Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.

394
17.
BLEACHING AND PULP PROPERTIES CALCULATIONS
3.
A 50.0 ml aliquot of
CIO2
solution consumed
19.96 ml of 0.152 N thiosulfate solution.
What is the concentration of
CIO2
in g/L?
4.
In the Solvay process for making CIO2, how
much methanol is theoretically required per
ton of sodium chlorate?
5.
A softwood, unbleached kraft pulp has a
kappa number of 34. During the bleaching
process, all of the lignin and 2% of the
carbohydrates are removed, what is the pulp
yield of the bleach plant? If the yield from
the pulp plant is 46%, what is the overall
bleached pulp yield from wood?
6. Describe how to prepare and standardize 5
gallons of KMn04 solution of 0.1000 N
to
be
used in the pulp mill for quality control. The
laboratory has 2 M
H2SO4,
1 N
H2SO4,
2 M
KI,
0.1000 N Na2S204, and starch indicator
solution.
Paper machine calculations
7.
A paper machine with a width of 2.5 m has
a speed of 10 m/s and produces a paper with
a basis weight of 50 g/m^. Calculate the
weight of paper produced per second. From
this,
and assuming a consistency of 0.5% in
theheadbox, calculate the volumetric flow
rate necessary through the headbox. Since
one knows the width of the slice, and the
length per second going through the slice,
you should be able to easily solve the slice
height. Assume this is an open headbox with
100%
efficiency of conversion of potential
energy to kinetic energy (unlikely in the first
case;
impossible in the second case). Calcu-
late the height of the water above the slice
from the equations for potential energy (PE
= mgh, ^ = 9.8 m/s^) and kinetic energy
(KE = 0.5
mv^)
to obtain the equation.
8. A paper machine operates at 20 m/sec and is
10 m
wide.
Calculate
the
required equivalent
height of water in the headbox assuming all
of the potential energy of water is converted
to kinetic energy. For paper of basis weight
50 g/m^ what is the required slice height?
(The headbox consistency is
0.5%.)
What is
the maximum annual production in tons?
9. A paper machine has a speed of 5 m/s with
a basis weight of 100 g/m^ and stock consis-
tency of 0.6% at the headbox. What should
the slice height be?
Strength of
wet
fiber mats
10.
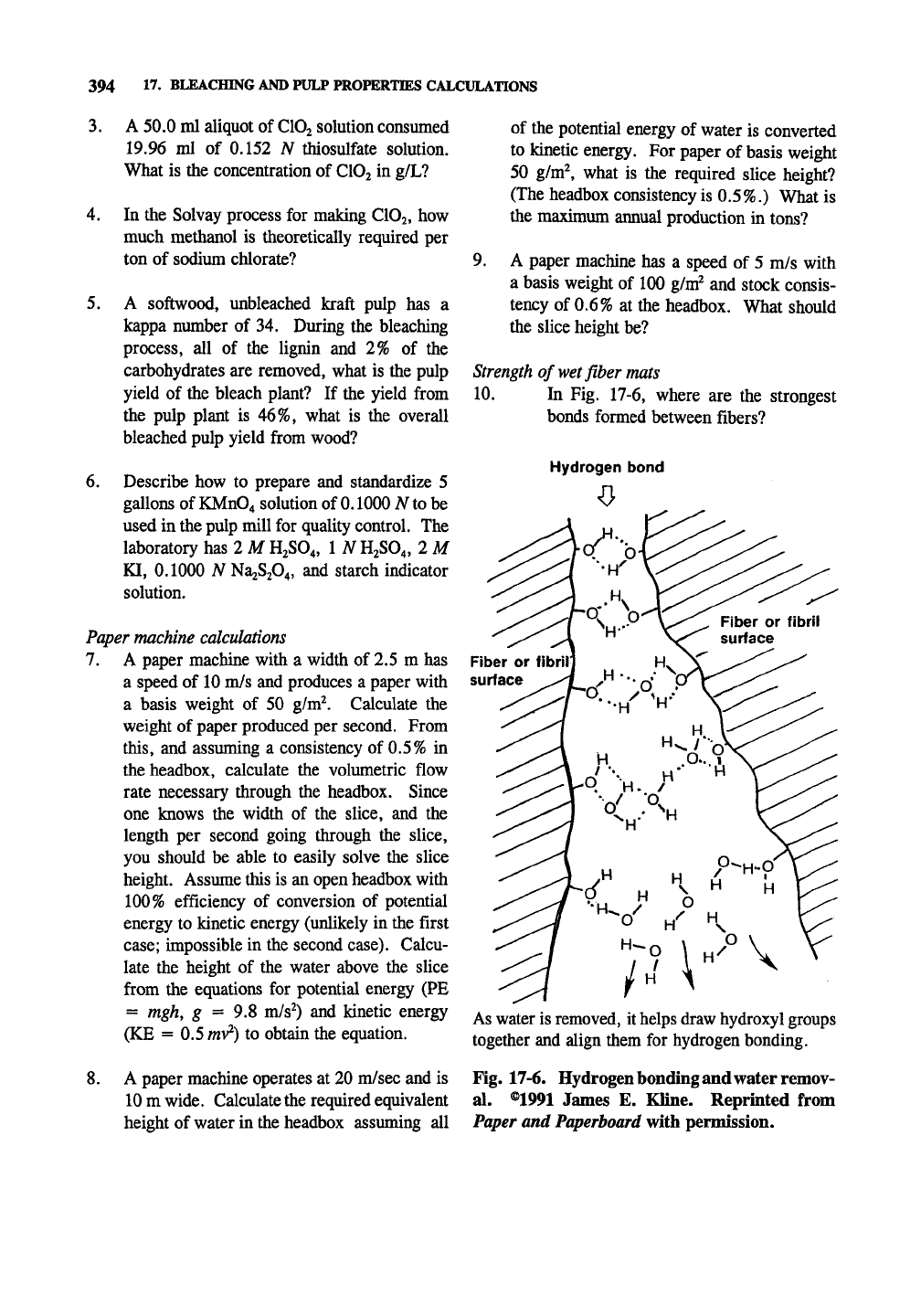
In Fig. 17-6, where are the strongest
bonds formed between fibers?
Hydrogen bond
Fiber or
surface
As water is removed, it helps draw hydroxyl groups
together and align them for hydrogen bonding.
Fig. 17-6. Hydrogen bonding and water remov-
al. ®1991 James E. Kline. Reprinted from
Paper and Paperboard vfith permission.

18
POLYMER CHEMISTRY
18.1 INTRODUCTION AND TYPES OF
POLYMERS
Introduction
This chapter is an introduction to polymer
chemistry with emphasis on topics related to pulp
and paper. Polymers are very important to many
aspects of pulp and paper, including wet end
chemistry, surface sizing, and coating. Of course,
the principal constituents of pulp fibers are all
polymers. Like other chapters in this book, many
of the introductory details are not included; for
further information, one should consult a textbook
on polymer science.
History of polymers
One of the earliest industrial developments
was the use of natural, soft rubber (poly-cw-
isoprene) in the early 1800s; the development of
the vulcanization process (reaction of the carbon
double bonds with sulfur to form crosslinked
chains) by Goodyear in 1839 led to hard rubber.
The first human-made plastic may be consid-
ered to be cellulose nitrate, which was discovered
in 1846 by Schonbein of Switzerland by the action
of a mixture of nitric and sulfuric acids on cotton.
Cellulose nitrate was used as a propel-
lant/explosive by the Austrian army in 1852 (now
called guncotton or smokeless powder), with amyl
acetate solvent as the first modern lacquer in the
United States in 1882, and in early photographic
films (plasticized with camphor to form Celluloid,
and as the first artificial silk by Count de
Chardonnet in France in 1884. Cellulose acetate
(discovered in 1865), being much safer since it is
less flammable, soon replaced cellulose nitrate for
most uses. Regenerated cellulose (see cellulose
xanthate) was invented by Cross and Bevan in
1892;
this is the viscose rayon process and was
used by Brandenberger to make cellophane,
marking the beginning of modern packaging with
transparent, plastic films in 1924 when the first
cellophane plant started operation in Buffalo, New
York. Indeed the cellulose-based plastics domi-
nated the synthetic plastics field for about 50
years.
The development of purely synthetic poly-
mers began with the discovery of the phenol-
formaldehyde resins by Baekland with the trade
name of Bakelite, small scale production of which
began in 1907. Other developments included the
use of styrene in synthetic rubbers in the 1930s,
the appearance of nylon (invented by Carothers) in
1939,
and the appearance of Teflon in 194L
Polymers
Polymers are high molecular weight chemi-
cals made of repeating units, called monomers,
which are linked by covalent
bonds.
The physical
properties of polymers depend on 1) the chemical
composition of the monomeric units, 2) stereo-
chemistry, if present, between the monomeric
units,
3) the mechanical configuration of the
polymer chain (that is, is it coiled or linear), and
4) the chain length of the polymer, that is, the
number of monomers, known as the degree of
polymerization (DP), of the polymer. Typical DP
values of commercial polymers range from several
hundred to many tens of thousands.
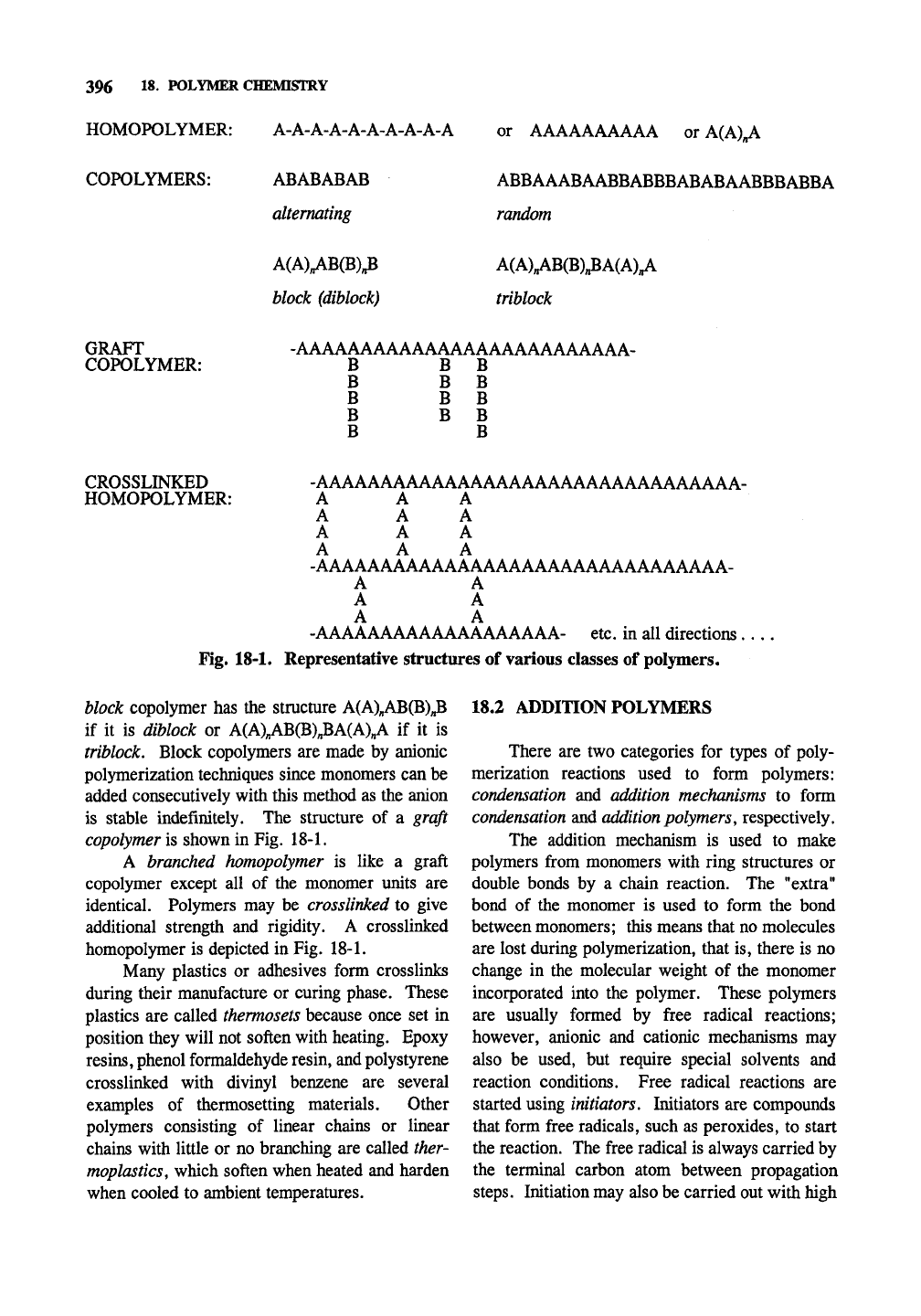
Polymers may be grouped according to their
component monomers. The simplest type of
polymer is the
homopolymer,
a polymer containing
only one type of monomer. Fig. 18-1. For conve-
nience, letters of the alphabet are assigned to
different types of monomers so that a homopoly-
mer may be described as one having the configu-
ration of: A-A-A-A-A-A-A-A-A-A. This can also
be written: A-(A)^-A; if « = 8 the DP of these
polymers is 10. A simple example of this is
polyethylene, made from the ethylene monomer
CH2=CH2; the structure of the polymer is CH3-
(CH2-CH2)n-CH3. Copolymers are polymers
containing two types of monomer, and terpolymers
contain three types of monomers. A copolymer
with an alternating structure is of the form
ABABABABABABABABABABABABA; a
copolymer with a random structure is
ABBAAABAABBABBBABABAABBBABA; a
395
396 18. POLYMER CHEMISTRY
HOMOPOLYMER: A-A-A-A-A-A-A-A-A-A
or AAAAAAAAAA or A(A)„A
COPOLYMERS:
ABABABAB
alternating
ABBAAABAABBABBBABABAABBBABBA
random
A(AUB(B)^
block (diblock)
A(AUB(B)^A(AU
triblock
GRAFT
COPOLYMER:
-AAAAAAAAAAAAAAAAAAAAAAAAAA-
B B B
B B B
B B B
B B B
B B
CROSSLINKED
HOMOPOLYMER:
-AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA-
A A A
A A A
A A A
A A A
-AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA-
A A
A A
A A
-AAAAAAAAAAAAAAAAAAA-
etc.
in all
directions.
Fig. 18-1. Representative structures of various classes of polymers.
block copolymer has the structure A(AXAB(B);3
if it is diblock or A(AXAB(B)„BA(A)„A if it is
triblock. Block copolymers are made by anionic
polymerization techniques since monomers can be
added consecutively with this method as the anion
is stable indefinitely. The structure of a graft
copolymer is shown in Fig. 18-1.
A branched homopolymer is like a graft
copolymer except all of the monomer units are
identical. Polymers may be cross linked to give
additional strength and rigidity. A crosslinked
homopolymer is depicted in Fig. 18-L
Many plastics or adhesives form crosslinks
during their manufacture or curing phase. These
plastics are called thermosets because once set in
position they will not soften with heating. Epoxy
resins,
phenol formaldehyde resin, and polystyrene
crosslinked with divinyl benzene are several
examples of thermosetting materials. Other
polymers consisting of linear chains or linear
chains with little or no branching are called ther-
moplastics, which soften when heated and harden
when cooled to ambient temperatures.
18.2 ADDITION POLYMERS
There are two categories for types of poly-
merization reactions used to form polymers:
condensation and addition mechanisms to form
condensation and
addition
polymers, respectively.
The addition mechanism is used to make
polymers from monomers with ring structures or
double bonds by a chain reaction. The "extra"
bond of the monomer is used to form the bond
between monomers; this means that no molecules
are lost during polymerization, that is, there is no
change in the molecular weight of the monomer
incorporated into the polymer. These polymers
are usually formed by free radical reactions;
however, anionic and cationic mechanisms may
also be used, but require special solvents and
reaction conditions. Free radical reactions are
started using initiators. Initiators are compounds
that form free radicals, such as peroxides, to start
the reaction. The free radical is always carried by
the terminal carbon atom between propagation
steps.
Initiation may also be carried out with high
ADDITION POLYMERS
397
energy radiation, photolysis, or thermal energy.
The reaction of a vinyl monomer, which has the
form CH2=CHR, is shown in Fig. 18-2.
Monomers almost invariably add head to
head, -CH2CHR-CH2CHR-, as opposed to head to
tail,
which is -CHjCHR-CHRCHj-. Head to head
addition can form three types of polymers which
differ in rotation around a carbon-carbon single
bond: in atactic polymers the R groups are ran-
domly oriented around the longitudinal axis of the
polymer; in isotactic polymers the R groups
branch out on one side of the polymer chain; and
in syndiotacticipo\ymtxs the R groups alternate on
one side of the axis to the opposite side of the
axis.
Atactic polymers are usually amorphous and
less dense than their often crystalline syndiotactic
or isotactic counterparts. Their structures are:
—
CH^
ISOTACTIC
—
CH^
-CHo
-CHo
-CHo
H
H
H
R
H
H
-CHo
SYNDIOTACTIC H
-CHo
-CHo
H
Addition polymers are characterized by high
molecular weight averages, rubbery or brittle
solids, and, usually, amorphous structures. The
chain reaction goes very quickly and the entire
polymer is built in a matter of seconds. It should
be noted that polymerization proceeds until all of
the monomer is removed or the free radicals are
all terminated. It is quite possible that residual
monomer will be present after polymerization.
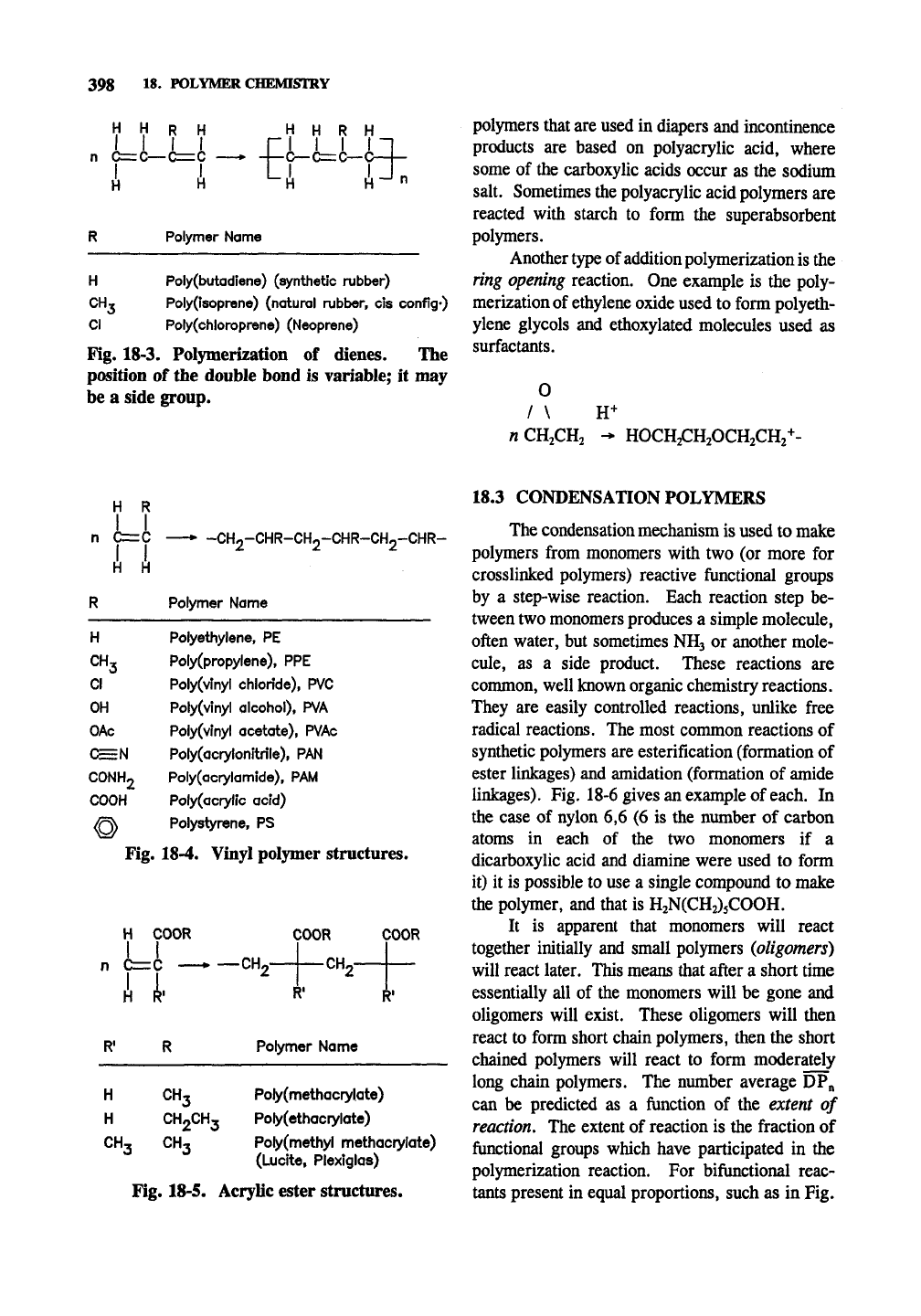
Fig. 18-3 shows a variety of vinyl diene struc-
tures.
Fig. 18-4 shows a variety of vinyl poly-
mers,
and Fig. 18-5 shows a variety of acrylic
polymers all of which are usually formed by the
addition mechanism. Two other polymers include
-CH2CCI2- [poly(vinylidene chloride), Saran] and
-CF2CF2- [poly(tetrafluoroethylene), Teflon]. The
diene polymers are very elastic because the double
bonds cause the backbone to assume a kinked,
random coil shape; these are rubber materials.
Tensile forces allow these coils to be partially
straightened; relaxation of the forces allow the
original structure to be assumed.
One other type of acrylic polymer is
polyacrylic acid where R and R' in Fig. 18-5 both
consist of H atoms. The so-called superabsorbent
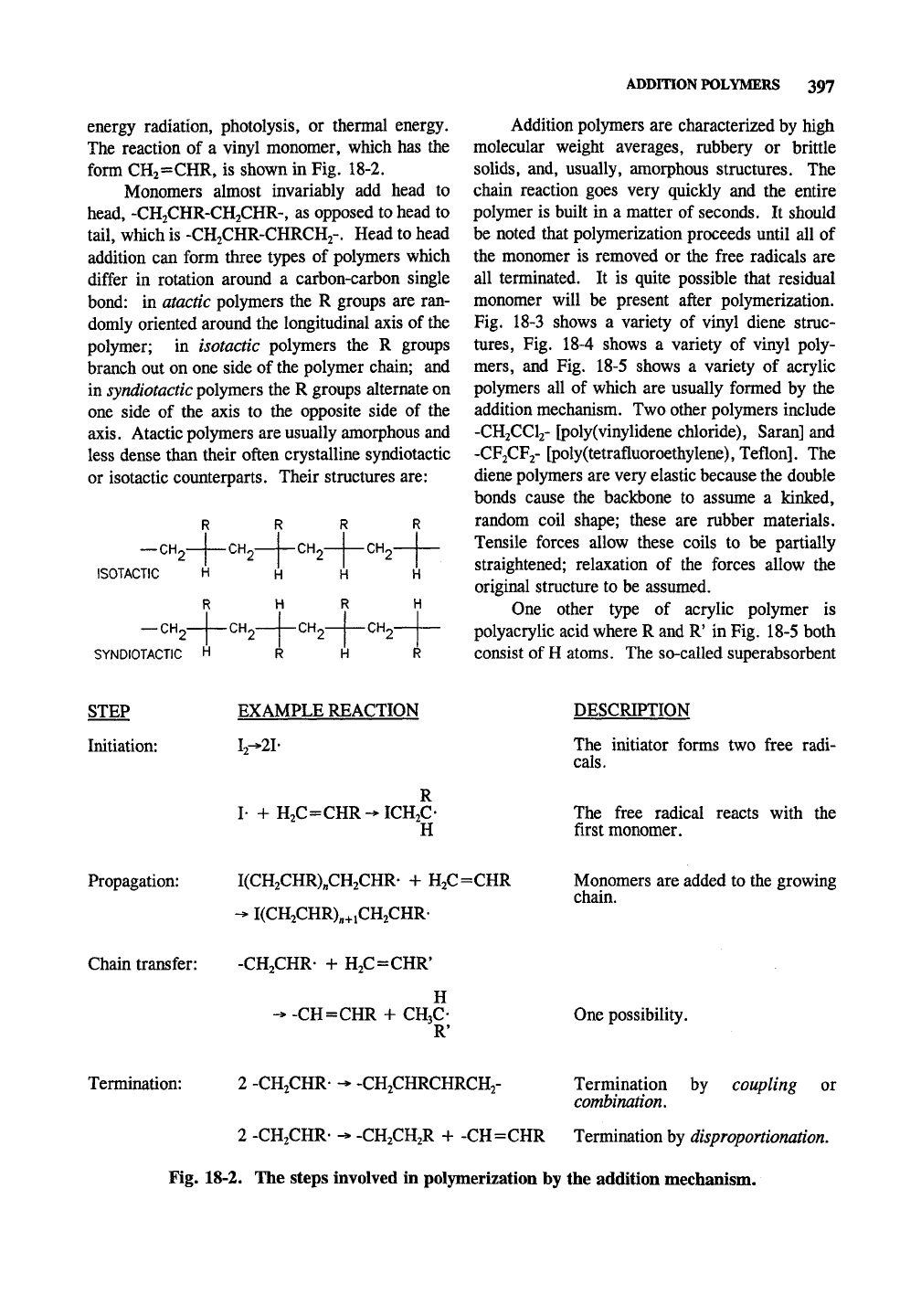
STEP
Initiation:
EXAMPLE REACTION
12-^21-
DESCRIPTION
The initiator forms two free radi-
cals.
I- +H2C=CHR-^ICH2C-
H
The free radical reacts with the
first monomer.
Propagation: I(CH2CHR)„CH2CHR- + H2C=CHR
-> I(CH2CHR)„+iCH2CHR-
Monomers are added to the growing
chain.
Chain transfer:
-CH2CHR- + H2C=CHR'
H
->-CH=CHRH-CH3C-
R'
One possibility.
Termination:
2 -CH.CHR- -* -CH.CHRCHRCHy
Termination by coupling or
combination.
2 -CH2CHR- -* -CH2CH2R + -CH=CHR Termination by disproportionation.
Fig. 18-2. The steps involved in polymerization by the addition mechanism.
3<)8 18. POLYMER CHEMISTRY
H H R
u
Polymer Name
H Poly(butadiene) (synthetic rubber)
CHT
Poly(isoprene) (natural rubber, cis conflg*)
CI Poly(chloroprene) (Neoprene)
Fig. 18-3. Polymerization of dienes. The
position of the double bond is variable; it may
be a side group.
polymers that are used in diapers and incontinence
products are based on polyacrylic acid, where
some of the carboxylic acids occur as the sodium
salt. Sometimes the polyacrylic acid polymers are
reacted with starch to form the superabsorbent
polymers.
Another
type
of addition polymerization
is
the
ring opening reaction. One example is the poly-
merization of ethylene oxide used to form polyeth-
ylene glycols and ethoxylated molecules used as
surfactants.
O
/ \
n CH2CH2
H^
HOCH2CH20CH2CH2^-
H R
1 1
1 1
n Cz=:C
1 1
i i
R
H
CH3
CI
OH
OAc
C=ti
CONH2
COOH
0
Fig.
—• -CH2-CHR-CH2-CHR-CH2-CHF
Polymer Name
Polyethylene, PE
Poly(propylene), PPE
Poly(vlnyl chloride), PVC
Poly(vinyl alcohol), PVA
Poly(vinyl acetate), PVAc
Poly(acrylonitrile), PAN
Poly(acrylamfde), PAM
Poly(acrylic acid)
Polystyrene, PS
18-4. Vinyl polymer structures.
H COOR
„u —
COOR
COOR
CHo
H R'
-CH^
Polymer Name
H
CH,
12 Poly(methacrylate)
H CH2CH3 Poly(ethacrylate)
Poly(methyl methacrylate)
(Luclte, Plexiglas)
CH3 CH3
Fig. 18-5. Acrylic ester structures.
18.3 CONDENSATION POLYMERS
The condensation mechanism is used to make
polymers from monomers with two (or more for
crosslinked polymers) reactive functional groups
by a step-wise reaction. Each reaction step be-
tween two monomers produces a simple molecule,
often water, but sometimes NH3 or another mole-
cule,
as a side product. These reactions are
common, well known organic chemistry reactions.
They are easily controlled reactions, unlike free
radical reactions. The most common reactions of
synthetic polymers are esterification (formation of
ester linkages) and amidation (formation of amide
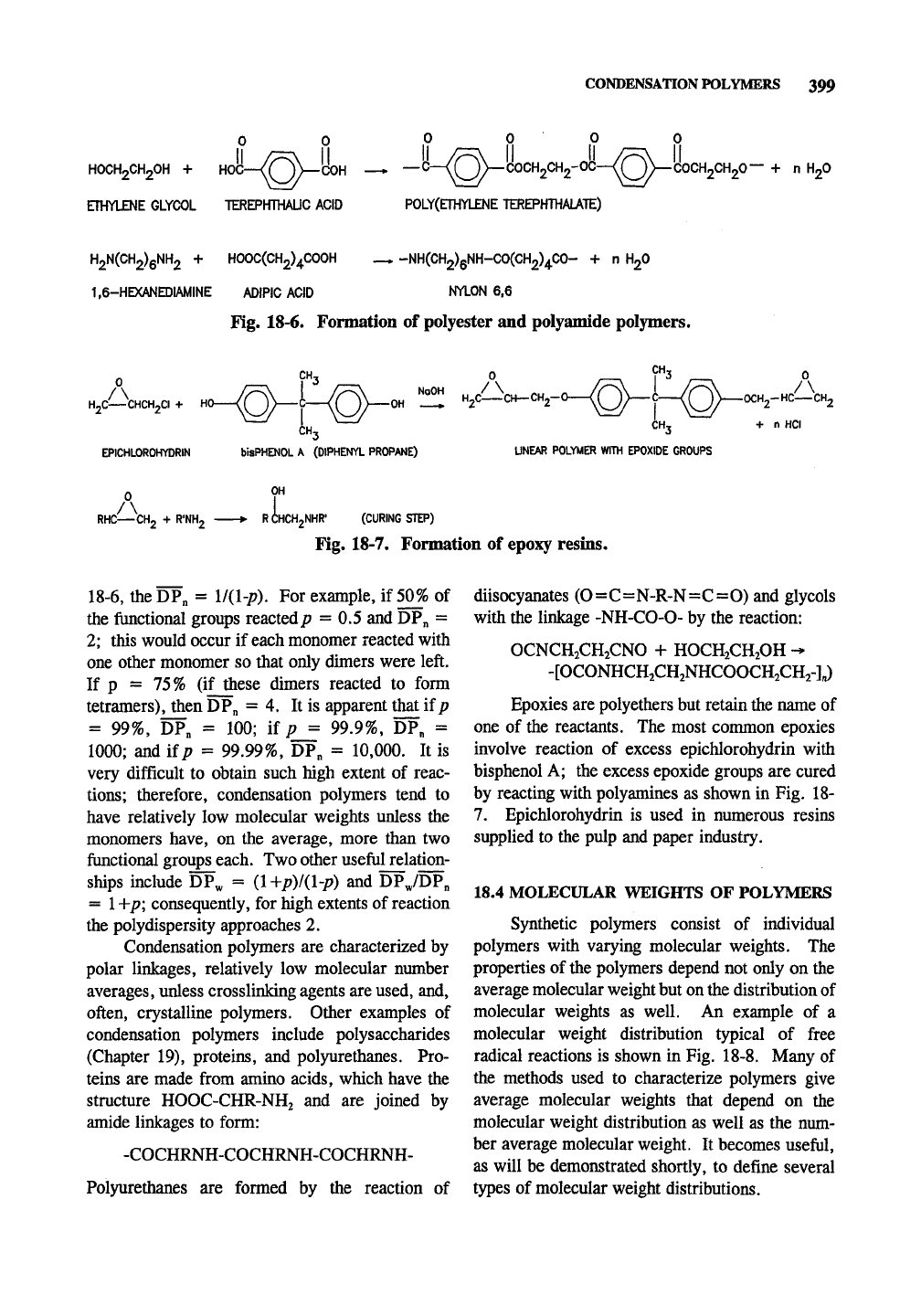
linkages). Fig. 18-6 gives an example of
each.
In
the case of nylon 6,6 (6 is the number of carbon
atoms in each of the two monomers if a
dicarboxylic acid and diamine were used to form
it) it is possible to use a single compound to make
the polymer, and that is H2N(CH2)5COOH.
It is apparent that monomers will react
together initially and small polymers (oligomers)
will react later. This means that after a short time
essentially all of the monomers will be gone and
oligomers will exist. These oligomers will then
react to form short chain polymers, then the short
chained polymers will react to form moderately
long chain polymers. The number average DP„
can be predicted as a fiinction of the extent of
reaction. The extent of reaction is the fraction of
fiinctional groups which have participated in the
polymerization reaction. For bifimctional reac-
tants present in equal proportions, such as in Fig.
CONDENSATION POLYMERS
399
HCX:H2CH20H
+
ETHYLENE GLYCOL
0
HOC-
•-^L - -t^^L^,-<L^.
TEREPKHHAUC ACID
COCH2CH2-
POLYCETHYLENE TEREPHTHALATE)
-COCH2CH2O—
+ n
H2O
H2N(CH2)eNH2
+
H00C(CH2)4C00H
1,6-HEXANEDIAMINE ADIPIC ACID
—. -NH(CH2)6NH-C0(CH2)4C0-
+ n
H2O
NYLON
6.6
0
EPICHLOROHYDRIN
Fig.
18-6.
Formation
of
polyester and polyamide polymers.
CH3
Q
0^H0^<"
-"
0
I2C
CH—CH2--'
CH3
bisPHENOL
A
(DIPHENYL PROPANE)
CH,
+
n
HCI
UNEAR
POLYMER WITH EPOXIDE GROUPS
0
/\
OH
RCHCH2NHR'
(CURING STEP)
Fig.
18-7.
Formation
of
epoxy resins.
18-6,
the DP„
=
l/(l-p).
For
example,
if
50%
of
the functional groups reacted/?
=
0.5
and DP^
=
2;
this would occur
if
each monomer reacted with
one other monomer
so
that only dimers were left.
If
p =
75%
(if
these dimers reacted
to
form
tetramers), then DP^
= 4. It
is
apparent tiiat if p
=
99%,
DP„ = 100; if
p_=
99.9%,
DP„ =
1000;
and
ifp =
99.99%,
DP„
=
10,000.
It is
very difficult
to
obtain such high extent
of
reac-
tions;
therefore, condensation polymers tend
to
have relatively
low
molecular weights unless
the
monomers have,
on
the
average, more than
two
functional groups each. Two other useful relation-
ships include
DP^
=
(l+p)/(l-p)
and
DPJDP„
=
1
-i-p;
consequently,
for
high extents
of
reaction
the polydispersity approaches
2.
Condensation polymers
are
characterized
by
polar linkages, relatively
low
molecular number
averages, unless crosslinking agents are used,
and,
often, crystalline polymers. Other examples
of
condensation polymers include polysaccharides
(Chapter
19),
proteins,
and
polyurethanes. Pro-
teins
are
made from amino acids, which have
the
structure HOOC-CHR-NH2
and are
joined
by
amide linkages
to
form:
-COCHRNH-COCHRNH-COCHRNH-
Polyurethanes
are
formed
by the
reaction
of
diisocyanates (O=C=N-R-N=C=O) and glycols
with the linkage -NH-CO-0-
by the
reaction:
OCNCH2CH2CNO
-h
HOCH2CH2OH
-*
-[OCONHCH2CH2NHCOOCH2CH2-];^
Epoxies are polyethers but retain the name
of
one
of
the reactants.
The
most common epoxies
involve reaction
of
excess epichlorohydrin with
bisphenol A;
the
excess epoxide groups
are
cured
by reacting with polyamines
as
shown
in
Fig.
18-
7.
Epichlorohydrin
is
used
in
numerous resins
supplied
to the
pulp
and
paper industry.
18.4 MOLECULAR WEIGHTS OF POLYMERS
Synthetic polymers consist
of
individual
polymers with varying molecular weights.
The
properties
of
the polymers depend
not
only
on the
average molecular weight but on the distribution of
molecular weights
as
well.
An
example
of a
molecular weight distribution typical
of
free
radical reactions
is
shown
in
Fig.
18-8.
Many
of
the methods used
to
characterize polymers give
average molecular weights that depend
on the
molecular weight distribution
as
well
as the
num-
ber average molecular weight.
It
becomes useful,
as will
be
demonstrated shortly,
to
define several
types
of
molecular weight distributions.
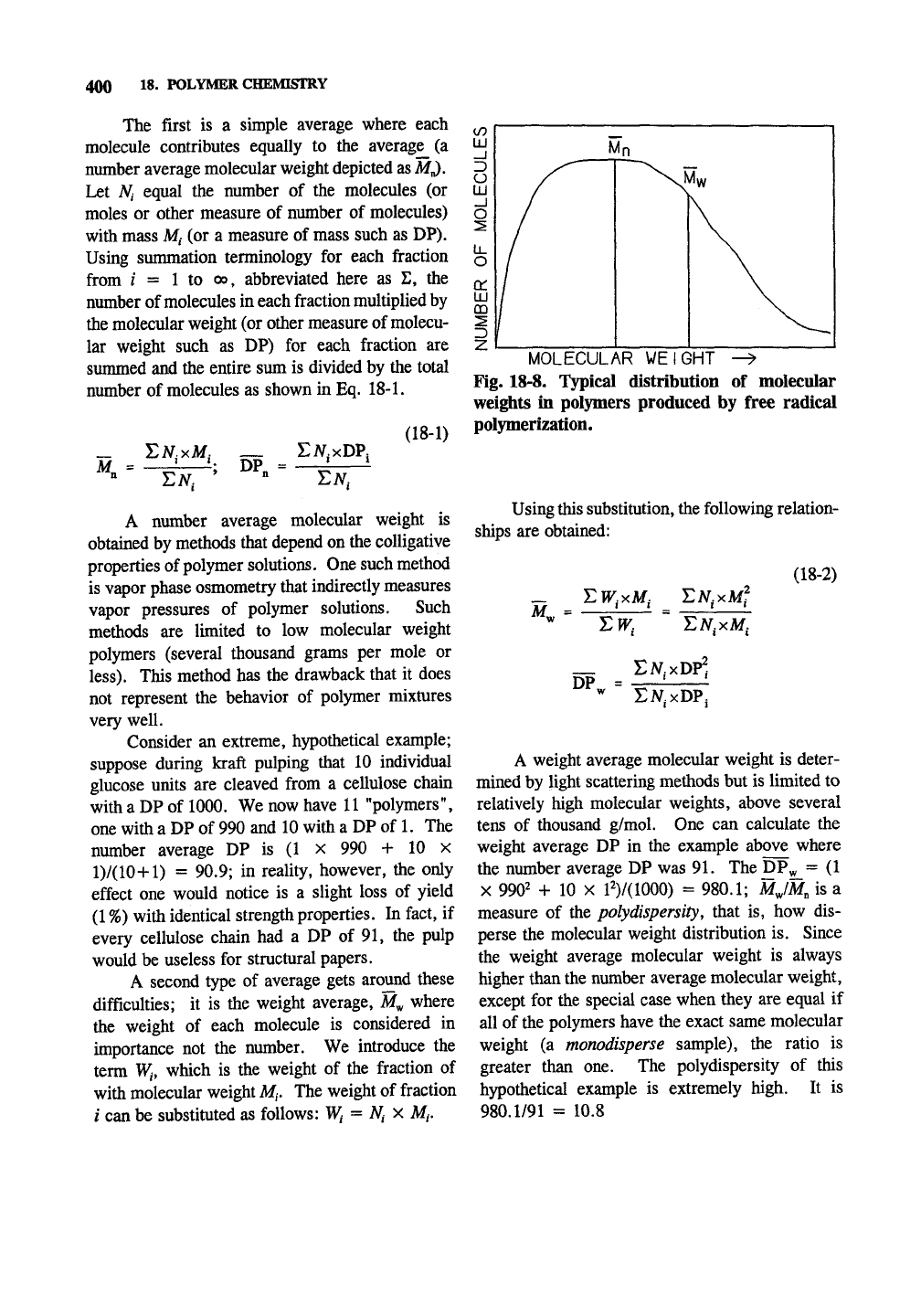
400 18. POLYMER CHEMISTRY
The first is a simple average where each
molecule contributes equally to the average^ (a
number average molecular weight depicted as M^,
Let Ni equal the number of the molecules (or
moles or other measure of number of molecules)
with mass M, (or a measure of mass such as DP).
Using summation terminology for each fraction
from / = 1 to 00, abbreviated here as E, the
number of molecules in each fraction multiplied by
the molecular weight (or other measure of molecu-
lar weight such as DP) for each fraction are
summed and the entire sum is divided by the total
number of molecules as shown in Eq. 18-1.
M„ = -^^ k DP. =
(18-1)
EM
EM
A number average molecular weight is
obtained by methods that depend on the colligative
properties of polymer solutions. One such method
is vapor phase osmometry that indirectly measures
vapor pressures of polymer solutions. Such
methods are Ihnited to low molecular weight
polymers (several thousand grams per mole or
less).
This method has the drawback that it does
not represent the behavior of polymer mixtures
very well.
Consider an extreme, hypothetical example;
suppose during kraft pulping that 10 individual
glucose units are cleaved from a cellulose chain
with a DP of 1000. We now have 11 "polymers",
one with a DP of 990 and 10 with a DP of 1. The
number average DP is (1 x 990 + 10 x
1)/(10+1) = 90.9; in reality, however, the only
effect one would notice is a slight loss of yield
(1 %)
with identical strength properties. In fact, if
every cellulose chain had a DP of 91, the pulp
would be useless for structural papers.
A second type of average gets aroimd these
difficulties; it is the weight average, M^ where
the weight of each molecule is considered in
importance not the number. We introduce the
term W;., which is the weight of the fraction of
with molecular weight
M,.
The weight of fraction
/ can be substituted as follows: W; = A/; x M^,
</)
UJ
-J
D
O
UJ
—1
o
:^
u.
o
(T
LLI
CD
2
D
Mn
*s.
\v
MyV
1
MOLECULAR WEIGHT
Fig. 18-8. Typical distribution of molecular
weights in polymers produced by free radical
polymerization.
Using
this
substitution, the following relation-
ships are obtained:
(18-2)
EPF,
TN.XM.
DP„
EN.XDP^
EN.XDP,
A weight average molecular weight is deter-
mined by light scattering methods but is limited to
relatively high molecular weights, above several
tens of thousand g/mol. One can calculate the
weight average DP in the example above where
the number average DP was 91. The DPj^= (1
X 9902 + 10 X P)/(1000) =
980.1;
MJM^ is a
measure of the polydispersity, that is, how dis-
perse the molecular weight distribution is. Since
the weight average molecular weight is always
higher than the number average molecular weight,
except for the special case when they are equal if
all of the polymers have the exact same molecular
weight (a monodisperse sample), the ratio is
greater than one. The polydispersity of this
hypothetical example is extremely high. It is
980.1/91 = 10.8
MOLECULAR WEIGHTS OF POLYMERS 401
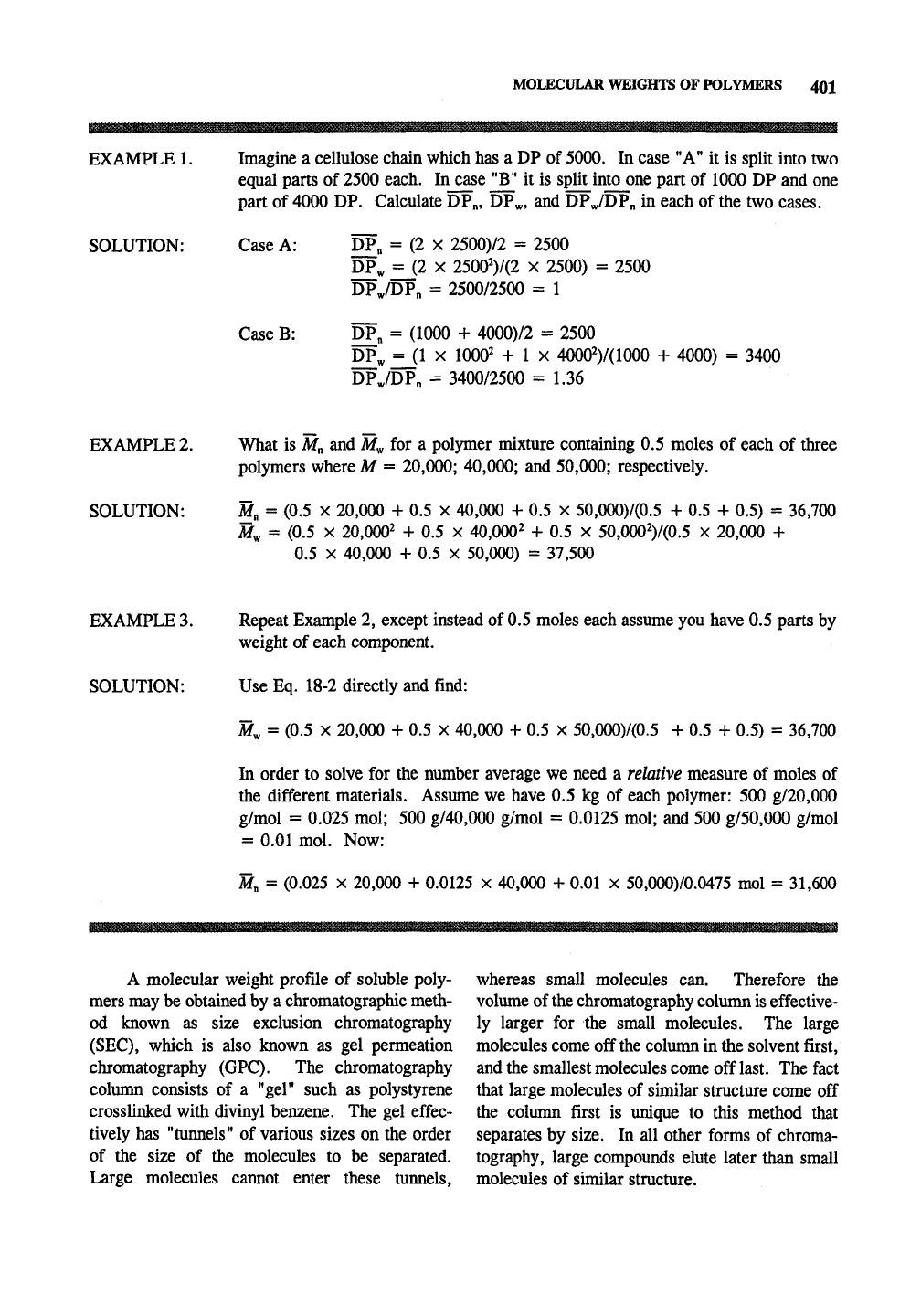
EXAMPLE 1.
SOLUTION:
Imagine a cellulose chain which has a DP of 5000. In case "A" it is split into two
equal parts of 2500 each. Incase
"B"
it is split into one part of 1000 DP and one
part of 4000 DP. Calculate DPn, DP^, and DP^/DP„ in each of the two cases.
Case A: DP„ = (2 x 2500)/2 = 2500
pP^
=J2 X 25002)7(2 X 2500) = 2500
DP,/DP„ = 2500/2500 = 1
Case B: DP„ = (1000 + 4000)/2 = 2500
DP^
=jl X 1000^ + 1 X 40002)7(1000 + 4000) = 3400
DPJDPn = 340072500 = 1.36
EXAMPLE 2. What is
M^
and
M^
for a polymer mixture containing 0.5 moles of each of three
polymers where M = 20,000; 40,000; and 50,000; respectively.
SOLUTION: M„ = (0.5 x 20,000 + 0.5 x 40,000 + 0.5 x 50,000)7(0.5 + 0.5 + 0.5) = 36,700
M^ = (0.5 X 20,0002 + 0.5 x 40,000^ + 0.5 x 50,000^)7(0.5 X 20,000 +
0.5 X 40,000 + 0.5 X 50,000) = 37,500
EXAMPLE 3. Repeat Example 2, except instead of 0.5 moles each assume you have 0.5 parts by
weight of each component.
SOLUTION: Use Eq. 18-2 directly and find:
M^ = (0.5 X 20,000 + 0.5 x 40,000 + 0.5 x 50,000)7(0.5 + 0.5 + 0.5) = 36,700
In order to solve for the number average we need a relative measure of moles of
the different materials. Assume we have 0.5 kg of each polymer: 500 g720,000
g7mol =
0.025
mol; 500 g740,000 g7mol = 0.0125 mol; and 500 g750,000 g7mol
= 0.01 mol. Now:
M„ = (0.025 X 20,000 + 0.0125 x 40,000 + 0.01 X 50,000)70.0475 mol = 31,600
A molecular weight profile of soluble poly-
mers may be obtained by a chromatographic meth-
od known as size exclusion chromatography
(SEC),
which is also known as gel permeation
chromatography (GPC). The chromatography
colunm consists of a "gel" such as polystyrene
crosslinked with divinyl benzene. The gel effec-
tively has "tunnels" of various sizes on the order
of the size of the molecules to be separated.
Large molecules cannot enter these tunnels,
whereas small molecules can. Therefore the
volume of
the
chromatography colunm is effective-
ly larger for the small molecules. The large
molecules come off
the
colunm in the solvent first,
and the smallest molecules come off
last.
The fact
that large molecules of similar structure come off
the colunm first is unique to tiiis method that
separates by size. In all other forms of chroma-
tography, large compounds elute later than small
molecules of similar structure.
402
18.
POLYMER CHEMISTRY
Solution viscosity
Strength^
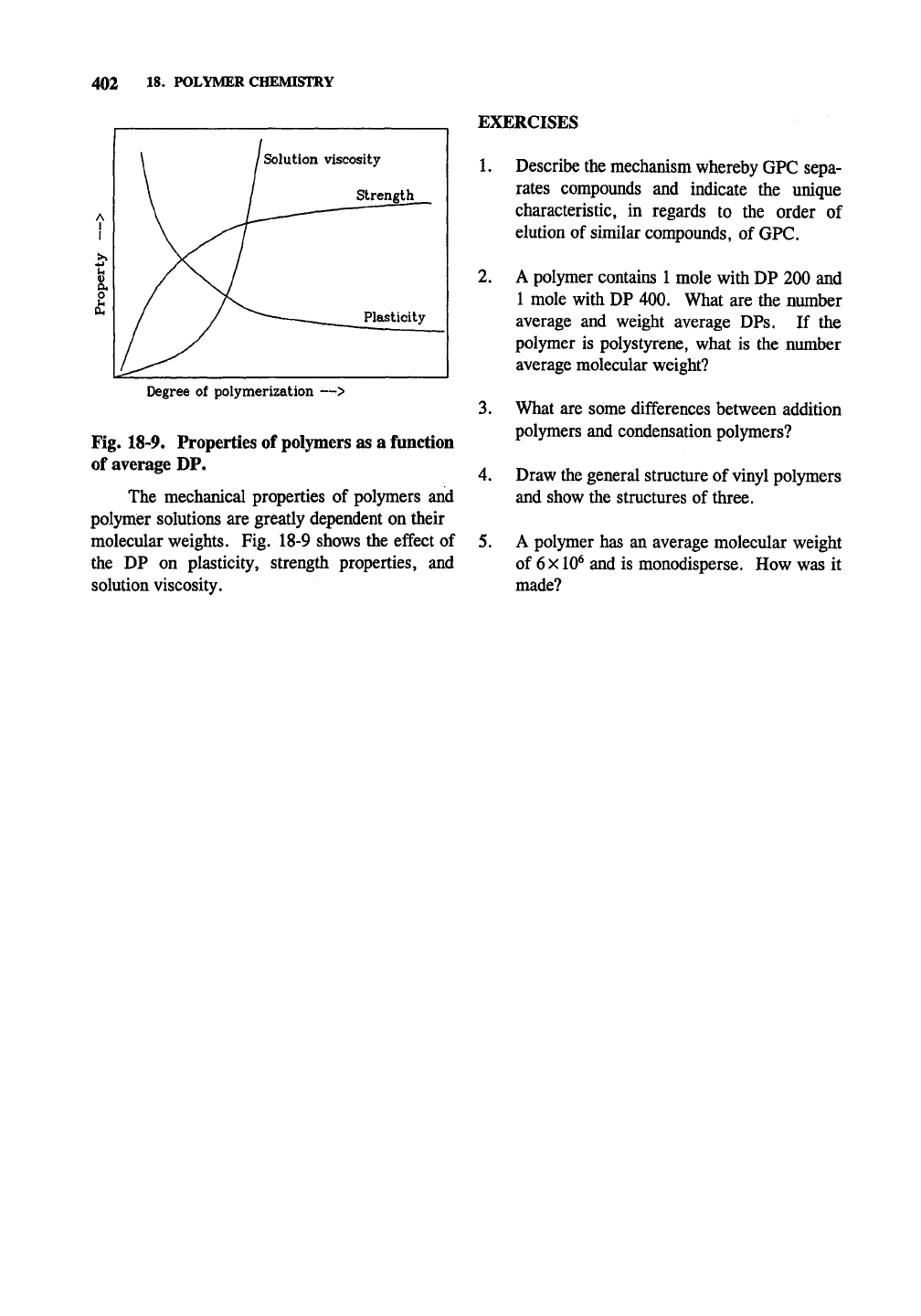
Degree of polymerization —>
Fig. 18-9, Properties of polymers as a function
of average DP.
The mechanical properties of polymers and
polymer solutions are greatly dependent on their
molecular weights. Fig. 18-9 shows the effect of
the DP on plasticity, strength properties, and
solution viscosity.
EXERCISES
1.
Describe the mechanism whereby GPC sepa-
rates compounds and indicate the unique
characteristic, in regards to the order of
elution of similar compounds, of GPC.
2.
A polymer contains 1 mole with DP 200 and
1 mole with DP 400. What are the number
average and weight average DPs. If the
polymer is polystyrene, what is the number
average molecular weight?
3.
What are some differences between addition
polymers and condensation polymers?
4.
Draw the general structure of vinyl polymers
and show the structures of three.
5.
A polymer has an average molecular weight
of 6x
10^
and is monodisperse. How was it
made?

19
CARBOHYDRATE CHEMISTRY
19.1 INTRODUCTION
The field of carbohydrate chemistry has seen
many advances in the past thirty years. The
interest in carbohydrate chemistry is not surprising
considering the importance of carbohydrates in
most aspects of our environment. It is only in the
last few decades that the significance of carbohy-
drates, in their many forms, has truly been recog-
nized. Carbohydrates have long been recognized
for their roles as structural materials and sources
of energy in the biological world, but their role as
informational molecules has only relatively recent-
ly been understood, and we have much more to
learn. Some interesting facts regarding carbohy-
drates only hint at their remarkable properties.
1.
Cellulose is the most abundant organic chem-
ical on the face of the earth.
2.
Some 400 billion tons of carbohydrates are
produced annually by photosynthesis.
3.
The typical diet consists of more than 60%
(dry weight) of carbohydrates.
4.
The major blood group types are determined
by sequences and branching of carbohy-
drates.
5.
Clearance of erythrocj^es from the blood
stream by the spleen is determined by the
structure of oligosaccharides on the erythro-
cyte membrane.
Generally, carbohydrates may be defined as
polyhydroxy aldehydes or polyhydroxy ketones,
occurring in their open chain forms or in their
heterocyclic ring forms (the acetal or ketal forms).
The simplest types of carbohydrates are called
neutral sugars. Sugars may be chemically modi-
fied to form derivatives. Some examples include,
reduction of the carbonyl group to give alditols,
oxidation of either terminal carbon to form sugar
acids,
acetylation or methylation of hydroxy 1
groups. The chemistry of carbohydrates is pre-
sented here to the extent that it is of importance to
the understanding of cellulose and hemicelluloses
during pulping and papermaking. For further
information, introductory biochemistry, wood
chemistry, and organic chemistry texts should be
consulted. Many of these texts contain chapters
devoted to carbohydrate chemistry.
19.2 NOMENCLATURE
The monosaccharides, simple sugars that
cannot be easily hydrolyzed into smaller
units,
are
classified according to the number of carbon atoms
in the molecule. This classification is used for
carbohydrates with three to seven carbon atoms;
that is, with trioses, tetroses, pentoses, hexoses,
and heptoses. Aldoses are monosaccharides that
have an aldehyde when in the acyclic form (in the
absence of the hemiacetal form); ketoses are
monosaccharides with a ketone when in the acyclic
form (absence of the hemiketal bond). Glucose is
an example of an aldohexose, and fructose is an
example of a ketohexose or hexulose, a six-carbon
ketose, as shown in Fig. 19-1.
If the terminal R-CHjOH (at the C-6 posi-
tion) of an aldose is oxidized to a carboxylic acid,
then the monosaccharide is known as an uronic
acid;
if the aldehyde is oxidized to a carboxylic
acid, the compound is referred to as an aldonic
acid; and if both terminal carbon atoms are oxi-
dized to carboxylic acids, the compound is re-
ferred to as an aldaric acid. Monosaccharide
constituents of particular importance in woody
plant cell wall polysaccharides are the pentoses
arabinose and xylose; the hexoses glucose,
mannose, and galactose; and the uronic acid (4-0-
methyl) glucuronic acid; these structures are
shown with the hemicelluloses in Section 2.6.
19.3 FORMS OF MONOSACCHARIDES
Monosaccharides may be represented, as in
Fig.
19-1,
by the Fischer projection, introduced by
Emil Fischer in the late 19th century. The Fischer
projection and the ball-and-stick model of D-
glucose are presented in Fig. 19-2, as well as the
Fischer projection of L-glucose. Horizontal lines
of the Fischer projection represent H and OH
403
