Czichos H., Saito T., Smith L.E. (Eds.) Handbook of Metrology and Testing
Подождите немного. Документ загружается.

428 Part C Materials Properties Measurement
to C [7.272]
D
eff
(C
2
) =
P
A
+ p
dP
A
dp
p
2
dp
dC
2
p
2
, (7.128)
which requires the pressure dependence of the per-
meability and solubility to calculate D
eff
. Methods to
measure pressure dependence of permeability and solu-
bility are discussed in this chapter.
Gas sorption isotherms in polymers are typically
described using simple models, such as Henry’s law,
the Flory–Huggins theory or the dual-mode sorption
model [7.264]. These models have been extensively in-
vestigated in the literature [7.273] and, therefore, will
only be briefly introduced. Once the gas sorption in
a polymer has been measured, values of dp/dC
2
can
be easily calculated for use in (7.128).
Henry’s law corresponds to a linear relationship be-
tween C and p [7.274]
C = k
D
p , (7.129)
where k
D
is the solubility coefficient, or Henry’s law
constant. This equation is typically valid for light gas
sorption in rubbery polymers at low concentrations,
such as 1 cm
3
(STP)/cm
3
polymer. For example, N
2
and O
2
sorption in poly(dimethylsiloxane) (PDMS)at
35
◦
C obey Henry’s law over a rather wide pressure
range [7.272].
For highly sorbing penetrants in rubbery polymers,
such as organic vapors, penetrant concentration in the
polymer is often described using the Flory–Huggins
model [7.274]
ln a = ln φ
2
+(1 −φ
2
)+χ(1 −φ
2
)
2
, (7.130)
where a is the penetrant activity, χ is the Flory–Huggins
interaction parameter, and φ
2
is the volume fraction of
penetrant dissolved in the polymer matrix. For ideal
gases, the activity is equal to p/p
o
, where p
o
is the
penetrant vapor pressure at the experiment temperature.
The volume fraction φ
2
is given by [7.274].
φ
2
=
C/22 414
V
2
1+
C/22 414
V
2
, (7.131)
where V
2
is the partial molar volume (cm
3
/mol) of
the penetrant in the polymer, and C has units of
cm
3
(STP)/(cm
3
polymer). For low concentrations of
sorbed gas, φ
2
1, (7.130)and(7.131) reduce to
Henry’s law [7.275]. For high concentrations of sorbed
gas, this model predicts an increasing value of solubil-
ity (i. e., C/ p) as pressure increases. Equation (7.130)
successfully describes the sorption of strongly sorb-
ing penetrants in rubbery polymers, such as CO
2
in
poly(ethylene oxide) at 35
◦
C[7.275].
The dual-mode sorption model was originally used
to describe gas sorption in glassy polymers, which
contain both equilibrium volume (i. e., Henry’s law
sorption sites) and nonequilibrium excess volume (i. e.,
microvoids or Langmuir sorption sites). The model is
expressed as follows [7.276]
C =C
D
+C
H
=k
D
p +
C
H
bp
1 +bp
, (7.132)
where C
D
and C
H
are the gas concentrations in the
Henry’s law sorption sites and Langmuir sites, re-
spectively, b is the Langmuir affinity constant, which
characterizes the affinity between penetrants and mi-
crovoids, and C
H
is the Langmuir capacity constant
[cm
3
(STP)/cm
3
polymer], which characterizes the
maximum sorption capacity of the nonequilibrium ex-
cess volume. This model has also been applied to
describe the gas sorption in hybrid systems such as
blends of rubbery polymers and additives [7.277, 278].
These additives could be fillers that physically adsorb
the gas or dissolved species that chemically interact
with the gas.
The dependence of permeability on pressure is of-
ten described using empirical equations. For example,
the following equation is often used to describe the
pressure dependence of gas permeability in rubbery
polymers [7.272,275]
P
A
= P
A0
(1 +mΔp) , (7.133)
where P
A0
and m are constants, and Δp = p
2
− p
1
.For
glassy polymers at p
1
=0 (or, equivalently, Δ p = p
2
),
the following equation, based on the dual-mode model,
has been used [7.279]
P
A
=k
D
D
1 +
FK
1+bp
2
, (7.134)
where K =C
H
b/k
D
,andD and F are constants ob-
tained by fitting the model to experimental sorption and
permeation data. For example, Koros and coworkers ob-
tained k
D
, b and C
H
by fitting gas sorption isotherms
to (7.132) and then fitting the permeability data at var-
ious upstream pressures to (7.134) to obtain D and
F [7.279]. Equation (7.133)or(7.134) can be used to
calculate dP
A
/dp, which can be substituted into (7.128)
along with dp/dC
2
to calculate D
eff
.
The ideal selectivity of a membrane for gas A over
gas B (i. e., α
A/B
) is the ratio of their pure-gas perme-
Part C 7.6
Mechanical Properties 7.6 Permeation and Diffusion 429
abilities [7.262]
α
A/B
=
P
A
P
B
=
D
A
D
B
×
S
A
S
B
, (7.135)
where D
A
/D
B
is the diffusivity selectivity, which is the
ratio of the diffusion coefficients of gases A and B. The
ratio of the solubility coefficients of gases A and B,
S
A
/S
B
, is the solubility selectivity. For a binary gas
mixture, the selectivity of gas A to gas B (α
∗
A/B
)is
defined as
α
∗
A/B
≡
y
A
/y
B
x
A
/x
B
=α
A/B
Δp
A
/x
A
Δp
B
/x
B
, (7.136)
where y
i
and x
i
are the mole fractions of component i in
the upstream and downstream gas phases, respectively,
and Δp
i
is the partial pressure difference of compo-
nent i across the membrane. When the downstream
pressure is much less than the upstream pressure, α
∗
A/B
approaches the ideal separation factor α
A/B
.
7.6.2 Kinetic Measurement
The kinetics of gas transport through a thin uniform film
have been modeled, and such measurements can yield
solubility, diffusivity and permeability in a single ex-
periment. The penetrant concentration as a function of
time and position in the film is given by Fick’s second
law [7.270]
∂C
∂t
=
∂
∂x
D
eff
∂C
∂x
. (7.137)
The analytical solution of (7.137) depends on initial
conditions, boundary conditions and the concentra-
tion dependence of the diffusion coefficient, if any.
The diffusion coefficient could be a function of con-
centration, which makes the solution to (7.137)more
complex [7.261]. However, Crank and Park have shown
that solutions to (7.137) assuming constant D
eff
val-
ues are also applicable when the diffusion coefficient
depends on concentration [7.268]. In general, this
approximation is sufficiently accurate to model experi-
mental data [7.268], except that the diffusion coefficient
obtained from such an analysis should be interpreted
as an average effective diffusion coefficient as defined
in (7.126).
Constant Diffusion Coefficient
In the simplest scenario, the gas diffusion coefficient
is constant over the concentration range of interest.
This assumption is usually valid for low-sorbing pene-
trants in rubbery polymers. However, care must be taken
since gas diffusion coefficients can exhibit concentra-
tion dependence at concentrations where gas sorption
still obeys Henry’s law, i. e., when gas solubility is in-
dependent of pressure [7.280]. Nevertheless, in a typical
experiment, initially (i. e., at t = 0) the membrane is at
a uniform concentration C
0
.Att > 0, one face (x =0)
of the membrane is exposed to a constant concentra-
tion C
2
by changing the gas pressure in contact with
the membrane, and the other face at x =l is exposed
to C
1
[7.261]. The analytical solution to (7.137) under
these conditions is [7.261]
C = C
2
+
C
1
−C
2
x
l
+
2
π
∞
n=1
C
1
cos nπ −C
2
n
×sin
nπ x
l
exp
−Dn
2
π
2
t/l
2
+
4C
0
π
∞
m=0
1
2m +1
sin
2m +1
πx
l
×exp
−D
2m +1
2
π
2
t/l
2
. (7.138)
The direct measurement of penetrant concentration in
the film as a function of position and time is very difficult
and is usually not done. Instead, a much more straight-
forward experiment, measurement of the gas flux out of
the film is usually performed. Two methods are typically
used to monitor this kinetic process: (1) transient perme-
ation, and (2) sorption (or desorption).
In a transient permeation study, C
0
and C
1
are
zero. The gas molecules diffusing out of the mem-
brane at x =l (i. e., the downstream side) are collected
in a closed volume of known size. The gas flux [i. e.,
−D(∂C/∂x)
x=l
] can be easily calculated from the rate of
gas accumulation in the closed volume. The total amount
of gas transported across the membrane at time t, Q
t
,is
given by
Q
t
=
t
0
−D
∂C
∂x
x=l
dt
=
DtC
2
l
−
lC
2
6
−
2lC
2
π
2
×
∞
n=1
−1
n
n
2
exp
−Dn
2
π
2
t/l
2
, (7.139)
which reduces to the following equation when the gas
flux reaches a steady state or, equivalently, as t →∞
Q
t
=
DC
2
l
t −
l
2
6D
.
(7.140)
Part C 7.6
430 Part C Materials Properties Measurement
Verification
curve
Time
θ
Q
t
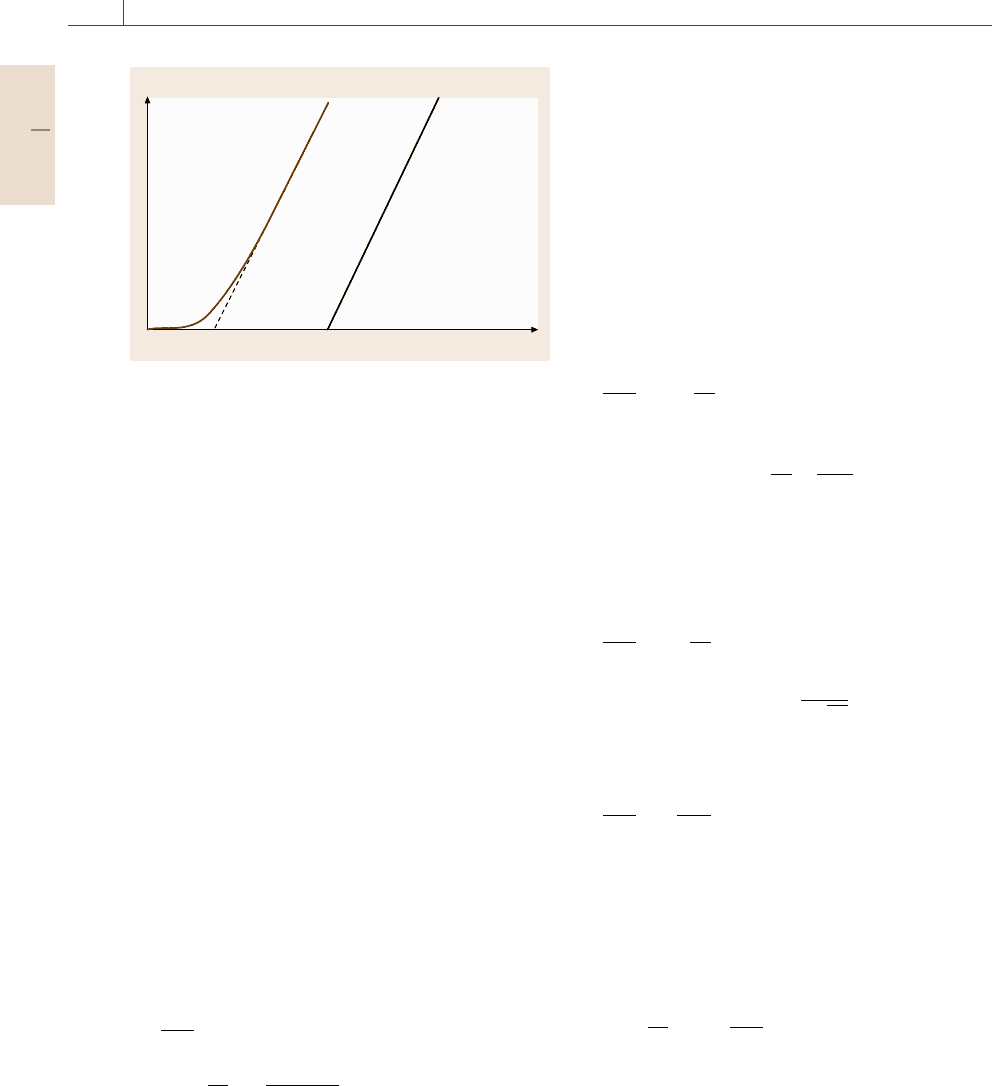
Fig. 7.106 A typical curve for the amount of accumulated
permeate Q
t
as a function of time in a transient permeation
experiment
A typical curve for the amount of accumulated gas as
a function of time appears in Fig. 7.106. The intercept
of the steady-state region of Q
t
with the time axis (i. e.,
l
2
/6D) is defined as the time lag θ. The diffusion coeffi-
cient is given by
D =l
2
/6θ. (7.141)
The above theoretical analysis was developed by Daynes
in 1920 [7.281]. In 1939, Barrer and coworkers reported
a system to measure the time lag and steady-state flow
in polymers. Using this instrument, permeability, dif-
fusivity and solubility can be estimated from a single
experiment [7.282].
In a kinetic sorption or desorption study, the migra-
tion of gas into or out of a film is monitored by measuring
the weight change of the polymer film containing the
sorbed gas as a function of time. Typically, a polymer
film with an initial gas concentration C
0
is suddenly ex-
posed to an environment with a constant gas pressure
p
2
,i.e.,att > 0, C = C
2
at x = 0andl. C
2
is the con-
centration of gas in the polymer in equilibrium with the
applied gas pressure p
2
.WhenC
2
> C
0
, sorption occurs,
and penetrant diffuses into the film. Desorption happens
when C
2
< C
0
. The kinetics of these processes are given
by [7.261]
M
t
M
∞
=1−
8
π
2
∞
n=0
1
2n +1
2
exp
−D
2n +1
2
π
2
t/l
2
,
(7.142)
where M
t
and M
∞
denote the total amount of penetrant
sorbed or desorbed in the film at time t and at equilib-
rium, respectively. For a film of cross-sectional area A
and thickness l, M
t
is given by
M
t
=
A
l
0
C
x, t
dx −C
0
Al
(7.143)
and M
∞
is given by
M
∞
=
|
C
2
−C
0
|
Al . (7.144)
Therefore, by determining the weight change of the
sample with time, diffusion coefficients can be ob-
tained by fitting the experimental data to (7.142). When
M
t
/M
∞
> 0.7, all terms with n > 0in(7.142) can be
neglected, and (7.142) becomes
M
t
M
∞
=1 −
8
π
2
exp
−Dπ
2
t/l
2
, (7.145)
which can be rearranged to [7.261]
ln
1−M
t
/M
∞
= ln
8
π
2
−
Dπ
2
l
2
t . (7.146)
In this way, the diffusion coefficient can be easily ob-
tained by fitting the experimental data to (7.146).
Another way to analyze kinetic sorption data is based
on an alternative analytical solution to (7.138), which
is [7.261]
M
t
M
∞
=4
Dt
l
2
1/2
π
−1/2
+2
∞
n=1
−1
n
i erfc
nl
2
√
Dt
. (7.147)
The above solution can be simplified to the following
equation at short times (i. e., M
t
/M
∞
< 0.6)
M
t
M
∞
=
16D
πl
2
1/2
t
1/2
. (7.148)
Equation (7.148) predicts that the amount of penetrant
sorbed or desorbed exhibits a linear dependence on t
1/2
,
and the diffusion coefficient can be calculated from the
slope of the fractional uptake (or desorption) kinetics
versus t
1/2
.
In general, penetrant solubility can be derived from
M
∞
and the initial concentration C
0
S
A
=
1
p
2
C
0
±
M
∞
Al
, (7.149)
where p
2
is the gas-phase pressure at equilibrium. The
+sign is for sorption, and the −sign is for desorption.
Variable Diffusion Coefficient
In many situations, penetrant diffusion coefficients
vary with concentration [7.261]. For example, strongly
Part C 7.6

Mechanical Properties 7.6 Permeation and Diffusion 431
sorbing penetrants in rubbery or glassy polymers
often swell the polymer at sufficiently high activ-
ity, leading to changes in polymer properties (e.g.,
glass-transition temperature, fractional free volume,
etc.) and penetrant diffusion coefficients [7.264].
Frisch developed an explicit expression for the time
lag when the diffusion coefficient is concentration-
dependent [7.283]. Crank treated this subject in detail
using both analytical and numerical methods [7.261].
Strictly speaking, most of these treatments require
knowledge of the functional form of the relation-
ship between diffusion coefficient and concentra-
tion [7.261]. In contrast, the steady-state measure-
ments discussed in Sect. 7.6.1 can provide a reliable
way to evaluate diffusion, solubility and permeability
coefficients without making a priori assumptions re-
garding the concentration dependence of the diffusion
coefficient.
Various experimental approaches have been re-
ported to measure gas transport through polymeric
films. In principle, most of the techniques directly mea-
sure gas flux through the film or gas uptake by the
film (i. e., solubility). Diffusion coefficients are typi-
cally derived indirectly from permeability and solubility
measurements based on the theory introduced earlier. In
the following sections, general strategies are described
for measuring gas transport properties in polymers.
7.6.3 Experimental Measurement
of Permeability
Film Preparation
The measurement of gas flux requires the success-
ful preparation of a uniform, nonporous thin film. In
general, polymer films for permeation testing have
thicknesses of less than 250 μm, unless special situa-
tions require a thicker film. For example, thicker films
may make it easier to prepare nonporous specimens
for study, or they may be used to increase the time
lag when studying penetrants with high diffusion co-
efficients (e.g., He or H
2
). Uniformity of thickness is
important for reducing the uncertainty in permeability,
as illustrated in (7.124). A nonporous film is required
if one wishes to determine the inherent properties of
the material under study, because gas flow through
a pinhole obeying Knudsen diffusion can be orders of
magnitude faster than that through a dense film obeying
the solution–diffusion mechanism [7.258]. Thus, any
pinhole defects in a sample can compromise the accu-
rate measurement of gas flux through a polymer film.
The first challenge in successfully measuring gas per-
meation properties typically lies in the ability to prepare
uniform, pinhole-free films.
Polymer films are typically prepared in the labora-
tory by melt-pressing and solvent-casting [7.284, 285].
Melt-pressing is used less often than solvent-casting
to prepare films for study. In melt-pressing, one ap-
plies heat to melt a polymer powder, and then a film is
made by pressing the molten polymer under high pres-
sure. Solvent-casting is a widely used method to prepare
films. In this method, solid polymer is dissolved in a sol-
vent, the resulting solution is cast on a leveled support,
and the solvent is allowed to evaporate slowly, leaving
a solid film behind.
Gas permeation properties of films are often influ-
enced by many processing factors. One critical factor
is the solvent used in preparing the film, which can
strongly affect polymer morphology and, in turn, gas
permeation properties. For example, poly(4-methyl-1-
pentene) films were prepared by solvent-casting using
chloroform and carbon tetrachloride [7.285]. The N
2
permeability at 35
◦
C and 2 atm is 1.1 Barrers for
a film prepared from chloroform solution, but it is
6.0 Barrers for a similar film prepared from carbon
tetrachloride solution, even though the two films ex-
hibit almost the same crystallinity values (i. e., 57%
and 56%, respectively) [7.285]. Other possible factors
influencing gas transport properties include polymer
concentration, evaporation temperature, annealing con-
ditions, etc. Pinholes in a solid film can be introduced
during the solvent-casting process by bubbles present
in the solution before casting, the presence of insoluble
impurities in the solution (such as dust), and very rapid
solvent evaporation. Typically, before casting, the solu-
tion is filtered to remove any impurities, and the solvent
evaporation rate is controlled during film drying. For
highly crystalline polymers such as poly(ethylene ox-
ide), thermal annealing above the melting temperature
could be critical to form defect-free films, although the
reason for this is not clear [7.275].
Uniform thickness films can be obtained by spread-
ing the solution onto a support using tools such as
a Gardner knife or a doctor blade, which controls the
liquid film thickness [7.284]. Alternatively, the solution
may be poured into a glass ring resting on a flat solid
support. The glass ring is typically stuck to the sup-
port using silicon caulk to achieve a leak-free boundary.
The film thickness is controlled by the amount of liquid
solution added and its concentration. The solid support
needs to be leveled to obtain films of uniform thickness.
The choice of the support material can be important. If
the solution cannot wet the support, the liquid polymer
Part C 7.6
432 Part C Materials Properties Measurement
solution will bead up, and it will not form a continu-
ous film. If the solid film adheres too strongly to the
support, it can be difficult or impossible to remove the
film intact from the support. Common supports are glass
plates, Teflon plates, metal plates, plastic plates (which
do not interact with the solvent) and even liquid surfaces
such as water and mercury [7.284].
Permeation Cell
Gas permeability is the steady-state flux through a film
normalized by the pressure difference and film thick-
ness, as indicated in (7.124). Pressure is measured using
commercial sensors or gauges with high precision in
different pressure ranges. For example, Dresser Instru-
ments (Shelton, USA) provides a digital indicator for
pressure measurement with an accuracy of 0.025% of
full scale over the pressure range 0–20 atm. Film thick-
ness can be measured using a digital micrometer (Mi-
tutoyo Corp., Japan), which can measure thickness to
the nearest micrometer. To measure the thickness of rub-
bery polymers, the film should be coveredwith a uniform
thin plate (quartz, glass or other flat, rigid materials) to
prevent direct contact of the measuring tip of the digital
micrometer with the soft film. The measurement tip can
press into flexible films or even penetrate them; in either
case, the thickness value provided by the micrometer is
an underestimate of the true film thickness.
The gas flux through the film, which is the key
parameter for permeability measurements, is typically
measured from the permeate side (x =l) using a perme-
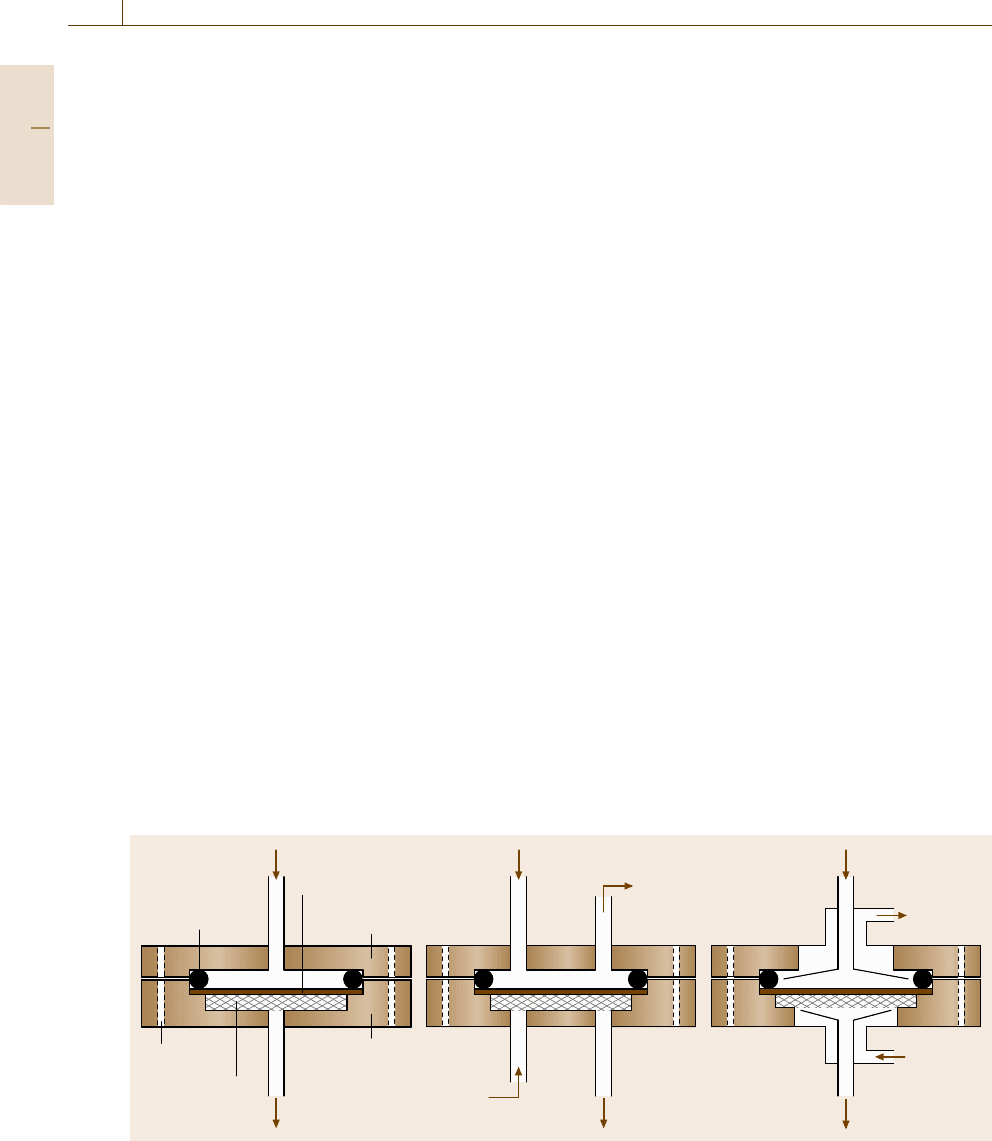
ation cell. Figure 7.107a–c presents schematics of typ-
ical permeation cell designs for constant-pressure var-
iable-volume, constant-volume variable-pressure and
mixed-gas systems, respectively. These cells are com-
O-ring
Feed
Membrane
Permeation
cell
Feed
Residue
Feed
Residue
Sweep in
Sweep out
Permeate
Purge
Permeation
cell
Permeate
Metal mesh
support
Flange
a) c)b)
Fig. 7.107a–c Schematic of a typical permeation cell (a) for pure-gas measurements using the continuous flow method;
(b) for pure-gas measurements using the constant-volume variable-pressure method and (c) for mixed-gas measurements
mercially available from Millipore Corporation (Biller-
ica, USA) under the tradename of high-pressure filter
holder. A flat polymer film with the same size as the inner
diameter of the cell is placed on a sintered-metal sup-
port inside the cell, dividing the system into upstream
(i. e., above the film) and downstream (i. e., below the
film) compartments. A silicon or Viton O-ring is often
used to prevent gas leakage at the film edge from the
upstream (i. e., high-pressure) to the downstream (i. e.,
low-pressure) side of the cell and to avoid gas exchange
between the upstream portion of the cell and the exterior
atmosphere. In this way, only the feed gas permeating
through the film is collected in the downstream section
of the cell. For a pure-gas study, the upstream portion of
the cell can be designed as a dead end, and gas permeat-
ing into the downstream side of the film will be collected
as illustrated in Fig. 7.107a and b. For mixed-gas stud-
ies, the gas flow pattern requires a special cell design to
minimize gas composition changes in the upstream and
downstream chambers of the permeation cell. The up-
stream gas enters the cell at the center of the film, flows
radially to the film edge and then flows out of the cell,
which eliminates any trapped gas or dead zones in the
cell [7.286]. Typically, trapped gas does not mix well
with the entering feed gas, which results in gas concen-
tration variations across the film and, therefore, errors in
estimating the partial pressure that the film experiences
during the measurement. The design of the permeate side
follows similar principles. Additionally, to reduce the
change of feed gas composition during flow from the
film center to the edge, due to the preferential permeation
of a gas component, the gas flow rate in the upstream
is much higher than the gas flow rate due to permeation
through the film. Typically, the ratio of the permeate gas
Part C 7.6
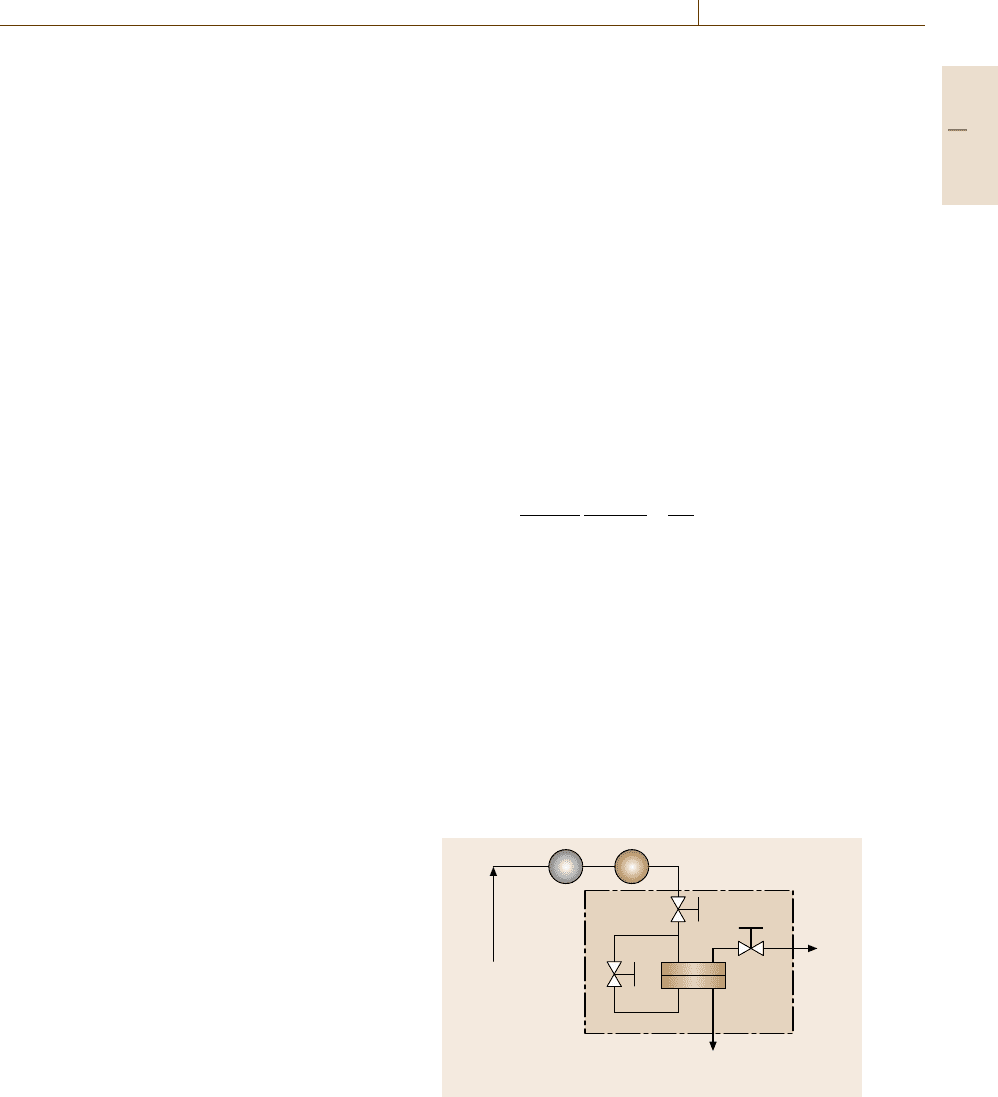
Mechanical Properties 7.6 Permeation and Diffusion 433
flow rate to feed gas flow rate (the so-called stage cut)
wouldbe1%orless.
Films are typically supported on filter paper and then
on a sintered-metal support. In general, the mass transfer
resistance of the paper and the metal support should be
negligible relative to that of the polymer film so that the
measured gas flux reflects only the inherent properties of
the film. The practical film area available for gas diffu-
sion is not the same as the area of the entire film, because
the contact area between the O-ring and the film area is
not accessible to gas diffusion. Typically, after the cell
is clamped in place for measurements, the O-ring would
leave an imprint on the film. The inside diameter of the
imprint is often used to calculate the active test area. In
many cases (for example, films that are too soft or vis-
cous or when the area of the films is smaller than the cell
size), films need to be partially masked using imperme-
able aluminum tape so that there is no direct contact of
the O-ring with the films [7.275,287, 288].
Temperature Control
Thermal uniformity is an important factor since gas per-
meability can depend strongly on temperature [7.265].
Two typical media are used as temperature-control flu-
ids: air and liquids. An air bath can conveniently provide
operational temperatures above room temperature. Such
a system includes a fan to circulate air past a heating
element and the permeation instrumentation, a heater
element (which can be as simple as a light bulb for
temperatures near ambient or commercially available
heating elements from, for example, Omega Engineering
Inc. (Stamford, USA)) and a temperature control system.
Commercially available temperature controllers, such as
a CN76000 microprocessor-based temperature/process
controller made by Omega Engineering, work well
for this purpose. A liquid bath, such as a mixture of
methanol and water (50/50 by weight) can provide tem-
peratures as low as −30
◦
C. For low-temperature opera-
tions, cooling is typically accomplished using a chiller.
Generally, good circulation in the bath is required to
achieve a uniform temperature around the cell and other
components of the measurement system.
7.6.4 Gas Flux Measurement
Gas flux is commonly measured using one of the fol-
lowing three methods: (1) constant-pressure variable-
volume, (2) constant-volume variable-pressure and
(3) a method using a special sensor for mixed-gas per-
meation measurements. These techniques are discussed
below.
Constant-Pressure Variable-Volume Method
A schematic of a constant-pressure variable-volume
(or continuous flow) permeation system is shown in
Fig. 7.108. A schematic of a typical cell for such a sys-
tem is given in Fig. 7.107b. The feed gas enters the cell
and leaves in the residue stream, and the downstream
is typically purged using the feed gas before beginning
measurements to remove any impurities from the down-
stream. This apparatus operates by applying a target gas
at a constant pressure to the upstream face of the film
and measuring the resulting steady-state gas flux on the
downstream side of the film or membrane being tested
using a flow meter. Electronic flow meters are commer-
cially available, such as the Agilent model ADM1000
(Wilmington, USA). However, a simple and convenient
device is a soap-bubble flow meter (Alltech Associates,
Inc. Deerfield, USA). By timing the movement of the
soap film in a graduated capillary tube, the steady-state
permeability (cm
3
(STP)cm/cm
2
scmHg)isgivenby
P
A
=
l
p
2
− p
1
273p
atm
TA76
dV
dt
, (7.150)
where the downstream pressure p
1
is atmospheric pres-
sure (cmHg) in this case, p
atm
is the atmospheric
pressure (cmHg), T is the absolute temperature of the
gas in the bubble flow meter (K), and dV/dt is the
steady-state volumetric displacement rate of the soap
film (cm
3
/s). The accuracy of this method depends
on the sensitivity of the bubble flow meter. Typically,
the accuracy of the bubble flow meter becomes higher
as the capillary tube diameter decreases. This method
is typically used to study polymeric films with high
gas fluxes, such as poly(dimethylsiloxane) (PDMS),
poly(1-trimethlsilyl-1-propyne) (PTMSP) and compos-
Gas cylinder
Vent
Cell
Valve
PR
Bubble flow
meter
Fig. 7.108 Schematic of a constant-pressure variable vol-
ume apparatus for gas permeability measurements (R: reg-
ulator; P: pressure transducer). The parts within the dashed
box are in a temperature-controlled chamber
Part C 7.6
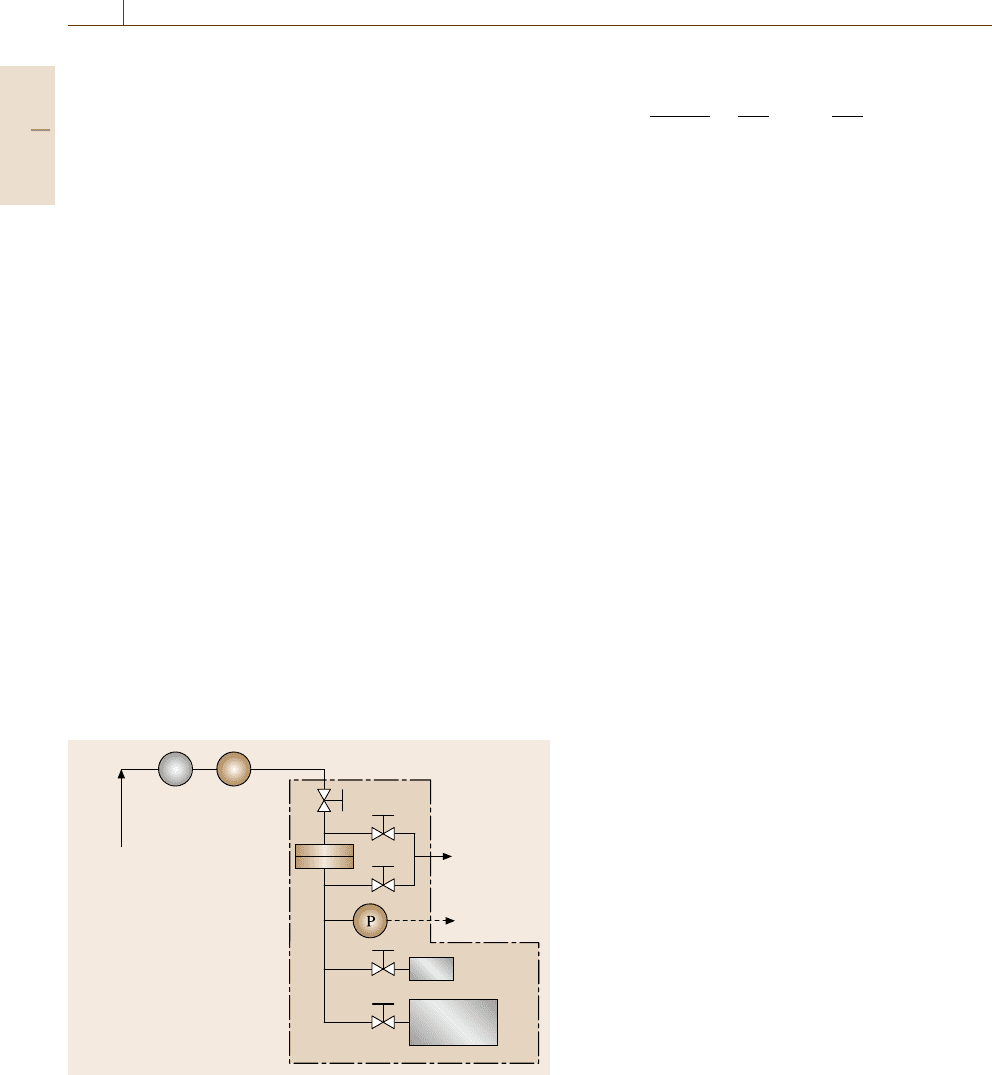
434 Part C Materials Properties Measurement
ite membranes with very thin polymer coatings on
porous membrane supports [7.289]. The authors have
good experience with such materials and bubble flow
meters with ranges such as 0–1.0ml, 0–10ml and
0–100 ml, which are made by Alltech Associates, Inc.
(Deerfield, USA).
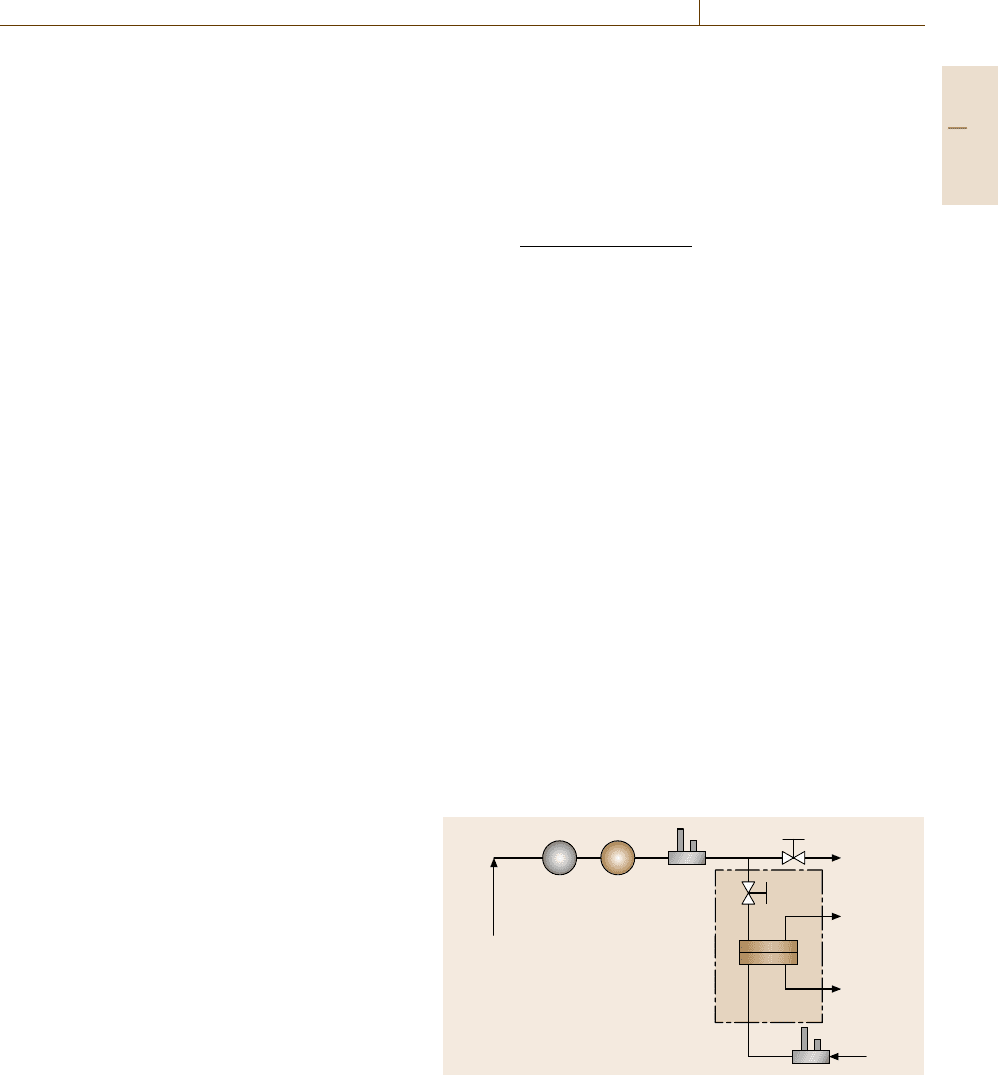
Constant-Volume Variable-Pressure Method
As illustrated in Fig. 7.109, a constant-volume variable-
pressure system measures permeate flux by monitoring
the pressure increase of collected permeate gas in
a closed volume using a pressure transducer. A typi-
cal cell is presented in Fig. 7.107a. Unlike the cell used
for the continuous-flow method, the cell used here does
not require a residue or purge stream since any volatile
impurities or air gases can be removed from the sam-
ple by exposing the whole system to vacuum prior to
beginning a measurement. The apparatus operates by
initially evacuating the upstream and downstream vol-
umes to degas the film. Then, the valve connecting the
permeation cell to the vacuum pump is closed, and
a slow pressure rise in the downstream volume (i. e.,
the leak rate of the system) is observed. This rate of
pressure rise should be at least ten times less than the
estimated steady-state rate of pressure rise due to per-
meation to obtain accurate permeability estimates. The
feed gas is then introduced to the upstream side of the
membrane, and the pressure rise in the downstream vol-
ume is recorded as a function of time. Gas permeability
(cm
3
(STP)cm/(cm
2
s cmHg)) is calculated from the
R
P
Gas cylinder
Cell
1
2
Va lve
Vacuum
pump
Computer
V
0
V
1
V
2
Fig. 7.109 Schematic of a constant-volume variable-pressure appa-
ratus for gas permeability measurements (R: regulator; P: pressure
transducer; V
0
, V
1
and V
2
: tubing volume, volumes 1 and 2,
respectively). The parts within the dashed box are in a temperature-
controlled chamber
following expression [7.275, 290]
P
A
=
V
d
l
p
2
ART
dp
1
dt
ss
−
dp
1
dt
leak
, (7.151)
where V
d
is the downstream volume (cm
3
), l is the film
thickness (cm), p
2
is the upstream absolute pressure
(cmHg), A is the film area available for gas trans-
port (cm
2
), the gas constant R is 0.278 cmHg cm
3
/
(cm
3
(STP)K), T is absolute temperature (K) and
(dp
1
/dt)
ss
and (dp
1
/dt)
leak
are the steady-state rates
of pressure rise (cmHg/s) in the downstream vol-
ume at fixed upstream pressure and under vacuum,
respectively. The downstream pressure must be kept
much lower than the upstream pressure to maintain an
effectively constant pressure difference across the mem-
brane. In our laboratory, the downstream pressure is
typically lower than 0.03 atm, compared with a typi-
cal upstream pressure of 4.4 atm or more. If steady-state
permeation cannot be achieved before the downstream
pressure increases to a significant value, the down-
stream should be evacuated using the vacuum pump,
and the recording of the pressure rise in the downstream
volume is restarted. Additionally, the design of the
downstream in Fig. 7.109 allows one to choose a suit-
able downstream volume to achieve a reasonable rate of
pressure rise for films of different fluxes. If the flux is
low, the smallest volume (i. e., the volume of the down-
stream tubing only) can be used to observe the pressure
rise. On the other hand, one or two additional volumes
can be used if the flux is high.
In this method, the upstream and downstream pres-
sures can be accurately measured using commercially
available pressure gauges or transducers. The key pa-
rameter to be measured is the downstream volume, for
example V
0
(defined as the tubing volume), which in-
cludes part of the permeation cell (i. e., beneath the
film), tubing, tubing connections and valves, as illus-
trated in Fig. 7.109. The direct measurement of V
0
is
impossible. One way to circumvent this issue is to mea-
sure volume 1, V
1
, including a short section of tubing
and a vessel (as illustrated in Fig. 7.109) first by liquid
filling followed by Burnett gas expansion [7.291]. Vol-
ume 1 is filled with a volatile liquid, such as methanol,
and the value of V
1
equals the volume of the liquid re-
quired to completely fill volume 1. To obtain the value
of V
0
, an impermeable aluminum foil film is installed in
the permeation cell to isolate the downstream from the
upstream. Initially, V
0
and V
1
are under vacuum (i. e.,
the pressure is 0 cmHg). Volume 1 is isolated from V
0
by turning off valve 1, and gas is introduced into the tub-
ing at a pressure of p
0
(which is typically much lower
Part C 7.6
Mechanical Properties 7.6 Permeation and Diffusion 435
than 1 atm). Light gases such as N
2
and He are typically
used since they exhibit ideal gas behavior at subatmo-
spheric pressure. After the value of p
0
stabilizes, the
valve is opened, and the gas expands into volume 1; the
pressure reaches p
f
in both volumes. Assuming ideal
gas behavior, the ratio of the two volumes is given by
V
1
/V
0
= p
0
/p
f
−1 , (7.152)
which permits one to obtain an accurate value of the
tubing volume. Following the same procedure, V
2
can
be estimated. V
2
can also be estimated using the liquid-
filling method.
To simplify the data acquisition process, automatic
recording of the downstream pressure can be employed.
Typically, transducers (such as those from MKS Instru-
ments, Wilmington, MA, USA) used in the downstream
sense the pressure and give an analog voltage output
signal. The pressure is typically linearly related to the
transducer output voltage, and this relationship can be
obtained by calibrating the transducer using a standard
pressure indicator. The voltage signal can be recorded
using a chart recorder. More conveniently, the signal
can be digitized and monitored using a computer via
data-acquisition programs such as Labtech Notebook
(Laboratory Technologies Corp., Andover, MA, USA)
or Labview (National Instruments Corp., Austin, TX,
USA), which show and record the pressure in real time
and allow one to analyze the data with widely used soft-
ware such as Microsoft Excel. The required hardware,
including computer boards, terminals, readouts etc., is
provided by commercial sources, such as Measurement
Computing Corp. (Middleboro, MA, USA).
Due to the ability to measure pressure accurately,
the constant-volume variable-pressure system is able to
measure a wide range of flux values, including mater-
ials exhibiting flux values too low to be measured by
a constant-pressure variable-volume system.
Mixed-Gas Permeability Measurement
The measurement of mixed-gas permeability coeffi-
cients follows principles similar to those used for
pure-gas permeability measurements, except that the
mixed-gas measurement requires a sensor to detect gas
concentration in the feed, residue and permeate streams.
A gas chromatograph (GC) is the most widely used con-
centration detection method [7.290, 292]. A schematic
of a mixed-gas permeation instrument is provided
in Fig. 7.110 [7.289]. In general, the permeate is swept
away from the membrane surface using a carrier gas
(e.g., He, H
2
or N
2
) at a pressure of about 1 atm. The
preferred carrier gas should have a thermal conductivity
that is very different from that of the permeate gas, since
thermal conductivity detectors are widely used for con-
centration detection in the GC. Consequently, helium or
hydrogen is often used as the carrier gas [7.293]. By
measuring the permeate gas concentration in the sweep
gas and the sweep gas flow rate (i. e., S), permeability
can be calculated as follows [7.289]
P
A
=
x
1A
S
F
l
x
P
He
A
p
2
x
2A
− p
1
x
1A
(7.153)
where x
1A
and x
P
He
are the mole fractions of compo-
nent A and helium in the permeate stream, respectively;
x
2A
is the mole fraction of component A in the feed gas.
Mixed-gas measurements require a specially de-
signed permeation cell, as illustrated in Fig. 7.107c, to
ensure good mixing in the gas phase above and below
the membrane. Low stage-cut values (the stage cut is the
ratio of the permeate gas flow rate to the feed gas flow
rate) are used to improve mixing efficiency on the feed
side of the membrane. Typically, the stage cut is set to
be less than 1%; that is, the permeate flow rate is less
than 1% of the feed flow rate. Additionally, this method
can be used for pure-gas studies. Compared with the
continuous flow system described earlier (i. e., constant
pressure/variable volume system), this apparatus is able
to measure low permeate flux, mainly because of the
high sensitivity of GC for gas composition detection
and the ease of measurement of the high sweep gas flux
using a bubble flow meter.
An alternative technique can also be used for mixed-
gas permeability measurement [7.294]. Instead of using
a carrier gas on the permeate side of the membrane,
a closed downstream volume is used. When the gas flux
Gas cylinder
R P
MFC
Cell
Carrier gas
GC
Vent/GC
Vent/GC
Fig. 7.110 Schematic of a mixed-gas permeability apparatus for gas
permeability measurements using a gas chromatograph for con-
centration detection (R: regulator; P: pressure transducer; MFC:
mass-flow controller; GC: gas chromatograph). The parts within the
dashed box are in a temperature-controlled chamber
Part C 7.6

436 Part C Materials Properties Measurement
reaches steady state, as detected by monitoring the rate
of downstream pressure rise, the downstream is evacu-
ated to remove the gas accumulated during nonsteady-
state permeation. Afterwards, the downstream volume
is isolated, and pressure in this volume increases. Then,
a valve is opened, and the gas expands into an evacuated
line connecting the gas chromatograph to the down-
stream volume. The gas permeability can be calculated
from the steady-state rate of pressure rise in the down-
stream volume and the corresponding composition of the
permeate gas determined using the GC.
Besides GC, other sensors have been employed for
gas permeation studies. For example, oxygen transmis-
sion across films is an important property in food pack-
aging, since the exposure of food and beverages to oxy-
gen can significantly influence their shelf life [7.257].
The American Society for Testing and Materials
(ASTM) standard F1307 describes a constant-volume
variable-pressure system which employs an oxygen sen-
sor to detect the oxygen concentration in the downstream
volume [7.295]. Coupled with the rate of pressure rise
in the downstream volume, O
2
permeability can be eas-
ily calculated using (7.151). This method is particularly
convenient for mixed gas feeds containing O
2
and other
species, where only the O
2
permeability is of interest.
Transient Transport Measurement
Based on the pioneering work by Daynes and Bar-
rer [7.281,282], transient transport measurements (time
lag) have come to be widely practised. The most
common system used to detect the time lag is the
constant-volume variable-pressure system [7.266], al-
though systems employing a gas chromatograph have
also been reported [7.292]. As illustrated in Fig. 7.106,
the time lag is defined as the intercept of the ex-
trapolated linear pseudo-steady-state region of the
downstream pressure rise back to the time axis. A key
issue is to ensure that the experiment has reached steady
Gas
cylinder
Vacuum
Valve
Volume
Polymer
Computer
PP
Fig. 7.111 Schematic of a barometric pressure decay apparatus for
gas sorption measurement (dual-volume dual-transducer system)
(P: pressure transducer). The parts within the dashed box are in
a temperature-controlled chamber
state. Koros and Zimmerman proposed a procedure il-
lustrated in Fig. 7.106 [7.266]. The experiment is run
for a period that is approximately five to six time lags,
then the downstream volume is evacuated, and the mea-
surement is restarted. If the slope of the downstream
pressure rise versus time is consistent with the rate of
downstream pressure rise measured before evacuation,
then the experiment is considered to be at steady state.
This technique requires films having good mechan-
ical properties to sustain the pressure difference across
the sample and a leak-free downstream volume. To in-
crease the sensitivity of this technique, i. e., to increase
the time lag, thicker films can be used. As illustrated
in (7.141), time lag increases with the square of the film
thickness. This strategy is especially useful for studies
of small penetrants such as hydrogen and helium, which
typically have high diffusion coefficients and, therefore,
exhibit time lag values that are often too short to be
detected. On the other hand, (7.141) suggests that film
thickness uniformity is very important for reducing the
uncertainty of this measurement.
7.6.5 Experimental Measurement of Gas
and Vapor Sorption
The sorption of gas in a polymer film is usually detected
by either a decrease of gas pressure (i. e. gas-phase con-
centration) in the environment surrounding a polymer
in a closed chamber or the weight increase of a polymer
film due to gas sorption. An apparatus is typically de-
signed to measure changes in either the amount of gas in
the gas phase or the weight change of the polymer film
due to the sorption or desorption of the penetrant. In the
following section, common methods of measuring gas
sorption are described. They include the dual-volume,
pressure-decay method, the gravimetric method, and in-
verse gas chromatography.
The Dual-Volume Pressure-Decay Method
The dual-volume pressure-decay method employs dual
transducers and dual cells, and is widely used to deter-
mine pure-gas solubility in polymeric samples [7.296,
297]. A schematic diagram of such a system is shown
in Fig. 7.111. The system consists of a sample cell con-
taining a polymer sample and a charge cell connected to
a gas cylinder. In the first step, the sample cell is evac-
uated and then isolated from the charge cell, which is
subsequently charged with a target gas at a known pres-
sure. The valve between the charge and sample cells is
opened briefly to introduce gas to the sample cell. The
valve between the sample and charge cells is closed, and
Part C 7.6
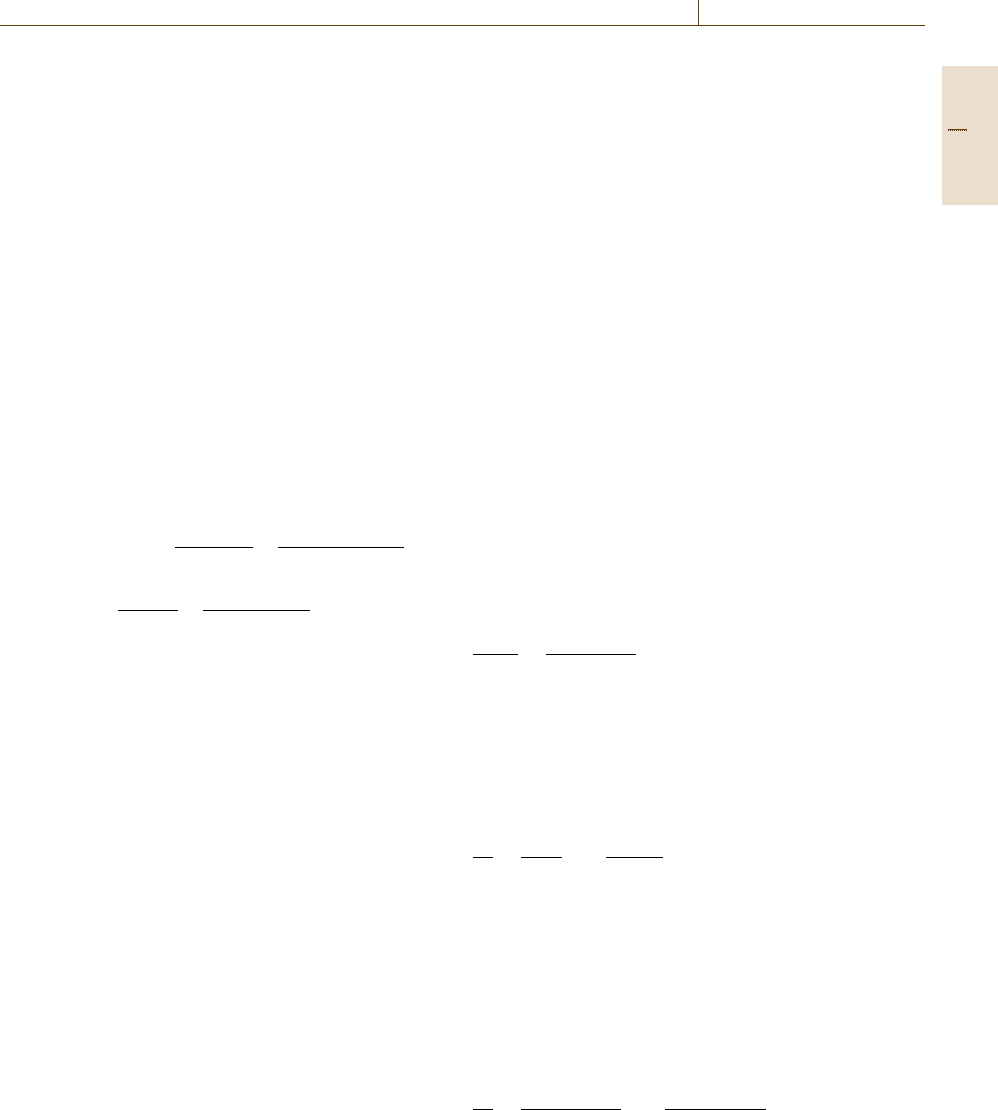
Mechanical Properties 7.6 Permeation and Diffusion 437
the pressure in the sample cell is monitored as a func-
tion of time. The difference between the initial and final
pressure in the charge cell can be used to calculate the
number of moles of gas admitted into the sample cell.
As the polymer sample sorbs gas, the pressure in the
gas phase of the sample cell decreases. When the sam-
ple cell pressure reaches a stable value, the polymer has
sorbed all of the gas that it can at the final pressure in
the sample cell (i. e., equilibrium is achieved). From the
final pressure in the sample cell, the number of moles of
gases in the gas phase of the sample cell can be calcu-
lated. The difference between the moles of gas admitted
to the sample cell from the charge cell and the final
number of moles of gas in the gas phase of the sample
cell is the number of moles of gas sorbed by the sample
at the final pressure of the sample cell. The second step
is to add more gas to the charge cell and repeat the pro-
cedure outlined above to obtain a data point at a higher
pressure. At step m, the number of moles of gas sorbed
in the polymer n
p,m
is given by [7.296]
n
p,m
=n
p,m−1
+
p
c,m−1
V
c
RTZ
c,m−1
+
p
s,m−1
V
s
−V
p
RTZ
s,m−1
−
p
c,m
V
c
RTZ
c,m
+
p
s,m
V
s
−V
p
RTZ
s,m
, (7.154)
where V
c
is the volume of the charge cell, V
s
is the vol-
ume of the sample cell, and V
P
is the volume of polymer
sample. Z
c,m
, Z
s,m
, Z
c,m−1
and Z
s,m−1
are the com-
pressibility factors of gas in the charge cell at step m,
sample cell at step m, charge cell at step (m −1) and
sample cell at step (m −1), respectively. The subscripts
m and m −1 represent the properties at step m and
step (m −1), respectively. For example, n
p,m−1
is the
number of moles of gas sorbed in the polymer at step
(m −1). In the first step (i. e., m = 1), the sample cell is
under vacuum, and n
p,0
is zero. From these sequential
measurements, the gas sorption isotherm in the polymer
can be obtained, and the gas solubility as a function of
gas pressure can be calculated based on the definition of
solubility: S =C/ p.
Compressibility factors are related to pressure
by [7.298]
Z =1 +B
∗
p +C
∗
p
2
, (7.155)
where B
∗
and C
∗
are virial coefficients which depend
on temperature. They have been compiled by Dymond
and Smith for a large number of gases at various tem-
peratures [7.298]. Virial coefficients are also available
for some gas mixtures from the same source [7.298].
In general, the amount of gas in the gas phase is
much greater than that sorbed in the polymer, i. e., the
values of the two terms in brackets on the right-hand
side of (7.154) are much larger than n
p,m
or n
p,m−1
.In
this sense, the value of n
p,m
is the result of a subtrac-
tion between two much larger numbers. Therefore, the
accuracy of n
p,m
is sensitive to the calibration of the
pressure transducers and volumes. A typical transducer
used for such studies is the Super TJE model (Sensotec,
Columbus, OH, USA), which measures a pressure range
of 0–34 atm with an uncertainty as low as ±0.05% of
full scale (i. e., ±0.017 atm). It is one of the most ac-
curate transducers commercially available. Generally,
the transducers sense pressure and provide an analog
signal such as a voltage, which can be related to the
pressure measured by a standard pressure gauge (such
as the PM indicator made by Dresser Instruments, Shel-
ton, CT, USA) using a linear or polynomial equation.
The volume calibration can be performed by a two-step
Burnett expansion process [7.291]. In the first step, gas
(typically helium) in the charge cell at a pressure p
0
is
expanded to the empty sample cell, which is kept under
vacuum before the expansion. The pressure in both cells
reaches a stable value of p
f
. Based on a mass balance,
the following equation can be derived
p
0
V
c
RTZ
0
=
p
f
V
c
+V
s
RTZ
f
, (7.156)
where Z
0
and Z
f
are compressibility factors at pres-
sures of p
0
and p
f
, respectively. Substituting (7.155)
into (7.156) and letting the third virial coefficient,
C
∗
, equal zero (since C
∗
is a very small number for
helium [7.298]andC
∗
p
2
is typically negligible for he-
lium), yields
p
0
p
f
=
B
∗
V
s
V
c
p
0
+
V
c
+V
s
V
c
. (7.157)
By varying p
0
, one can generate data to prepare a plot
of p
0
/p
f
as a function of p
0
. The resulting line inter-
sects the p
0
/p
f
axis at (1 +V
s
/V
c
). The second step
is to vary the volume of the sample cell by inserting
some nonsorbing solid of known volume V
m
,suchas
stainless-steel spheres, into the sample cell. Repeating
the Burnett expansion step outlined above, the follow-
ing equation is obtained
p
0
p
f
=
B
∗
V
s
−V
m
V
c
p
0
+
V
c
+V
s
−V
m
V
c
. (7.158)
Again, one can perform gas expansions as a function of
pressure to fit this model and determine (V
s
−V
m
)/V
c
,
Part C 7.6
