Cao Z. (Ed.) Thin Film Growth: Physics, materials science and applications
Подождите немного. Документ загружается.

238 Thin film growth
© Woodhead Publishing Limited, 2011
eased by slow cooling of Ni from ~ 1000°C down to room temperature (after
Ni has been enriched with carbon at high temperatures if required) (Kim,
2009). On the contrary, Ir can store much less carbon in its bulk (Arnoult,
1972), which implies that graphene growth proceeds only by impinging
carbon adspecies supplied to the surface.
a variety of carbon sources can be employed for the growth, and it is
probable that others will be used in the future. The most convenient source
is a gas of carbon-containing molecules (Fig. 10.6a). Such molecules are
usually hydrocarbons (Oshima, 1997). Until 2009, the gases were injected
at low-pressure in ultra-high vacuum systems (UHV). In 2009, gas mixtures
(e.g. Ch
4
/ar/h
2
) were also employed at ambient pressure (Kim; 2009; Reina,
2009a), opening valuable perspectives for graphene production. In both cases
(UHV and ambient pressure) the carbon-containing molecules are cracked
at the metal surface; for this the metal surface is made catalytically active
by heating it. This process can be considered an heterogeneous chemical
reaction between a gas phase and a solid one, which is why the technique is
often referred to as chemical vapour deposition (CVD). Another option for
graphene growth is the use of an atomic carbon source (Loginova, 2008).
Finally, in the case of metals having a non-negligible carbon solubility,
carbon segregation from the bulk towards the surface might be an alternative
route (Sutter, 2008).
In the following we focus on the growth processes disregarding the way
the carbon ad-species have been formed at the metal surface (CVD, atomic
H
2
CH
4
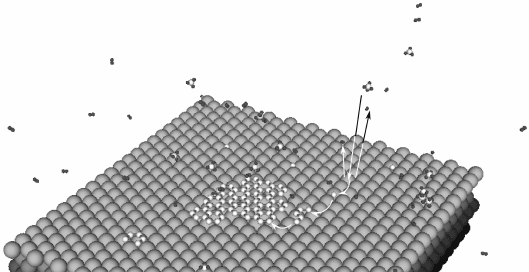
10.6 Schematics for the different elementary processed during
CVD growth of graphene on a metal, with a methane precursor as
an illustration. (a) A methane molecule is adsorbed at the surface,
cracked into a carbon adatom, leaving two hydrogen molecules
released in vacuum. (b) The carbon adatom diffuses at the surface
(or sub-surface). (c) The carbon adatom incorporates in a carbon
chain, forming a pentamer (on metal surfaces like Ir(111) or Ru(0001),
Loginova, 2008). (d) The carbon chain diffuses at the surfaces and (e)
is incorporated in the graphene flake.
(b)
(a)
(c)
(d)
(e)
ThinFilm-Zexian-10.indd 238 7/1/11 9:42:53 AM
239Epitaxial growth of graphene thin films on single crystal metal surfaces
© Woodhead Publishing Limited, 2011
carbon source, segregation). This is legitimate if the nature of the carbon
source only affects graphene growth through the rate of adatom formation.
This is at least the case in prototypical systems that are suited to address
elementary growth processes, for instance growth by CVD with ethene or
by an atomic carbon source at single-crystalline metal surfaces (loginova,
2009b).
10.3.1 Growing graphene nanoflakes
A derivative of the CVD growth for graphene consists in (i) adsorbing
carbon-containing molecules at the metallic surface, at such a temperature
that the molecules do not desorb to vacuum, and (ii) increasing the sample
temperature for promoting hydrocarbon decomposition and eventually
graphene growth (Coraux, 2009).
The surface is rst saturated with molecules, and the resulting adlayer
prevents the strong bonding of further molecules at the surface. In practice,
ethene molecules are transformed into ethyledine ones at Pt(111) and Ir(111)
(Nieuwenhuys, 1976) surfaces at room temperature. The amount of carbon
in the resulting adlayer is less than that in a full graphene layer, due to steric
effects.
In the next step the substrate is heated up. In the course of increasing the
sample temperature, dehydrogenation of the molecules proceeds, leaving
pure-carbon adspecies being mobile at the surface. Nanometer-scale carbidic
islands develop from this carbon sea. The electronic interaction between the
carbidic islands and the metal surface is strong, all the more as the islands
are small, as has been shown for graphene on Ir(111). This is because in
small islands the proportion of edge atoms is large: such low coordinated
atoms have a tendency to form bonds with the metal atoms underneath. This
strong bonding was detected through energy shifts in the carbon and metal
atoms core levels. as a result of the enhanced bonding of graphene edges
to the substrate, the small graphene islands were shown to be dome-shaped
and characterized by a small distance to the metal surface, noticeably below
that for a plain graphene sheet on Ir(111), for island radii smaller than 1–2
nm, and all the more as the radii is small (Fig. 10.7) (Lacovig, 2009). Such
small islands, as imaged by STM (Fig. 10.8a), are found at the terraces of
the metallic surface, and occasionally bond to substrate step edges. They are
characterized by a poorly dened height and rounded edges. Further increase
of the temperature modies their morphology (Fig. 10.8b–e). Their height
becomes well dened as seen by STM for islands radii larger than a few
nanometres.
Such islands with well-dened height are bound by straight edges that
are precisely oriented along the <11-20> directions of graphene, consistent
with the prominent proportion of zigzag portions composing these edges. at
ThinFilm-Zexian-10.indd 239 7/1/11 9:42:53 AM
240 Thin film growth
© Woodhead Publishing Limited, 2011
sufciently high growth temperature, it was shown for graphene on Ir(111)
that the island edges align to the <1-10> direction of Ir(111) (N’Diaye,
2008b). Graphene islands were also reported on Pt(111) (Land, 1992), Ir(111)
(N’Diaye, 2006; Coraux, 2009), and Co(0001) (Eom, 2009). The occurrence
of straight edges for graphene is the evidence that carbon mobility is active
at edges. In the explored growth temperature range (700–1300°C), these
effects are rather slow as the islands are most often not in their equilibrium,
hexagonal shape. Carbon diffusion at edges could proceed through that of
carbon pentagons continuously formed at graphene edges during growth
(Frenklach, 2004; Whitesides, 2010). Pentagon collisions result in hexagons,
1.62 Å
2.53 Å
(a)
(c)
(b)
(d)
[101
]
[01
1]
2.63 Å
3.13 Å
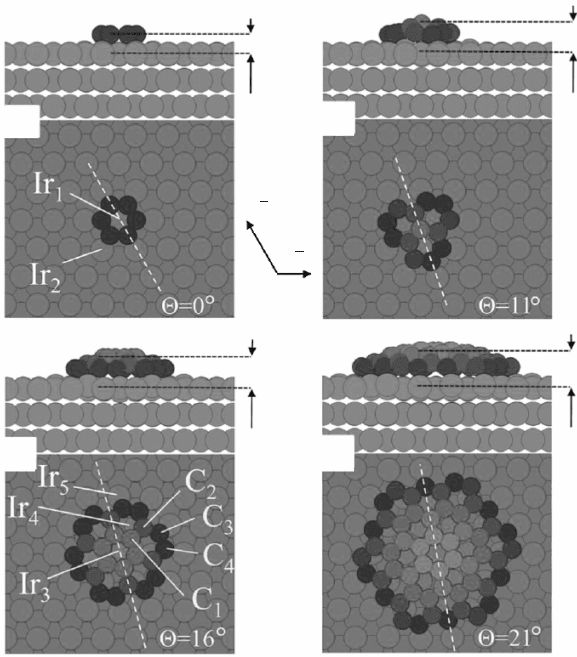
10.7 Calculated structural models for graphene nanoislands on Ir(111)
as a function of their (small) size, on a side view and on a top view:
for (a) one, (b) three, (c) seven, and (d) 19 carbon rings. Note that the
average height of the islands increases with their size (from Lacovig,
2009, © The American Physical Society, http://prl.aps.org/abstract/
PRL/v103/i16/e166101).
ThinFilm-Zexian-10.indd 240 7/1/11 9:42:54 AM
241Epitaxial growth of graphene thin films on single crystal metal surfaces
© Woodhead Publishing Limited, 2011
i.e. graphene building blocks (Whitesides, 2007). On the contrary, edge
smoothing through a 2D carbon gas surrounding the edges, that would be
formed via carbon atom detachment at edges (more readily at regions where
the 2D pressure is higher, i.e. at convex edges), is not a prominent process
at the temperature of interest.
The temperature to which the substrate is heated up determines the size
of the graphene islands: their average diameter can be adjusted between a
few and several tens of nanometres for 600°C and 1200°C respectively on
Ir(111). Through this temperature range, the island density drops by almost
three orders of magnitude, consistent with the fact that the total amount of
carbon at the surface is not modied. As the growth temperature increases,
the proportion of graphene islands bound to substrate step edges increases.
The density and size of the graphene islands vary because of a thermally
induced ripening process. Considering the strength of the C–C bond in
graphene, ostwald ripening, i.e. the preferential growth of large islands at
the expense of the dissolution of smaller ones through their higher pressure
of carbon adatoms, cannot account for the observed efcient ripening below
1300°C. The presence of large islands with irregularly shaped (though zigzag)
edges, exhibiting large vacancies, for growth temperature of 850°C during
(a)
(d)
(b)
(e)
(c)
(f)
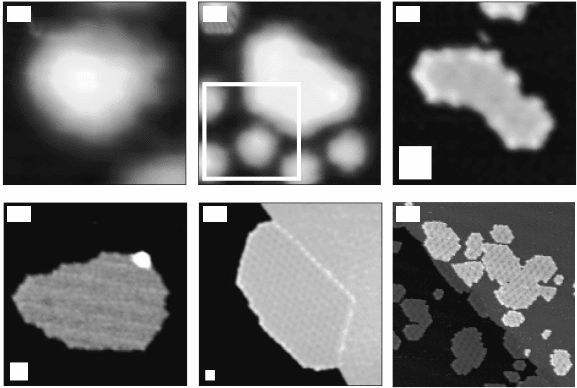
10.8 (a–e) STM topographs of typical carbidic (below 600°C) and
graphene (higher temperatures) islands on Ir(111) as a function of
the growth temperature: (a) 600°C (3.2 ¥ 3.2 nm
2
), (b) 700°C (6.2 ¥
6.2 nm
2
), (c) 850°C (18 ¥ 18 nm
2
), (d) 1050°C (32 ¥ 32 nm
2
), and (e)
1200°C (64 × 64 nm
2
). The size of the white box in (b–e) corresponds
to the size of (a) (from Coraux, 2009, © Institute of Physics). (f) STM
topograph (73 ¥ 73 nm
2
) of a graphene island grown on Ir(111) and
presumably formed upon the coalescence of smaller islands.
ThinFilm-Zexian-10.indd 241 7/1/11 9:42:54 AM
242 Thin film growth
© Woodhead Publishing Limited, 2011
a few seconds (Fig. 10.8f), suggests that large islands are formed upon the
coalescence of smaller ones that are mobile at the surface (Coraux, 2009).
This process is known as smoluchowski ripening. It is probably effective
due to the incommensuracy of the graphene and metal lattices. For a strictly
incommensurate island the activation energy for lateral motion would vanish,
as for every carbon moving away from its optimum binding site, another
one nds it. The existence of a migration barrier is probably linked to the
stronger binding of the graphene island edge atoms to the substrate. as larger
graphene islands display sizes consisting of an integer number of moiré units
it is likely that the graphene edges bind to specic energetically preferred
substrate sites. Prolonged heating results in islands becoming more compact,
presumably due to efcient carbon mobility at graphene edges.
10.3.2 Growing plain graphene sheets
We now consider the situation where carbon atoms, whatever their source,
are provided at the hot metallic surface.
From graphene islands to plain sheets
Graphene islands preferentially bind to the substrate step edges. The size
(density) of the islands is the larger (lower) the higher the growth temperature.
This is evidence that graphene nucleation along the steps is homogeneous
and not at a specic defect site. The nucleation at step edges was observed
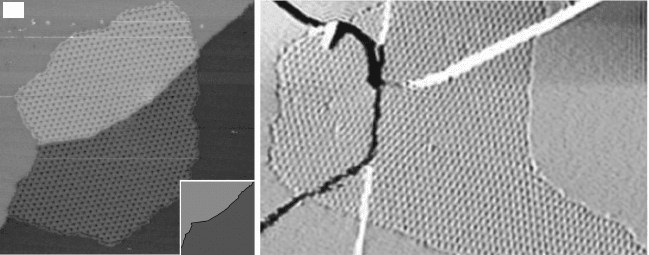
by STM (Fig. 10.9a) and low-energy electron microscopy (LEEM) on Ir(111)
(Coraux, 2009), Ru(0001) (Sutter, 2008), and Pt(111) (Sutter, 2009a), in a
wide range of growth temperatures, and for different types of carbon sources.
The graphene islands nucleate preferentially at the ascending side of the step
edge. as discussed in the next subsection, this is presumably promoted by the
fact that carbon atoms attach via s bonds to the ascending substrate step.
Providing more carbon adatoms at the surface results in the growth of
the islands. This growth is always anisotropic, faster at the lower terrace
than at the upper one. For graphene on Ru(0001), there is almost no growth
at the upper terrace (Sutter, 2008). On Ir(111) and Pt(111) (Coraux, 2009;
Loginova, 2009b; Sutter, 2009a) the onset of growth is delayed (Fig. 10.9b).
Probably it is delayed by an energetically costly transient state, for which the
carbon–metal bond is broken before a carbon–carbon bond is formed across
the substrate step edge. The growth of graphene over steps does not interrupt
the graphene lattice. The carbon rows are continuous and bent vertically
to adapt to the the terrace ow (Pan, 2007; Coraux, 2008); in other words,
graphene rests like a blanket on the substrate.
Growing islands will eventually meet. sTM showed that the moiré
displays continuous rows through the location where the islands coalesced,
ThinFilm-Zexian-10.indd 242 7/1/11 9:42:54 AM
243Epitaxial growth of graphene thin films on single crystal metal surfaces
© Woodhead Publishing Limited, 2011
which is evidence that the carbon rows are also continuous (Coraux, 2009).
This observation is puzzling at rst glance, as there is no reason why the
graphene lattices from the two lattices would be in registry. rather we would
expect a shift by a fraction of a lattice constant. lattice distortions, which
could be adapted either locally or at a larger scale, are therefore expected.
Dislocations (heptagon-pentagon pairs) are also found where the islands met
to accommodate for small tilts between the islands.
Prolonged growth will result in a lateral expansion of the graphene akes
which will meet and eventually cover the whole metallic surface. This is
discussed in more detail on page 245.
Graphene interaction with the substrate step edges
The nucleation of graphene at substrate step edges and the partial or total
hindering of uphill growth over step edges are evidence for the interaction
of graphene edges with substrate steps. This interaction is probably lowering
(a)
(b)
up
up
= down
20 µm
Down
0 100 200 300
Time (s)
Rate (arb. units)
1.0
0.8
0.6
0.4
0.2
0.0
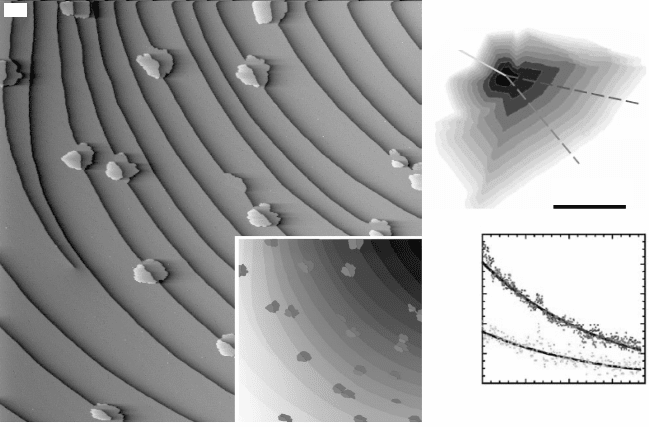
10.9 (a) Differentiated STM topograph (1 ¥ 1 mm
2
) of graphene
islands nucleated at Ir(111) atomic step edges (the islands are
highlighted in dark and light gray if nucleated at the upper or lower
terrace respectively) (from Coraux, 2009, © Institute of Physics). (b)
Contours of a growing graphene island on Pt(111) at the vicinity of
an atomic substrate step edge, as a function of the growth time, as
images with LEEM (top panel) and growth rate derived from such a
sequence of images at the upper and lower terrace around the step
edge (from Sutter, 2009a, © The American Physical Society, http://
prb.aps.org/abstract/PRB/v80/i24/e245411).
ThinFilm-Zexian-10.indd 243 7/1/11 9:42:55 AM
244 Thin film growth
© Woodhead Publishing Limited, 2011
the edge energies of both graphene and the metal by increasing the low
coordination of edge atoms.
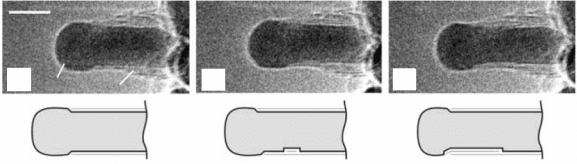
Graphene growth was also observed to induce reshaping of the substrate
step edges. The magnitude of this reshaping varies. On Ir(111) or Ru(0001)
(Coraux, 2009; Loginova, 2010), it is in the form of a reorientation of the
metal step edge that is in contact with one of the graphene island edges, so
that the metal edge transforms into segments aligning the zigzag direction
of graphene (Fig. 10.10a). On Pt(111) and Pd(111), the effects are much
stronger: a whole graphene island can penetrate the metal terraces so that all
graphene edges are bound to metal edges, with the latter aligning graphene’s
zigzag edges (Nakagoe, 2002; Fujita, 2005; Land, 1992; Kwon, 2009) (Fig.
10.10b). Such processes can only be effective if ow of metal atoms develops
at the step edges. The preferential orientation of the reshaped metal edges is
probably driven by maximization of the number of carbon–metal s-bonds.
So far only in the cases of Ni(111) (Helveg, 2004) and Ru(0001) (Loginova,
2010) supports has it been shown that a growth front of graphene is possible
also at the location where it is attached to a metallic step edge. accordingly
graphene grows at the expense of a retraction of the substrate step edge
(Fig. 10.11). It was argued (Loginova, 2010) that the metal atoms are then
displaced away from the step edge and can incorporate in the topmost metal
layer, causing the formation of a dislocation network pattern in this layer.
Metal step edge retraction may also result from the equilibrium between the
step edge and the 2D metal gas surrounding it at the growth temperature,
allowing for an efcient exchange between step atoms and adatoms.
(a)
(b)
10.10 STM topographs evidencing the interaction of metal step
edges upon graphene growth, on Ir(111) (a) and on Pd(111) (b) (from
Kwon, 2009, © The American Chemical Society). The superstructure
is the moiré of graphene/Ir(111) and graphene/Pd(111). The inset in
(a) highlights the structure of the substrate step edge reshaped by
the graphene island. The interaction of graphene with Pd(111) steps
is such that the graphene island is incorporated into a Pd terrace.
Image sizes are 125 ¥ 125 nm
2
for (a) and 380 ¥ 250 nm
2
for (b).
ThinFilm-Zexian-10.indd 244 7/1/11 9:42:58 AM
245Epitaxial growth of graphene thin films on single crystal metal surfaces
© Woodhead Publishing Limited, 2011
Elementary processes during the growth
as we already discussed, graphene growth starts at the step edges. It was
shown that the amount of graphene does not depend on the size of the terraces
surrounding the step edges (Coraux, 2009). In addition, at steps graphene
islands nucleate with random separations giving rise to earlier coalescence.
These observations indicate the absence of any signicant gradients induced
in the concentration of the adspecies from which the islands grow. Graphene
island growth is not diffusion limited.
on the contrary, it was proposed that the growth is interface limited
because carbon adatoms experience a large energy barrier for attaching to
graphene step edges (Loginova, 2008). A large carbon supersaturation is
needed at the surface so that several atom carbon clusters, being mobile at
the surface, can be formed. such clusters have been calculated to possess a
much lower energy barrier for attachment at graphene edges, and are thus
considered as building blocks for graphene growth. They are as large as
heptamers on Ru(0001) (Loginova, 2008) and Ir(111) (Loginova, 2009b).
These results are qualitatively very similar for two different carbon sources,
CVD with ethylene or atomic carbon ux.
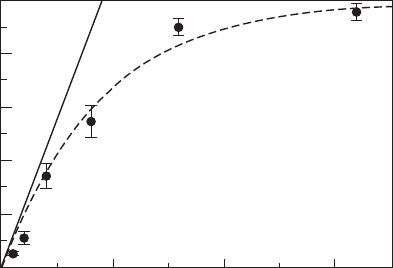
The analysis of the surface coverage with graphene as a function of the
carbon dose provides information about the stability of carbon adspecies
at the bare metal surfaces and regions covered with graphene. For growth
on Ir(111), it was shown that the experimental data are well reproduced by
a mere exponential law (Fig. 10.12), with a rapid increase at initial stages
followed by a slowing down of the growth rate towards zero as full coverage is
approached (Coraux, 2009). Quantitative analysis showed that the exponential
behaviour is well reproduced by assuming (i) the absence of desorption of
carbon adspecies from the Ir surface areas, (ii) the absence of C incorporation
to the bulk and (iii) no sticking of molecules or C adspecies to the graphene
areas. Therefore, under these conditions graphene growth is self-limiting
(a) (b) (c)
Ni
C
10.11 In situ observation of carbon nanotube growth at the surface of
a catalytic (Ni) particle with transmission electron microscopy, where
methane is provided with increasing doses from (a) to (c). Scale
bar is 5 nm. The schematics highlight the graphene growth at the
expense of the retraction of the substrate step edges (from Helveg,
2004, © Nature Publishing Group).
ThinFilm-Zexian-10.indd 245 7/1/11 9:42:58 AM
246 Thin film growth
© Woodhead Publishing Limited, 2011
and terminates upon monolayer completion. In the opposite situation where
the bulk of the support has the capability to store carbon and supply mobile
species, like it is the case from 1500°C on Ir(111) or 800–900°C on metals
like Ni or ru, the graphene growth is not self-limiting. a second and even
more layers may form underneath the monolayer graphene.
On the formation and stability of rotational variants
Graphene rotational variants are found at Ir(111) and Pt(111) surfaces (see
Section 10.2.3), and will presumably also be found at the surface of other
metals weakly interacting with graphene, typically those for which the average
graphene–metal distance is close to that between planes in graphite. On Ir(111)
the variant with graphene zigzag edges aligned to Ir dense-packed rows
grows is energetically preferred and dominates the growth; at high growth
temperatures and uxes, other variants may nucleate at its edges (Loginova,
2009b). For graphene on Pt(111) the different variants nucleate independently
and at the same time (Sutter, 2009a). On Ir(111), it was suggested that the
nucleation of the other variants is heterogeneous, taking place at defects
at the rst variant edges. Once the other variants are nucleated, they grow
faster than the rst variant. It was argued that this is due to different carbon
attachment kinetics at graphene edges for the different variants, as carbon
attachment is the limiting step in graphene growth (Lovinova, 2009b).
The growth of other variants can be suppressed if the development of
graphene edges not aligned to the dense-packed rows of the substrate can
be avoided, or at least largely postponed. For this, a fraction of the surface
Total amount
Graphene coverage (%)
100
80
60
40
20
0
0 1¥10
–7
2¥10
–7
3¥10
–7
Ethene dose (mbar.s)
10.12 Graphene coverage as a function of the ethene dose during
CVD growth of graphene on Ir(111), as estimated from STM
topographs, and exponential law fit (adapted from Coraux, 2009, ©
Institute of Physics).
ThinFilm-Zexian-10.indd 246 7/1/11 9:42:58 AM
247Epitaxial growth of graphene thin films on single crystal metal surfaces
© Woodhead Publishing Limited, 2011
might rst be covered by graphene islands having well-dened zigzag edges
following the recipe described in Section 10.3.2 (pre-adsorption of ethene at
room temperature followed by high temperature annealing). accordingly a
high density of islands is achieved, and the growth can be continued by CVD
at high temperature, yielding exclusively the more stable and energetically
preferred variant (van Gastel, 2009). In practice, the rst step may be
performed at 1200°C and the second at 800°C, which results in a single
crystallographic orientation of graphene across the whole sample surface. The
as-grown graphene has a low density of wrinkles (see Section 10.2.4) due
to the low growth temperature of 800°C during graphene layer completion.
The lower growth temperature induces less thermal lattice mismatch during
cooling and consequently less strain relieving defects.
The different variants have distinct stabilities, as suggested by the
preferential high temperature oxygen etching of the graphene variants of
which the zigzag edges do not align to dense-packed rows in the metal (van
Gastel, 2009; Starodub, 2010). This difference in reactivity was employed as
an alternative route to achieve macroscopic graphene samples with a single
orientation on Ir(111), via cycles of CVD growth with ethylene and oxygen
etching of the undesired variants (van Gastel, 2009) (Fig. 10.13).
10.3.3 Graphene multilayers on metals
Graphene multilayers are commonly obtained on metals which can store a
non-negligible amount of carbon in their bulk. This is the case for instance
for Ru, Ni or Co. Relatively slow cooling rates (typically 10°C/s, but this
gure depends on the amount of C stored in the metal) are usually employed
to promote the diffusion of carbon towards the bulk of the metal, leaving
only a limited amount of carbon close to the metal surface, which favours the
growth of few-layer and even single layer graphene (Yu, 2008; Reina, 2009b).
Too slow cooling rates leave too much time for carbon to diffuse towards
the metal bulk, resulting in negligible amounts of carbon near the surface
and accordingly no graphene growth by segregation. on the contrary, fast
cooling rates inhibit carbon diffusion towards the bulk, so that large amounts
of carbon are available close to the surface of the metal: this favours the
growth of multilayer and defective graphene. leeM showed that an additional
graphene layer starts to grow after each layer is completed (Sutter, 2009b,
2009c). So far the question of how the second layer of graphene grows after
the rst is completed remains open: on Pt(111) it was shown that the second
layer does not grow between the rst layer and the topmost Pt layer (Sutter,
2009a), pushing the rst layer upwards, which suggests that carbon atoms
are escaping the bulk towards the graphene surface via defects (e.g. holes)
present in the rst graphene layer. LEEM studies showed that the graphene
growth by segregation is mostly a bulk-diffusion limited process (McCarty,
ThinFilm-Zexian-10.indd 247 7/1/11 9:42:58 AM
