bicarbonate anion decreases from 100% to 0%,
while the carbon dioxide increases from 0 to
100%.
The first endpoint is titrated to a phenol-
phthalein endpoint and the second endpoint is
titrated to a methyl orange endpoint. A graph in
this form gives the relative concentration of two
species at any pH.
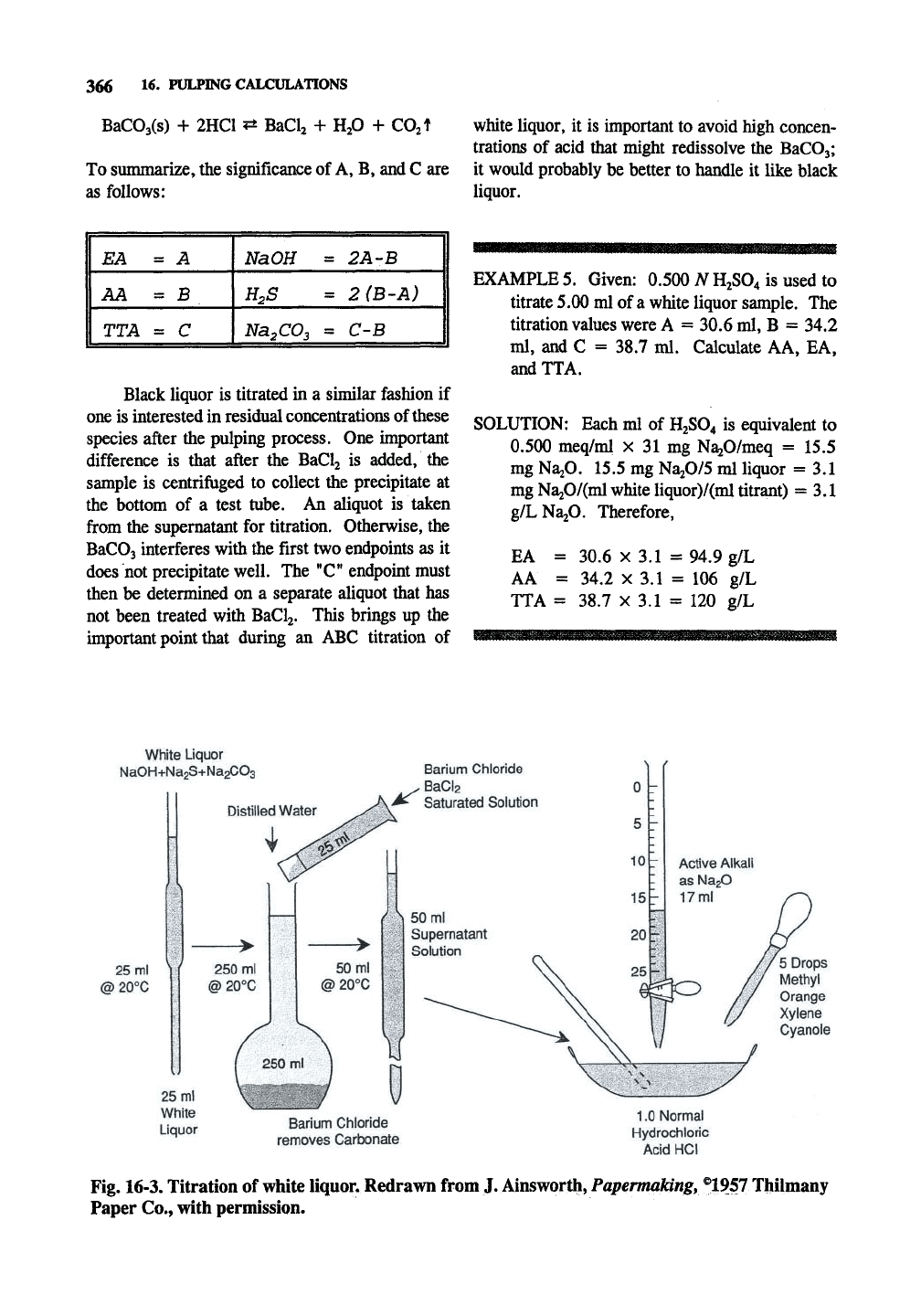
An ABC titration (shown in Fig. 16-3) is
carried out with three titrations. Before the first
titration, excess BaCl2 is added to the liquor to
precipitate carbonate anion according to the reac-
tion:
Ba^-^ + CO32- ^ BaCOsi K,^ = 8.1 x 10'^
The sample is then titrated with acid to the end-
point at 10.5 (phenolphthalein), the "A" titration.
This corresponds to all of the NaOH and half of
the NajS, since all of the NajS is converted to
NaHS in water with the liberation of
NaOH.
The
amount of acid corresponds to the effective alkali.
The titration curve up to this point is identical to
that of
Na2S
with HCl (Fig. 16-1). In the second
titration, excess formaldehyde is added to the same
sample to convert the NaHS to NaOH by the
reaction:
HS-
+ CH2O + H2O ^ CH2(0H)SH + OH-
The pH rises as NaOH is liberated upon addition
of formaldehyde and the solution is titrated again
to the phenolphthalein endpoint in the "B" titra-
tion. This corresponds to half of the original NagS
as all of the NaHS is converted to the complex
(avoiding
H2S
fumes that would be emitted without
formaldehyde addition). The acid consumed from
A to B corresponds to AA - EA. The solution is
then titrated with HCl to a methyl orange endpoint
of pH 4, the "C" titration. The acid consumed
from B to C corresponds to NajCOj. At this
endpoint the carbonate has redissolved and been
converted to CO2 according to the reaction:
366 1^. PULPING CALCULATIONS
BaCOsCs) + 2HC1 ^ BaCli + HjO + COjt
To summarize, the significance of A, B, and C are
as follows:
white liquor, it is important to avoid high concen-
trations of acid that might redissolve the BaCOj;
it would probably be better to handle it like black
liquor.
EA
AA
1 TTA
= A
= B
= C
NaOH
H2S
NazCOj
= 2A-B
= 2(B-A)
= C-B \
Black liquor is titrated in a similar fashion if
one is interested in residual concentrations of these
species after the pulping process. One important
difference is that after the BaCl2 is added, the
sample is centrifiiged to collect the precipitate at
the bottom of a test tube. An aliquot is taken
from the supernatant for titration. Otherwise, the
BaCOj interferes with the first two endpoints as it
does not precipitate well. The "C" endpoint must
then be determined on a separate aliquot that has
not been treated with
BaClj.
This brings up the
important point that during an ABC titration of
EXAMPLES. Given:
0.500
iVH2SO4 is used to
titrate 5.00 ml of
a
white liquor sample. The
titration values were A = 30.6 ml, B = 34.2
ml,
and C = 38.7 ml. Calculate AA, EA,
and TTA.
SOLUTION: Each ml of H2SO4 is equivalent to
0.500
meq/ml X 31 mg NaiO/meq = 15.5
mg NajO. 15.5 mg
N2ijO/5
ml liquor = 3.1
mg NajO/Cml white liquor)/(ml titrant) = 3.1
g/LNajO. Therefore,
EA = 30.6 X 3.1 =94.9g/L
AA = 34.2 X 3.1 = 106 g/L
TTA = 38.7 X 3.1 = 120 g/L
White Liquor
NaOH+NagS+NagCOa
Distilled Water
Barium Chloride
^ BaCl2
v^ Saturated Solution
25 ml
@20°C
25 mi
White
Liquor
Barium Chloride
removes Carbonate
Active Alkali
as NagO
17 ml
5 Drops
Methyl
Orange
Xylene
Cyanole
1.0 Normal
Hydrochloric
Acid HCI
Fig. 16-3. Titration of white liquor. Redrawn from J. Ainsworth, Papermaldng,®195T Thilmany
Paper Co., with permission.
SPECIFIC
GRAVITY AND VISCOSITY OF KRAFT LIQUORS 367
16.4 SPECIFIC GRAVITY AND VIS-
COSITY OF KRAFT LIQUORS
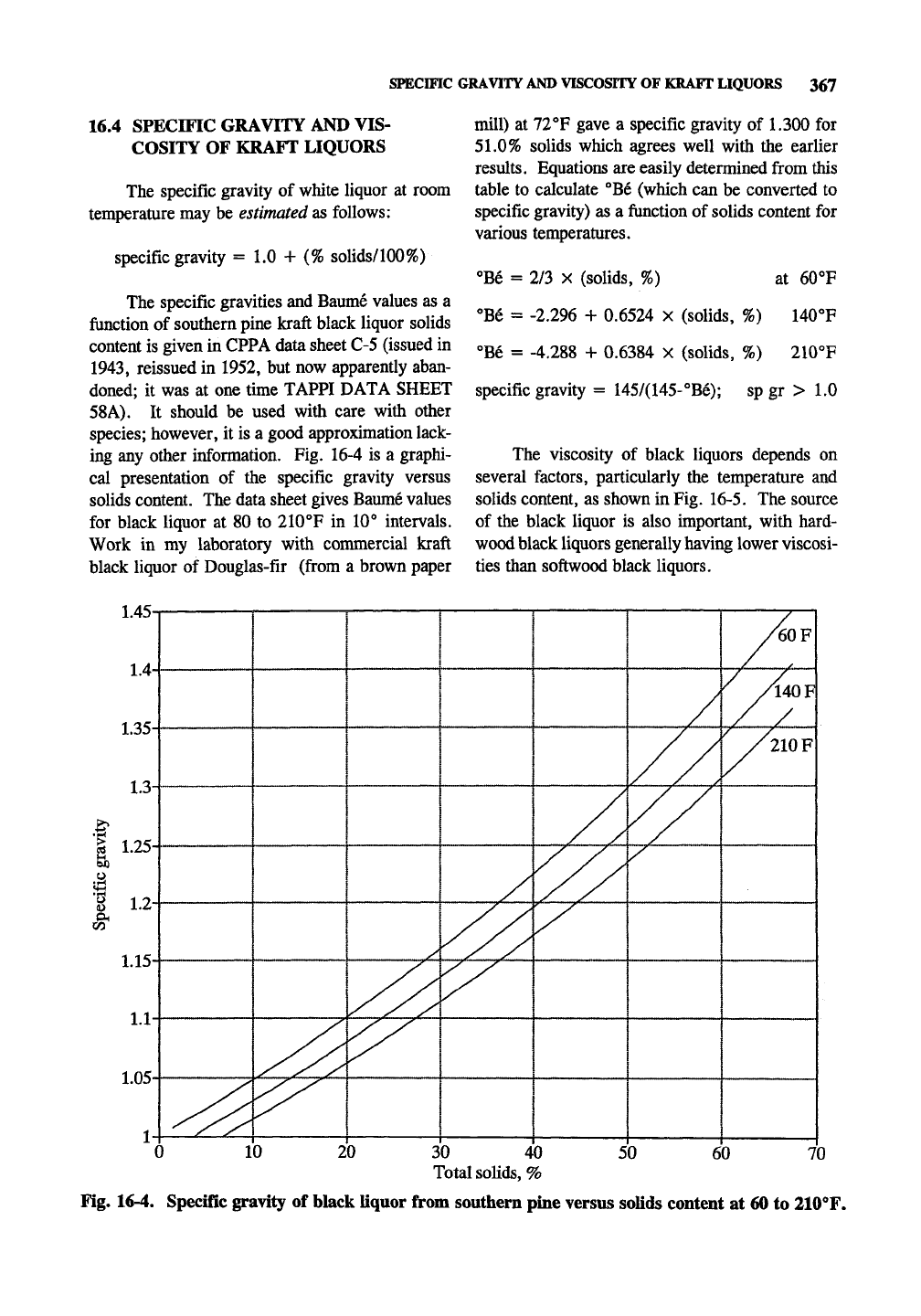
The specific gravity of white liquor at room
temperature may be estimated as follows:
specific gravity = 1.0 + (% solids/100%)
The specific gravities and Baume values as a
function of southern pine kraft black liquor solids
content is given in CPPA data sheet C-5 (issued in
1943,
reissued in 1952, but now apparently aban-
doned; it was at one time TAPPI DATA SHEET
58A).
It should be used with care with other
species; however, it is a good approximation lack-
ing any other information. Fig. 16-4 is a graphi-
cal presentation of the specific gravity versus
solids content. The data sheet gives Baume values
for black liquor at 80 to 210°F in 10° intervals.
Work in my laboratory with commercial kraft
black liquor of Douglas-fir (from a brown paper
mill) at 72°F gave a specific gravity of 1.300 for
51.0%
solids which agrees well with the earlier
results. Equations are easily determined from this
table to calculate °Be (which can be converted to
specific gravity) as a function of solids content for
various temperatures.
°Be = 2/3 X (solids, %) at 60°F
°Be = -2.296 + 0.6524 X (solids, %) 140°F
°Be = -4.288 + 0.6384 X (solids, %) 210°F
specific gravity = 145/(145-°Be); sp gr > 1.0
The viscosity of black liquors depends on
several factors, particularly the temperature and
solids content, as shown in Fig. 16-5. The source
of the black liquor is also important, with hard-
wood black liquors generally having lower viscosi-
ties than softwood black liquors.
1
A
1.4-
1
^C
1
1
1.3-
t
cj
I.ZJ-
p
O
1 O.
o
1«^
1 1
«J-
I.ID-
1
1-
1.1
1
n^-
1.U3
1-
^
z:^
^
y
XXy
yy
/
^
\y
/
/
//y
X
/
/
/
/60F
7
/
/140FI
/
^210
F
0
10
20
50 60
70
30 40
Total solids, %
Fig. 16-4. Specific gravity of black liquor from southern pine versus solids content at 60 to llOT.
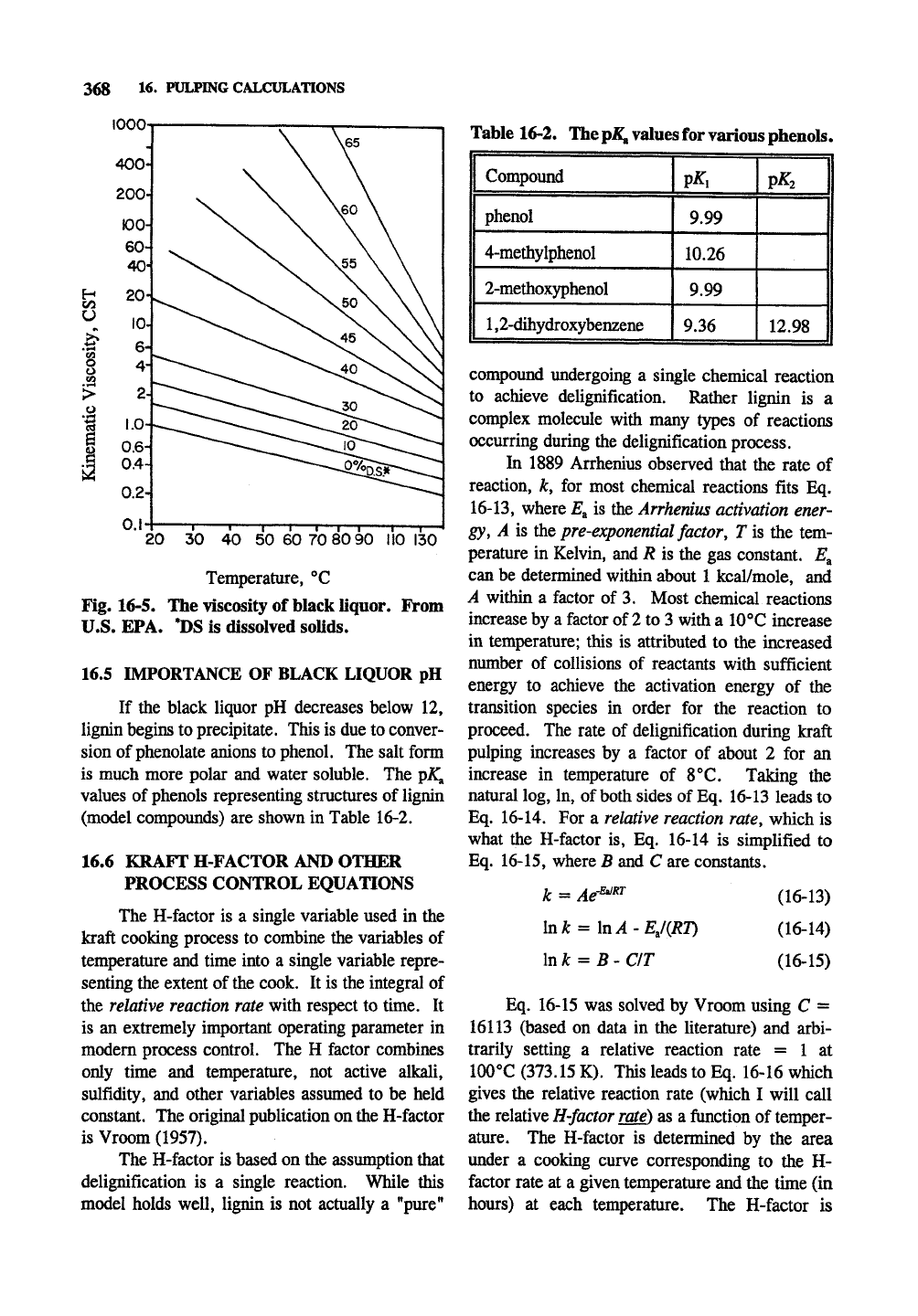
368 1^- PULPING CALCULATIONS
1000
H
C/3
u
.^
CO
O
o
Vi
>
P
1
%
•^
400-
200-
100-
60-
40-
20-
10-
6-
4-
2-
1.0-
0.6-
04-
0.2
0.1
Table 16-2.
The
p^,
values
for various phenols.
40 50 60 70 80 90 110 130
Temperature, °C
Fig. 16-5. The viscosity of black liquor. From
U.S. EPA. *DS is dissolved solids.
16.5 IMPORTANCE OF BLACK LIQUOR pH
If the black liquor pH decreases below 12,
lignin begins to precipitate. This is due to conver-
sion of phenolate anions to phenol. The salt form
is much more polar and water soluble. The pJ^^
values of phenols representing structures of lignin
(model compoimds) are shown in Table 16-2.
16.6 KRAFT H-FACTOR AND OTHER
PROCESS CONTROL EQUATIONS
The H-factor is a single variable used in the
kraft cooking process to combine the variables of
temperature and time into a single variable repre-
senting the extent of the cook. It is the integral of
the relative reaction rate with respect to time. It
is an extremely important operating parameter in
modem process control. The H factor combines
only time and temperature, not active alkali,
sulfidity, and other variables assumed to be held
constant. The original publication on the H-factor
is Vroom (1957).
The H-factor is based on the assumption that
delignification is a single reaction. While this
model holds well, lignin is not actually a "pure"
Compound
phenol
4-methylphenol
2-methoxyphenol
1,2-dihydroxybenzene
piST,
9.99
10.26
9.99
9.36
P^
1
12.98
1
compound undergoing a single chemical reaction
to achieve delignification. Rather lignin is a
complex molecule with many types of reactions
occurring during the delignification process.
In 1889 Arrhenius observed that the rate of
reaction, k, for most chemical reactions fits Eq.
16-13,
where
E^
is the Arrhenius activation ener-
gy, A is the
pre-exponential
factor, T is the tem-
perature in Kelvin, and R is the gas constant. E^
can be determined within about 1 kcal/mole, and
A within a factor of 3. Most chemical reactions
increase by a factor of 2 to 3 with a 10"^C increase
in temperature; this is attributed to the increased
number of collisions of reactants with sufficient
energy to achieve the activation energy of the
transition species in order for the reaction to
proceed. The rate of delignification during kraft
pulping increases by a factor of about 2 for an
increase in temperature of 8°C. Taking the
natural log. In, of both sides of Eq. 16-13 leads to
Eq. 16-14. For a relative reaction rate, which is
what the H-factor is, Eq. 16-14 is simplified to
Eq. 16-15, where B and C are constants.
k =
Ae-^^"""^
lni;:
= InA'EJiRT)
Ink = B-C/T
(16-13)
(16-14)
(16-15)
Eq. 16-15 was solved by Vroom using C =
16113 (based on data in the literature) and arbi-
trarily setting a relative reaction rate = 1 at
100°C (373.15 K). This leads to Eq. 16-16 which
gives the relative reaction rate (which I will call
the relative H-factor
rate)
as a function of temper-
ature. The H-factor is determined by the area
under a cooking curve corresponding to the H-
factor rate at a given temperature and the time (in
hours) at each temperature. The H-factor is
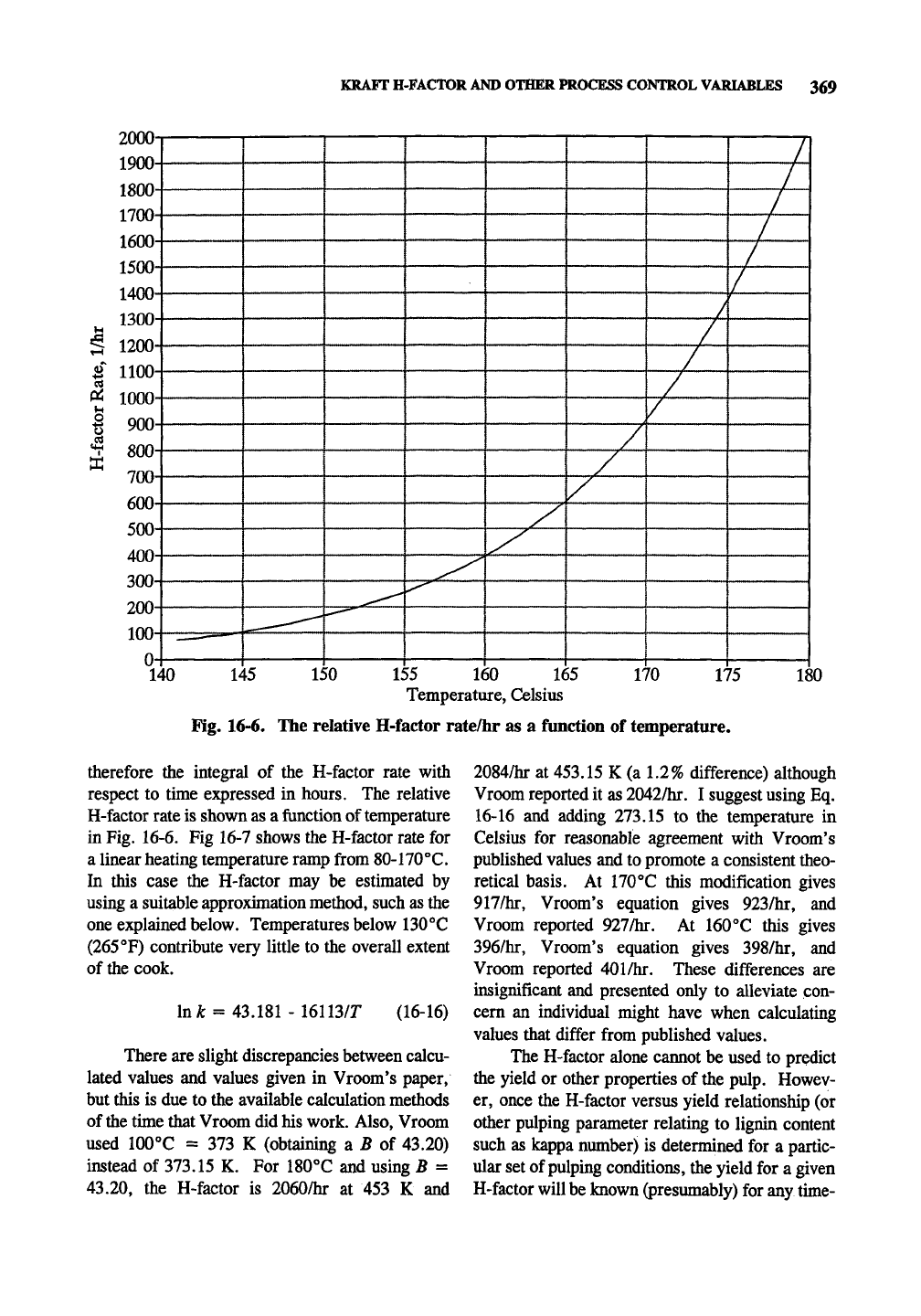
KRAFT H-FACTOR AND OTHER PROCESS CONTROL VARIABLES 369
2000
1900
1800
1700
1600
1500
1400
1300
^ 1200
I" 1100
^ 1000
J
900
800
700
600
500
400
300-
200
100
y
/
/
/
/
/
/
/
/
140
145 150 170
175
155 160 165
Temperature, Celsius
Fig. 16-6. The relative H-factor rate/hr as a function of temperature.
180
therefore the integral of the H-factor rate with
respect to time expressed in hours. The relative
H-factor rate is shown as a function of temperature
in Fig. 16-6. Fig 16-7 shows the H-factor rate for
a linear heating temperature ramp from 80-170°C.
In this case the H-factor may be estimated by
using a suitable approximation method, such as the
one explained below. Temperatures below 130**C
(265 °F) contribute very little to the overall extent
of the cook.
lnA: = 43.181-16113/r (16-16)
There are slight discrepancies between calcu-
lated values and values given in Vroom*s paper,
but this is due to the available calculation methods
of
the
time that Vroom did his work. Also, Vroom
used 100**C = 373 K (obtaining a S of 43.20)
instead of 373.15 K. For 180°C and using B =
43.20,
the H-factor is 2060/hr at 453 K and
2084/hr at 453.15 K (a 1.2% difference) although
Vroom reported it as 2042/hr. I suggest using Eq.
16-16 and adding 273.15 to the temperature in
Celsius for reasonable agreement with Vroom's
published values and to promote a consistent theo-
retical basis. At 170°C this modification gives
917/hr, Vroom's equation gives 923/hr, and
Vroom reported 927/hr. At 160°C this gives
396/hr, Vroom's equation gives 398/hr, and
Vroom reported 401/hr. These differences are
insignificant and presented only to alleviate con-
cern an individual might have when calculating
values that differ from published values.
The H-factor alone cannot be used to predict
the yield or other properties of the pulp. Howev-
er, once the H-factor versus yield relationship (or
other pulping parameter relating to lignin content
such as kappa number) is determined for a partic-
ular set of pulping conditions, the yield for a given
H-factor will be known (presumably) for any time-
370 1^. PULPING CALCULATIONS
temperature combination used to achieve that H-
factor.
EXAMPLE 6. Sawdust is pulped in a continuous
digester at a constant temperature (zero heat-
up time) of 180°C for 45 minutes to a kappa
number of 30. If the digester temperature is
increased by 5°C and the other pulping
conditions are held constant, solve the fol-
lowing:
a) What pulping time is required to reach the
same kappa number?
b) What is the percent increase in digester
output?
Note: the H-factor rate at 180°C = 2042/hr
and at 185°C = 3054/hr
SOLUTION, a) The H-factor of the cook at
180°C is 2042/hr x 0.75 hr = 1532. To
cook to the same H-factor at 185°C, solve
for time as: t = 1532/3054
hr"^
= 0.50 hr =
30 min. Cooking at the higher temperature
gives 3 batches/90 min or a 50% increase in
production.
^^^^M^lM^«l^e^i^^M^'lillli1ll1fl]l!lllililili1lll1l!ill1ll?
Although
Eq.
16-16 enjoys widespread
use
in
industry, there is no reason to assume that the
activation energy of the Arrhenius equation is the
same for hardwoods and softwoods or even among
species of either group. Furthermore, the compo-
sition of cooking chemical may influence the
activation energy somewhat. Taking the derivative
of the Arrhenius equation and solving for the
activation energy gives Eq. 16-17.
E^
=
RT^
d
Ink/dT
«
RT,T2(AT)-'
HkrSri)
(16-17)
EXAMPLE 7. Solve for
E^
based on the H-
factor relative reaction rates at 165^*0 and
175°C.
SOLUTION. (0.001987 kcal mol'^ K"^) x (438
K)(448 K)(10 K)-^ X ln(1387/610) = 32.0
kcal. Also, comparison of Eq. 16-14 to Eq.
16-16 shows that 16113 = EJR; therefore,
E^
= 32.0 kcal/mole and is independent of
temperature.
Often linear temperature ramps are used or
assumed during digester heating. It would be
ideal to integrate Eq. 16-16 with respect to time
using a linear temperature ramp to solve for the
H-factor during the heat-up time. By integrating
from absolute zero the form of the equation could
be written as follows, where T, is the rate of
temperature increase.
H-factor =
J
mdt =
-^^''''
J ^-^^iiwr') f
r^
dt
Leta = -16113/rr and^: = 1/r so,
dx =
-r^dt;
the integral is now in this form:
H-factor = C
J
ef^lx"
dx
Unfortunately this integral leads to a series
that requires several hundred terms before diverg-
ing, due to the large value of a, involving num-
bers on the order of 100! (factorial), making it
more difficult to solve than "manual" methods.
Below is a short program listed in BASIC which
can be used to solve the H-factor for linear tem-
perature ramps (or any ramp with suitable modifi-
cation). The program is set up for a temperature
ramp of 80-170°C during 90 minutes. Rimning
the program as is (with 6 time intervals of 15
minutes gives an H-factor of approximately 165.4
for the time period. Fig. 16-7 is a graphic depic-
tion of what the program accomplishes. The
program works by dividing the temperature inter-
val defined by the final temperature, TF, and
initial temperature, TI, into rectangles of time.
The reaction rate is determined at the temperature
corresponding to the center of the time interval
and applied to the entire time interval to give an
H-factor for that rectangle, TOTAL. The more
rectangles used, the closer the approximation.
Using 10 time intervals gives an H-factor of
171.6, while using 900 time intervals gives 175.2;
obviously only a few intervals are needed for
sufficient accuracy.
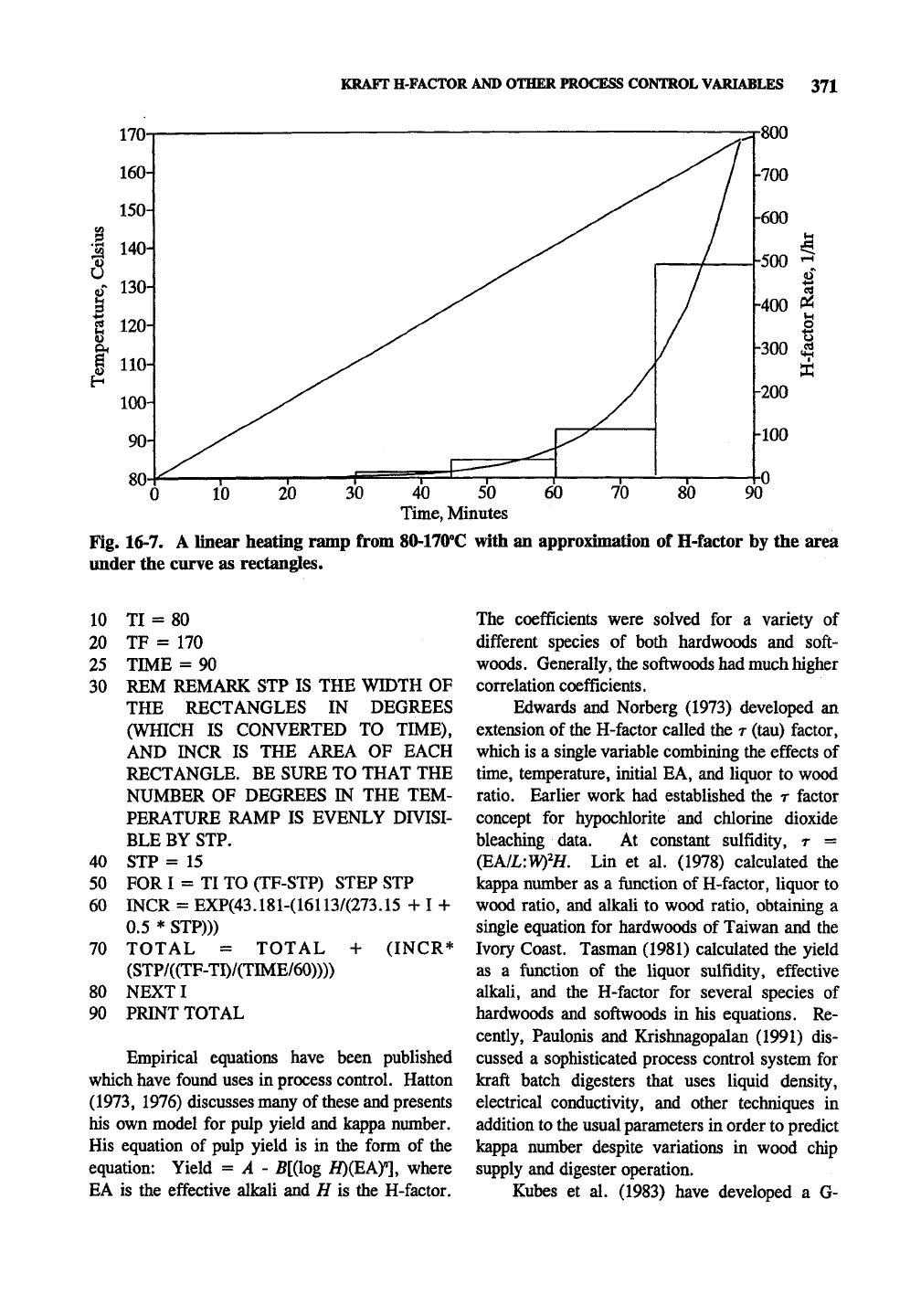
KRAFT H-FACTOR AND OTHER PROCESS CONTROL VARIABLES 371
800
0
Fig. 16-7.
under the
10
"T
20
30
60
70
80 90
40 50
Time, Minutes
A linear heating ramp from 80-170''C with an approximation of H-factor by the area
curve as rectangles.
10 TI = 80
20 TF = 170
25 TIME = 90
30 REM REMARK STP IS THE WIDTH OF
THE RECTANGLES IN DEGREES
(WHICH IS CONVERTED TO TIME),
AND INCR IS THE AREA OF EACH
RECTANGLE. BE SURE TO THAT THE
NUMBER OF DEGREES IN THE TEM-
PERATURE RAMP IS EVENLY DIVISI-
BLE BY STP.
40 STP = 15
50 FOR I = TI TO (TF-STP) STEP STP
60 INCR = EXP(43.181-(16113/(273.15 + I +
0.5 * STP)))
70 TOTAL = TOTAL + (INCR*
(STP/((TF-TI)/(TIME/60))))
80 NEXT I
90 PRINT TOTAL
Empirical equations have been published
which have found uses in process control. Hatton
(1973,
1976) discusses many of these and presents
his own model for pulp yield and kappa number.
His equation of pulp yield is in the form of the
equation: Yield = A - B[(log J9)(EA)"], where
EA is the effective alkali and H is the H-factor.
The coefficients were solved for a variety of
different species of both hardwoods and soft-
woods. Generally, the softwoods had much higher
correlation coefficients.
Edwards and Norberg (1973) developed an
extension of the H-factor called the r (tau) factor,
which is a single variable combining the effects of
time,
temperature, initial EA, and liquor to wood
ratio.
Earlier work had established the T factor
concept for hypochlorite and chlorine dioxide
bleaching data. At constant sulfidity, r =
(EA/L:W)^H. Lin et al. (1978) calculated the
kappa number as a function of H-factor, liquor to
wood ratio, and alkali to wood ratio, obtaining a
single equation for hardwoods of Taiwan and the
Ivory Coast. Tasman (1981) calculated the yield
as a function of the liquor sulfidity, effective
alkali, and the H-factor for several species of
hardwoods and softwoods in his equations. Re-
cently, Paulonis and Krishnagopalan (1991) dis-
cussed a sophisticated process control system for
kraft batch digesters that uses liquid density,
electrical conductivity, and other techniques in
addition to the usual parameters in order to predict
kappa number despite variations in wood chip
supply and digester operation.
Kubes et al. (1983) have developed a G-
372 16. PULPING CALCULATIONS
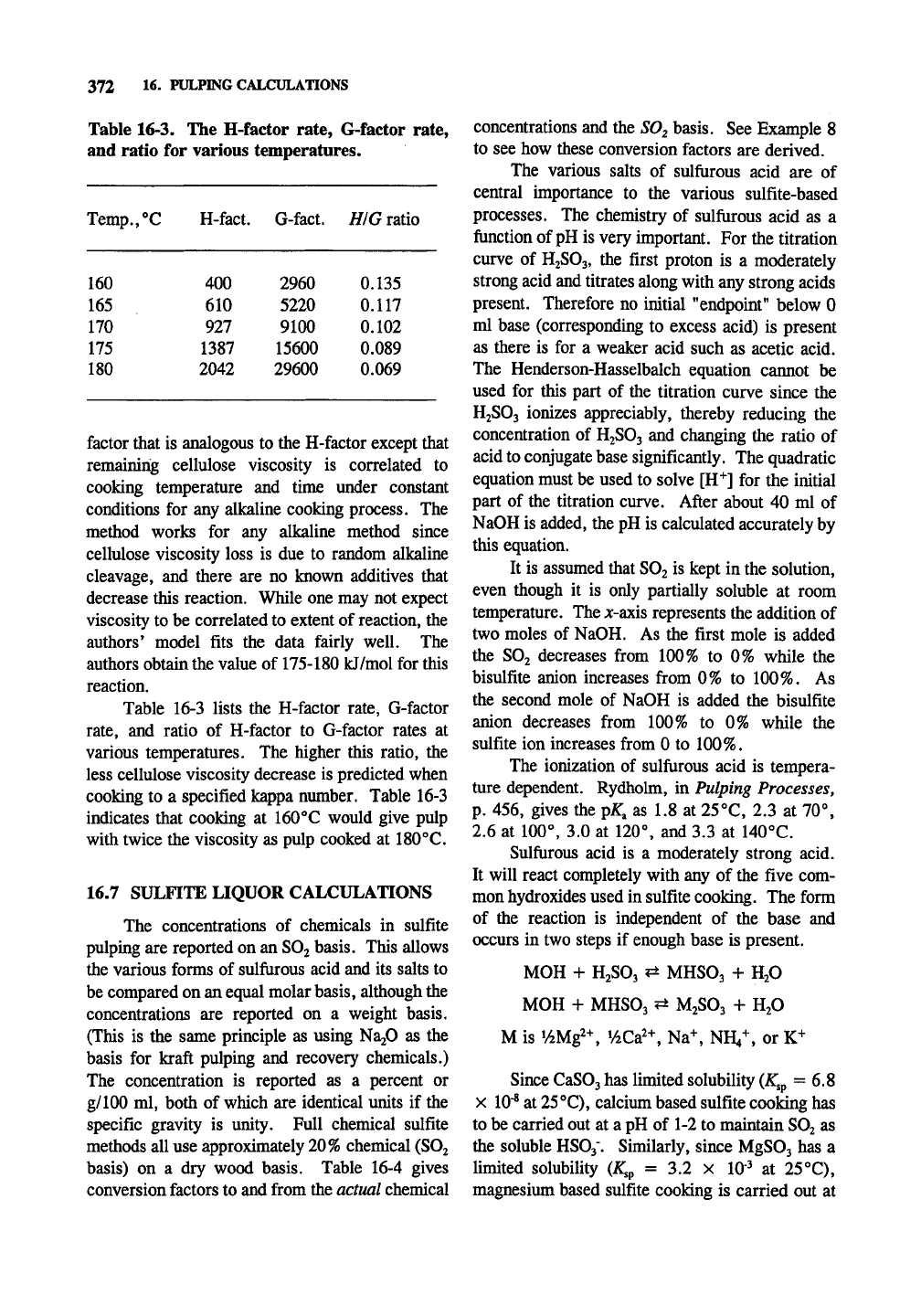
Table 16-3. The H-factor rate, G-factor rate,
and ratio for various temperatures.
Temp.,"’C
160
165
170
175
180
H-fact.
400
610
927
1387
2042
G-fact.
2960
5220
9100
15600
29600
HIG ratio
0.135
0.117
0.102
0.089
0.069
factor that is analogous to the H-factor except that
remaining cellulose viscosity is correlated to
cooking temperature and time under constant
conditions for any alkaline cooking process. The
method works for any alkaline method since
cellulose viscosity loss is due to random alkaline
cleavage, and there are no known additives that
decrease this reaction. While one may not expect
viscosity to be correlated to extent of reaction, the
authors' model fits the data fairly well. The
authors obtain the value of 175-180 kJ/mol for this
reaction.
Table 16-3 lists the H-factor rate, G-factor
rate,
and ratio of H-factor to G-factor rates at
various temperatures. The higher this ratio, the
less cellulose viscosity decrease is predicted when
cooking to a specified kappa number. Table 16-3
indicates that cooking at 160°C would give pulp
with twice the viscosity as pulp cooked at 180°C.
16.7 SULFITE LIQUOR CALCULATIONS
The concentrations of chemicals in sulfite
pulping are reported on an
SO2
basis. This allows
the various forms of sulfurous acid and its salts to
be compared on an equal molar basis, although the
concentrations are reported on a weight basis.
(This is the same principle as using NajO as the
basis for kraft pulping and recovery chemicals.)
The concentration is reported as a percent or
g/100 ml, both of which are identical units if the
specific gravity is unity. Full chemical sulfite
methods all use approximately
20%
chemical (SO2
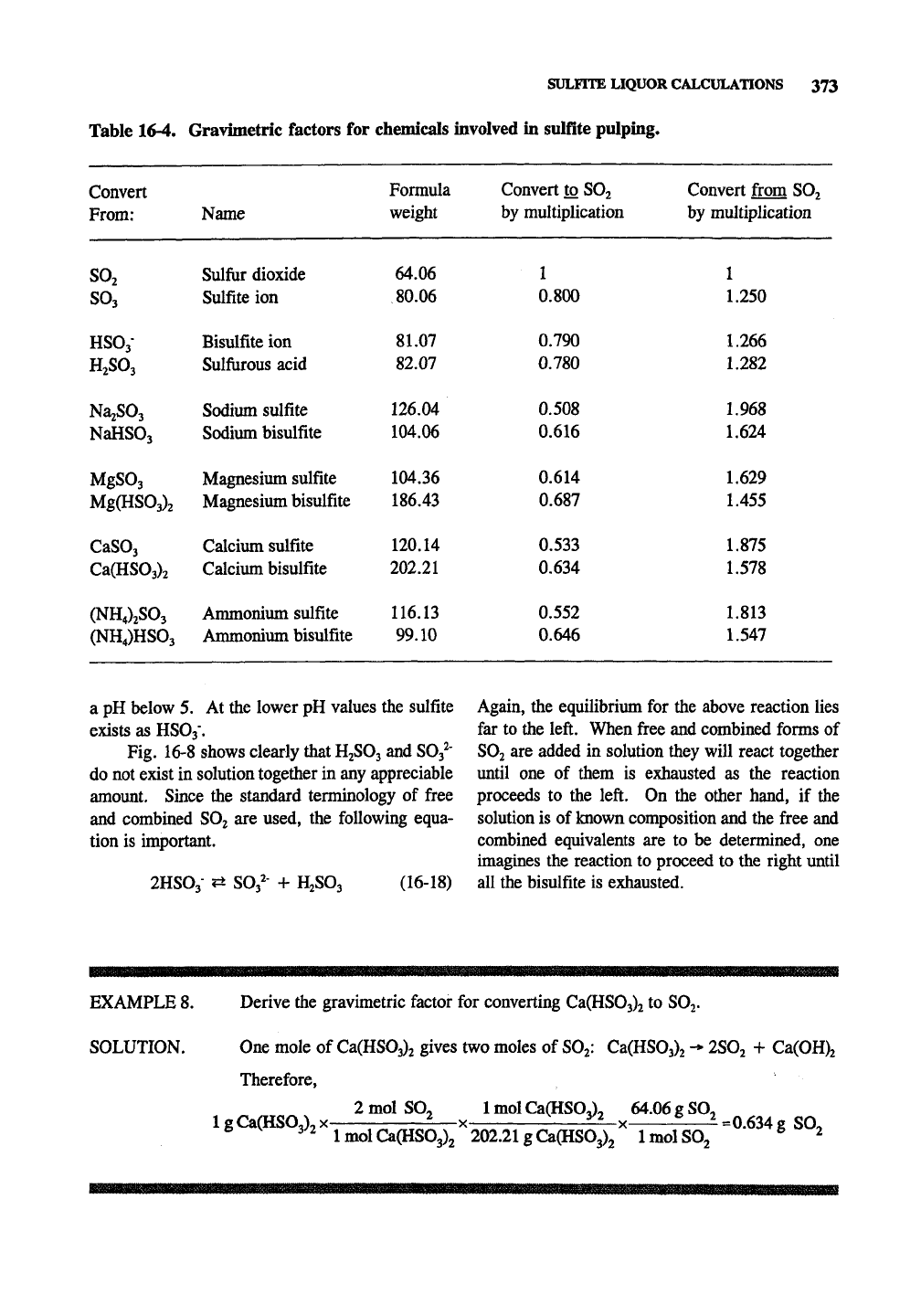
basis) on a dry wood basis. Table 16-4 gives
conversion factors to and from the actual chemical
concentrations and the
SO2
basis. See Example 8
to see how these conversion factors are derived.
The various sahs of sulfurous acid are of
central importance to the various sulfite-based
processes. The chemistry of sulfurous acid as a
function of
pH
is very important. For the titration
curve of
H2SO3,
the first proton is a moderately
strong acid and titrates along with any strong acids
present. Therefore no initial "endpoint" below 0
ml base (corresponding to excess acid) is present
as there is for a weaker acid such as acetic acid.
The Henderson-Hasselbalch equation cannot be
used for this part of the titration curve since the
H2SO3 ionizes appreciably, thereby reducing the
concentration of
H2SO3
and changing the ratio of
acid to conjugate base significantly. The quadratic
equation must be used to solve [H"^] for the initial
part of the titration curve. After about 40 ml of
NaOH is added, the pH is calculated accurately by
this equation.
It is assumed that
SO2
is kept in the solution,
even though it is only partially soluble at room
temperature. The
A:-axis
represents the addition of
two moles of NaOH. As the first mole is added
the SO2 decreases from 100% to 0% while the
bisulfite anion increases from 0% to 100%. As
the second mole of NaOH is added the bisulfite
anion decreases from 100% to 0% while the
sulfite ion increases from 0 to 100%.
The ionization of sulfurous acid is tempera-
ture dependent. Rydholm, in Pulping Processes,
p.
456, gives the ^K^ as 1.8 at 25^*0, 2.3 at 70°,
2.6 at 100°, 3.0 at 120°, and 3.3 at 140°C.
Sulfurous acid is a moderately strong acid.
It will react completely with any of the five com-
mon hydroxides used in sulfite cooking. The form
of the reaction is independent of the base and
occurs in two steps if enough base is present.
MOH + H2SO3
T±
MHSO3 + H2O
MOH + MHSO3 ^ M2SO3 4- H2O
M is ViMg^^,
ViCd^^,
Na-', NH4+, or K"'
Since CaS03 has limited solubility (^^p = 6.8
X
10*
at 25°C), calcium based sulfite cooking has
to be carried out at a pH of 1-2 to maintain
SO2
as
the soluble
HS03'.
Similarly, since MgS03 has a
limited solubility {K,^ = 3.2 x 10"^ at 25°C),
magnesium based sulfite cooking is carried out at
SULFITE LIQUOR CALCULATIONS 373
Table 16-4. Graviiiietric factors for chemicals involved in sulfite pulping.
Convert
From:
SO2
SO3
HSOj-
H2SO3
Na^SOj
NaHSOj
MgSOj
Mg(HS03)2
CaSOj
Ca(HS03)2
(NH4)jS03
(NH4)HS03
Name
Sulfur dioxide
Sulfite ion
Bisulfite ion
Sulfiirous acid
Sodium sulfite
Sodium bisulfite
Magnesium sulfite
Magnesium bisulfite
Calcium sulfite
Calcium bisulfite
Ammonium sulfite
Ammonium bisulfite
Formula
weight
64.06
80.06
81.07
82.07
126.04
104.06
104.36
186.43
120.14
202.21
116.13
99.10
Convert to SOj
by multiplication
1
0.800
0.790
0.780
0.508
0.616
0.614
0.687
0.533
0.634
0.552
0.646
Convert fi-om SO2
by multiplication
1
1.250
1.266
1.282
1.968
1.624
1.629
1.455
1.875
1.578
1.813
1.547
a pH below 5. At the lower pH values the sulfite
exists as HSOs".
Fig. 16-8 shows clearly that
H2SO3
and SOj^-
do not exist in solution together in any appreciable
amount. Since the standard terminology of free
and combined SOj are used, the following equa-
tion is important.
2HSO3-
<^ SO32- + H2SO3
(16-18)
Again, the equilibrium for the above reaction lies
far to the left. When free and combined forms of
SO2 are added in solution they will react together
until one of them is exhausted as the reaction
proceeds to the left. On the other hand, if the
solution is of known composition and the free and
combined equivalents are to be determined, one
imagines the reaction to proceed to the right until
all the bisulfite is exhausted.
EXAMPLE 8. Derive the gravimetric factor for converting Ca(HS03)2 to SOj.
SOLUTION. One mole of Ca(HS03)2 gives two moles of
SOj:
Ca(HS03)2 -* 2SO2 + Ca(0H)2
Therefore,
lgCa(HS03)2x
2 mol SO.
1
mol Ca(HSOA 64.06 g SO,
—X ^^ ^^^x —?-=0.634 g SO,
ImolCaOHSOj)^ 202.21 g Ca(HS03)2 ImolSO^ ^ ^
