Czichos H., Saito T., Smith L.E. (Eds.) Handbook of Metrology and Testing
Подождите немного. Документ загружается.

398 Part C Materials Properties Measurement
a high-sensitivity strain detector is necessary because
the change in the elastic strain is very small. In other
words, this is a tensile test with extremely low strain
rates, and the strain rate decreases continuously while
the stress is held.
7.4.2 Dynamic Loading
Dynamic means that the loading or deformation speed is
high compared with the quasistatic case. Several kinds
of dynamic loading are found. These include high-speed
tension or compression tests such as the Hopkinson
split-bar method, impact tests, and fatigue tests. These
three tests are explained below.
Computer
memorizer
etc.
Compressor
Booster
Valver
Gun
Strike bar
Input bar
Input bar
Output bar
Output
bar
Specimen
Absorber
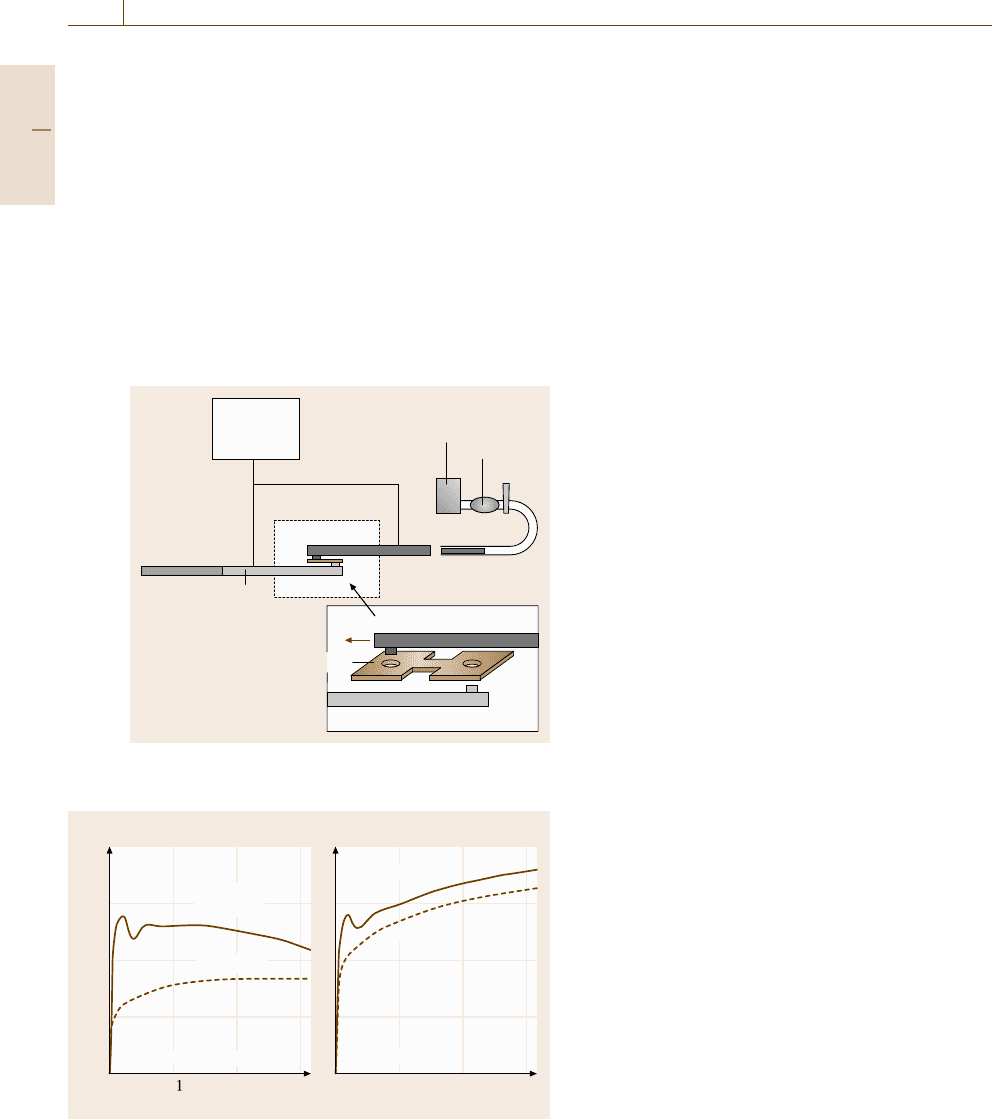
Fig. 7.52 Schematic illustration of a high-speed tension
test using the Hopkinson split-bar method
Nominal stress (MPa)
Low carbon steel SUS310S
Nominal strainNominal strain
800
600
400
200
0
00.10.20.300.10.20.3
Strain rate
2 × 10
3
s
–1
4 × 10
–3
s
–1
4 × 10
–3
s
–1
2 × 10
3
s
–1
a) b)
Fig. 7.53a,b Flow curves observed under high-speed deformation
(the Hopkinson split-bar tension test): (a) low-carbon steel and
(b) austenitic steel
High-Speed Tension or Compression Tests
These testing methods include the split pressure-bar
tester, the one-bar method, the load-sensing block-type
tester, and the servo-hydrostatic loading machine. The
most popular method is the Hopkinson split-bar method
in which either tension or compression impact defor-
mation can be carried out. The outline of the test is
shown in Fig. 7.52, where the input bar and the out-
put bar are pictured. A specimen is set between them.
To avoid the reflected stress pulse, the specimen gage
length should be sufficiently short. Examples of flow
curves obtained at a strain rate of 2× 10
3
s
−1
are pre-
sented in Fig. 7.53 [7.213,214]. As seen, the flow stress
becomes higher at a high speed, similarly to the case
of lowering test temperature. Very-high-speed deforma-
tion occurs almost under adiabatic conditions, so that
the temperature of a specimen increases with plastic
deformation. Hence workhardening decreases in a low
carbon steel with a large temperature dependence of
flow stress. Such workhardening behavior is the dif-
ference between the deformation at lower temperature
with a low strain rate and that at room temperature with
a high strain rate. These data are useful for the the safety
design of automobiles with respect to traffic collisions
or the safety of infrastructures such as buildings with
respect to the occurrence of earthquakes.
The dislocation structure that evolves during plastic
deformation is strongly dependent on the test tempera-
ture and strain rate. As shown in Fig. 7.54 [7.213]for
the case of a low-carbon steel, the dislocation cell struc-
ture evolves at room temperature under a low-strain-rate
deformation. However, planar dislocation arrays are ob-
served either during low-temperature deformation with
a low strain rate or at room temperature with a high
strain rate.
Impact Test
Impact testing is performed to evaluate the toughness of
materials. There are several loading methods, including
tension, compression, bending, and torsion. The typi-
cal test is the Charpy impact test in which three-point
bending is employed. As shown in Fig. 7.55, a hammer
is dropped to hit a rectangular specimen. A V- or U-type
notch is introduced to allow easy fracture due to stress
concentration for ductile materials. In the case of brit-
tle materials such as ceramics, cast iron etc., a notch is
not introduced. Parts of the hammer before and after hit-
ting (breaking) a specimen are compared. The balance
is considered to show the resistance to fracture, and the
energy needed to bend and fracture the specimen. The
impact tester can impart 300 J for metallic materials use,
Part C 7.4

Mechanical Properties 7.4 Strength 399
a) b) c)
200 nm 200 nm 200 nm
Fig. 7.54a–c Dislocation structures evolved by tensile deformation (10%) in ferritic steels: (a) 77 K, 3.3×10
−3
s
−1
,
(b) 295 K, 2 × 10
3
s
−1
and (c) 295 K, 3.3×10
−3
s
−1
Hammer
Charpy
Specimen
Izot
= Absorbed
energy
α
β
Fig. 7.55 Outline of impact testing: Charpy and Izot tests
Charpy impact energy (J)
350
300
250
200
150
100
50
0
0 100 150 200 250 300
Test temperature (K)
Low carbon steel
Lower shelf energy
Upper shelf energy
Fig. 7.56 Ductile-to-brittle transition behavior observed
by the Charpy impact test
and 3 J for resin or plastics. The Izot tester is another
typical device but is now rarely used. The specimen is
held in a different way, as shown in Fig. 7.55. The ab-
sorbed energy K is calculated from the angle of the
hammer and the starting (α) and maximum (β) height
after hitting,
K = WR(cos β −cos α) , (7.67)
where W and R refer to the weight and the arm length,
respectively.
Some materials fracture in a ductile manner under
quasistatic testing but fracture in a brittle manner under
impact testing. As the fracture mode is strongly depen-
dent on the testing temperature, the Charpy impact test
is carried out at a variable test temperature. The ab-
sorbed energy is then plotted as a function of the test
temperature, as presented in Fig. 7.56 [7.208]. In the
case of ferrite steel, for example, the energy changes
at a certain temperature, known as the ductile-to-brittle
transition temperature (DBTT). At higher tempera-
tures, specimens fracture in a ductile mode so that the
fracture surface exhibits a dimple pattern. However, in-
tergranular or cleavage (transgranular) fracture facets
are observed in specimens fractured at lower tempera-
tures. The DBTT is determined either from the absorbed
energy or the percentage of dimple facet on the fracture
surface. The results of impact tests are often used for
material selection. It is difficult to use the results quan-
titatively for machine design, e.g., for the determination
of design stress. On the other hand, the quantitative
resistance to fracture is determined by other tests us-
ing precracked specimens based on fracture mechanics
(see Sect. 7.4 for details).
Fatigue
Several fatigue testing methods have been utilized to
date.
Rotating (four-point) bending has been used to sim-
ulate a wheel axis, where a tension–compression stress
wave is repeated on the surface. The results are sum-
marized in a diagram of stress amplitude versus number
of cycles to fracture (S–N). As shown in Fig. 7.57,the
applied stress amplitude is plotted as a function of the
Part C 7.4

400 Part C Materials Properties Measurement
Stress (MPa)
1400
1200
1000
800
600
10
2
10
3
10
4
10
5
10
6
10
7
10
8
Number of cycles to failure
Spring steels
Conventional
fatigue limit
Fig. 7.57 Stress amplitude versus number of cycles to fail-
ure (S–N) curve for fatigue fracture
Stress amplitude σ
a
σ
w
σ
m
σ
a
σ
a
Mean stress σ
m
Yield stress UTS
Time
Stress
0
Fig. 7.58 Fatigue endurance-limit diagram where the in-
fluence of mean stress is given
number of cycles to failure [7.215]. As can be seen, the
relationship is almost linear in the beginning and tends
to saturate at a certain stress level near 10
6
–10
7
cycles.
In general, the stress at 10
7
cycles is called the fatigue
strength or endurance limit σ
w
. At stresses higher than
σ
w
, the number determines the time to fracture and is
called fatigue life. Thus, the fatigue strength and fatigue
life are utilized for the design of machines or structures.
Recent studies on very-long-life fatigue reveal that
some materials fracture even at stresses below the con-
ventional σ
w
. In the case of aged materials, this should
also be taken into consideration.
The influence of the mean stress on the fatigue stress
(stress amplitude) is discussed using the fatigue-limit
diagram shown in Fig. 7.58 where the stress amplitude
at 10
7
cycles is plotted as a function of the mean applied
stress. The diagram is constructed with consideration
of the yield strength σ
s
, tensile strength σ
B
,andthe
Extrusion and
intrusion
Slip
Free
surface
Crack
Striation
Final
fracture
Maximum tensile
stress direction
Stage 1 Stage 2
Fig. 7.59 Schematic illustration on fatigue fracture; crack
initiation, growth and final fracture
true fracture strength, σ
f
, obtained by tension testing
that corresponds to a quarter cycle of the fatigue test.
A hatched area is evaluated to be safe for both fatigue
fractures and macroscopic yielding (failure of elastic-
ity).
The mechanism for fatigue fracture is illustrated
schematically in Fig. 7.59. In the usual case, a fatigue
crack is initiated on the surface or a stress-concentrated
region such as a flaw, brittle inclusions etc. If a material
is nearly free from such defects, slip deformation occurs
preferentially near the surface. Because of cyclic loading
of small stress below the yield strength, slip occurs lo-
cally and repeatedly, resulting in intrusion and extrusion
at the surface. Since the intrusion is a kind of micro-
crack at which stress is concentrated (the first stage of
fatigue cracking), plastic flow occurs locally at the tip
of the micro-crack. The growth direction along the max-
imum shear stress then changes to be perpendicular to
the applied tensile stress. This crack-propagating regime
is called stage 2. When the crack length has increased
enough to satisfy the final fracture condition given by the
fracture mechanics approach (a detailed explanation of
which is given in Sect.7.4)
K
max
≥ K
F
th
, (7.68)
then final fracture takes place. Here, the left-hand side
of (7.68) is the maximum stress-intensity factor K
max
(=
ασ
max
√
πc), where α, σ
max
,andc refer to a constant re-
lated to the specimen’s geometry, the maximum stress
applied to the specimen and the crack length, respec-
tively, and K
F
th
is the fracture toughness against fatigue.
The crack growth speed during stage 2 is measured
using a prepared specimen in which a sharp prenotch is
introduced. With repeated application of the stress, the
length of the crack is measured and the growth rate is
then plotted as a function of the amplitude of the stress-
intensity factor ΔK(= Δσ
√
πc), as shown in Fig. 7.60.
Part C 7.4

Mechanical Properties 7.4 Strength 401
Crack growth rate log (da/dN)
Amplitude of stress intensity factor log (ΔK)
Stage 2a
(Microstructure
sensitive)
Stage 2b
(Striation:
(microstructure
insensitive)
Stage 2c
1
m
Fig. 7.60 Fatigue crack growth rate as a function of stress-
intensity amplitude
The growth rate in the low-ΔK region can be meas-
ured by decreasing Δσ continuously. Here, the engineer-
ing lower limit for crack growth is determined as the
threshold intensity factor ΔK
th
, which is sensitive to mi-
crostructural parameters. In the middle of the curve, it is
approximated by a linear relationship described by Paris’
equation [7.216,217]
dc/dN = CΔK
m
, (7.69)
where C and m are constants. The crack growth rate dur-
ing this stage 2 is insensitive to microstructure.
–200 0 200 400 600 800 1000 1200 1400
Temperature (°C)
Shear stress
at 300 K
(MN/m
2
)
Ferrite
Ferrite
RT
Plasticity
Power law
Diffusional flow
Homologous temperature T/T
m
Creep
Austenite
(Boundary)
(Lattice)
(Lattice)(Boundary)
Phase
change
Phase
change
Low tempera-
ture creep
High tempera-
ture creep
High tempera-
ture creep
Dynamic
recrystallization
Curie
temperature
Normalized
shear stress
τ/μ
10
–1
10
–2
10
–3
10
–10
10
–4
10
–5
10
–6
0
0.2 0.4 0.6 0.8 1
10
–1
10
1
10
2
10
3
10
1
10
–2
10
–4
10
–6
10
–6
10
–6
10
–8
10
–9
10
–10
10
–5
10
–4
10
–3
10
–2
10
–4
10
–1
1
1
10
–7
10
–8
10
–10
/s
1/s
/s
Fig. 7.61 Deformation map for iron
(d = 0.1mm)
If the initial crack length c
i
in a material is known,
for example, by using nondestructive inspection such as
x-ray transmission, the fatigue life N
f
of the material can
be predicted by combining (7.68)and(7.69). That is, by
integrating (7.68), we obtain,
N
f
=
c
f
c
i
dc
C(αΔσ
√
πc)
m
, (7.70)
where c
f
can be calculated by (7.68). In ductile materials,
the fracture surface during stage 2 consists of striation,
which is caused by repeated loading. Hence, the crack
growth speed is estimated from fractography.
7.4.3 Temperature and Strain-Rate Effects
Flow stress is influenced by the test temperature mainly
due to thermal activation mechanisms for dislocation
motion. The thermal vibration of atoms leads to the
vibration of the dislocation line. Therefore, the possibil-
ity per second of achieving a certain thermal activation
energy is proportional to the Boltzmann probability.
This means that the influence of temperature and strain
rate on strength should be understood simultaneously;
to achieve this, the deformation mechanism map has
been constructed for various materials [7.218]. As an
example, the deformation map for iron is presented
in Fig. 7.61. Because iron shows ferrite-to-austenite and
then austenite-to-ferrite transformations when heated,
Part C 7.4
402 Part C Materials Properties Measurement
a)
b)
Relative flow stress σ/E
Flow stress
Twinning
Phase transformation
Ferrite / pearlite
Austenite
Temperature
Dynamic strain
aging
σ
c
/E
Increase in
strain rate
Low temperature
deformation
Thermal
component
Athermal
component
High temperature
deformation
0
0
T
c
/T
m
Relative temperature T/T
m
α–γ
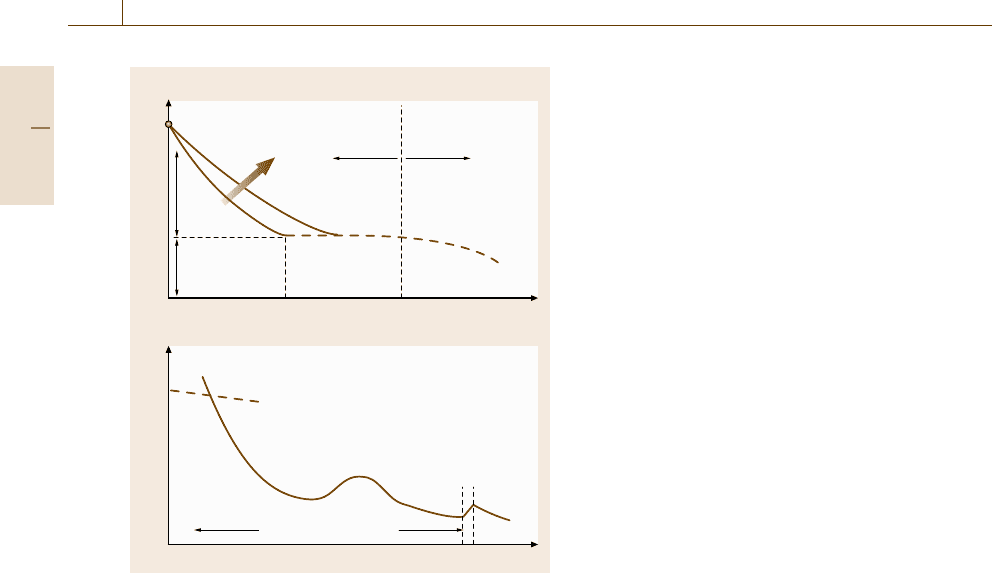
Fig. 7.62a,b Effect of temperature on the yield strength
(schematic illustration): (a) general case and (b) mild steel
the curves for body-centered cubic (BCC, ferrite) is
interrupted by a face-centered cubic (FCC, austenite)
regime. Depending on the temperature and strain rate,
various deformation mechanisms become dominant.
Taking a look at the map near room temperature with
a strain rate of 10
0
–10
−5
s
−1
, the dominant mechanism
is slip, i. e. dislocation motion. A simpler illustration to
explain the effect of temperature on the yield strength
isgiveninFig.7.62. At 0 K, the enhancement by ther-
mal activation disappears and the strength corresponds
to the theoretical strength. At a certain temperature,
the strength is decreased through due to thermal ac-
tivation of dislocation motion. At higher temperatures
near T
m
/2, all the short-range barriers for dislocation
motion can be overcome, but some long-range barriers
remain. The stresses related to overcoming these two
kinds of barriers are called thermal stress (or effective
stress) and athermal stress (internal stress), respectively.
Thus, as shown in Fig. 7.62a, thermal stress becomes
zero at T
0
.BelowT
0
the temperature dependence of the
yield strength is ascribed to thermal activation mech-
anisms for dislocation motion. Therefore, if the strain
rate is increased, its effect is equivalent to decreasing
temperature. The influence of strain rate on the yield
strength is also drawn schematically in Fig. 7.62a. Flow
stress can also be discussed using this approach, al-
though the evolution of the dislocation structure must
also be taken into account. With increasing of plastic
strain, the dislocation density (ρ) increases by ρ
+
and
then workhardening occurs. At the same time, dislo-
cations are annihilated with recovery to be decreased
by ρ
−
, which is influenced by temperature. Therefore,
the total dislocation density (ρ
+
+ρ
−
) is determined as
a function of the strain as well as temperature and time,
i. e., strain rate. The flow curve obtained at high strain
rate is therefore influenced by two thermal activation
mechanisms: dislocation motion and dynamic recovery.
When the test temperature is higher than T
0
,the
microstructure itself changes obviously during de-
formation, for example exhibiting to grain growth,
dynamic recovery and/or recrystallization. Such a re-
gion, as shown in Fig. 7.62a, is called high-temperature
deformation. As observed in Fig.7.61, creep deforma-
tion occurs strongly at high temperatures with small
strain rates.
The behavior of a real material is compli-
cated in comparison with that of the pure metal
in Fig. 7.62a. The example of mild steel is pre-
sented in Fig. 7.62b[7.219]. The curve in the lower-
temperature region corresponds to the deformation
of the BCC ferrite phase, while that in the higher-
temperature region corresponds to FCC austenite.
Moreover, due to the intrusion of deformation twinning
at cryogenic temperatures and dynamic strain aging
caused by solute carbon and nitrogen atoms in the
region slightly above room temperature, the typical be-
havior explained in Fig. 7.62a is not easy to discern.
7.4.4 Strengthening Mechanisms
for Crystalline Materials
As described above, flow stress is controlled by the
motion of dislocations. Hence, there are two ways to
strengthen a material: remove dislocations thoroughly or
introduce a large number of obstacles to prevent disloca-
tion motion. The former approach has been achieved in
the form of single-crystal whiskers. When the diameter
of the whisker increases, the strength decreased rapidly
due to the inevitable introduction of defects including
dislocations. The latter approach has been used widely,
including solid-solution hardening, precipitation hard-
ening or dispersion particles hardening, workharden-
ing, grain-refinement hardening and the duplex structure
(composite) hardening. These mechanisms to prevent
dislocation motion are described below.
Part C 7.4
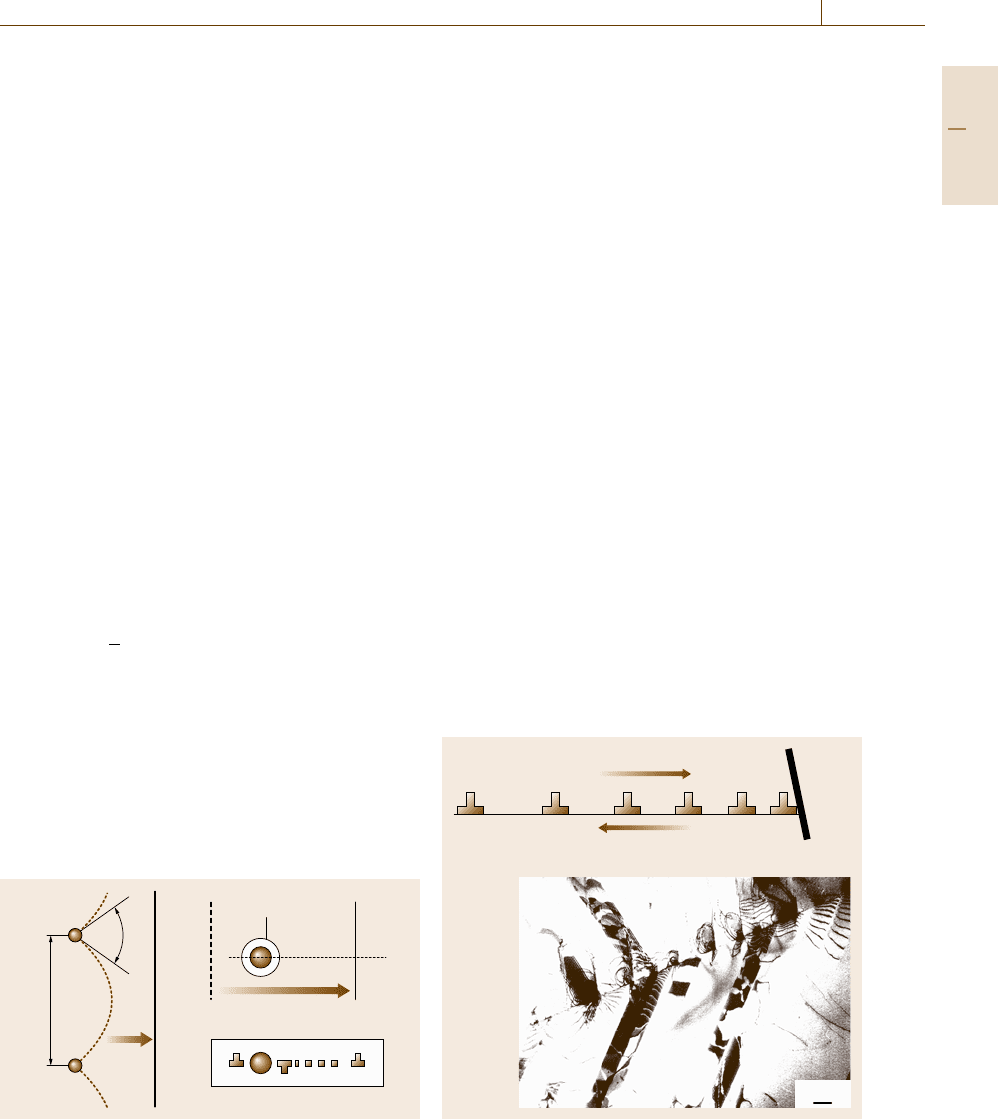
Mechanical Properties 7.4 Strength 403
Solid-Solution Hardening
The interstitial or substitutional solute atoms hinder the
motion of dislocations due to the size effect and/or the
effect of an inhomogeneous elastic modulus. These are
elastic interactions and hence can be overcome by ther-
mal activation for dislocation motion. The amount of
hardening is given by
Δσ = Ac
n
, (7.71)
where A and c refer to a material constant and the con-
centration of solute atoms, respectively. The constant n
is about 0.5–1.0 from experimental or theoretical con-
siderations.
Workhardening
As explained in the previous sections, the dislocation
density is increased by plastic deformation and mobile
dislocations have to pass through the resulting dislo-
cation structure. There are two types of dislocation–
dislocation interactions: short-range interactions, in-
cluding elastic interactions, cutting, reactions etc., and
long-range interaction due to internal stresses caused
by the dislocation structure. The back-stress caused by
piled-up dislocations is a typical example. Either model
gives strengthening according to
Δσ = B
√
ρ, (7.72)
where B is constant. This equation was firstly proposed
by Bailey and Hirsch [7.220].
Precipitation or Dispersion Hardening
When small precipitates are dispersed in a matrix, a dis-
location line interacts with them. When the particle is
weak, the dislocation line cuts through such particles and
passes through, as shown in Fig. 7.63. The threshold con-
Orowan loop
Dislocation movement
Cross sectional view
L
φ
b)a)
Fig. 7.63a,b Strengthening mechanisms for obstacles dis-
persion; (a) cut-through mechanism and (b) Orowan bypass
mechanism
dition is given by the following equation
Δσ =(aμb/λ)cos(φ/2) , (7.73)
where a is a constant, μ is the shear modulus, b is the
magnitude of the Burgers vector of a dislocation, λ is
the average distance between particles, and φ is the crit-
ical angle to cut the particle. If φ is 0
◦
, a dislocation line
passes through the particle, leaving a dislocation loop
known as an Orowan loop (Fig. 7.63b). This is the case
of a strong particle, leading to the following equation for
dispersion hardening (using φ = 0anda ≈2in(7.73))
Δσ =2μb/λ . (7.74)
Grain-Refinement Hardening
When a dislocation moves towards a grain boundary,
it is stopped and induces internal stress, as is shown
in Fig. 7.64. When dislocations pile up at the grain
boundary, the back-stress generated hinders the further
motion of dislocations, as described for the case of
workhardening. Because the number of piled-up disloca-
tions depends on the applied stress and slip distance, i.e.,
grain diameter (d), the amount of strengthening (Δσ)is
given by theoretical relations such as
Δσ =kd
−1/2
. (7.75)
Other interpretations have been proposed for the effect
of grain size in this mechanism. For instance, the dislo-
cation density is increased due to the occurrence of many
Grain boundary
Grain
boundary
200 nm
τ
τ
b)
a)
Fig. 7.64a,b Strengthening mechanism for grain boundary
or interface: (a) dislocation pile-up model and (b) TEM mi-
crograph for a high-nitrogen-bearing austenitic steel
Part C 7.4
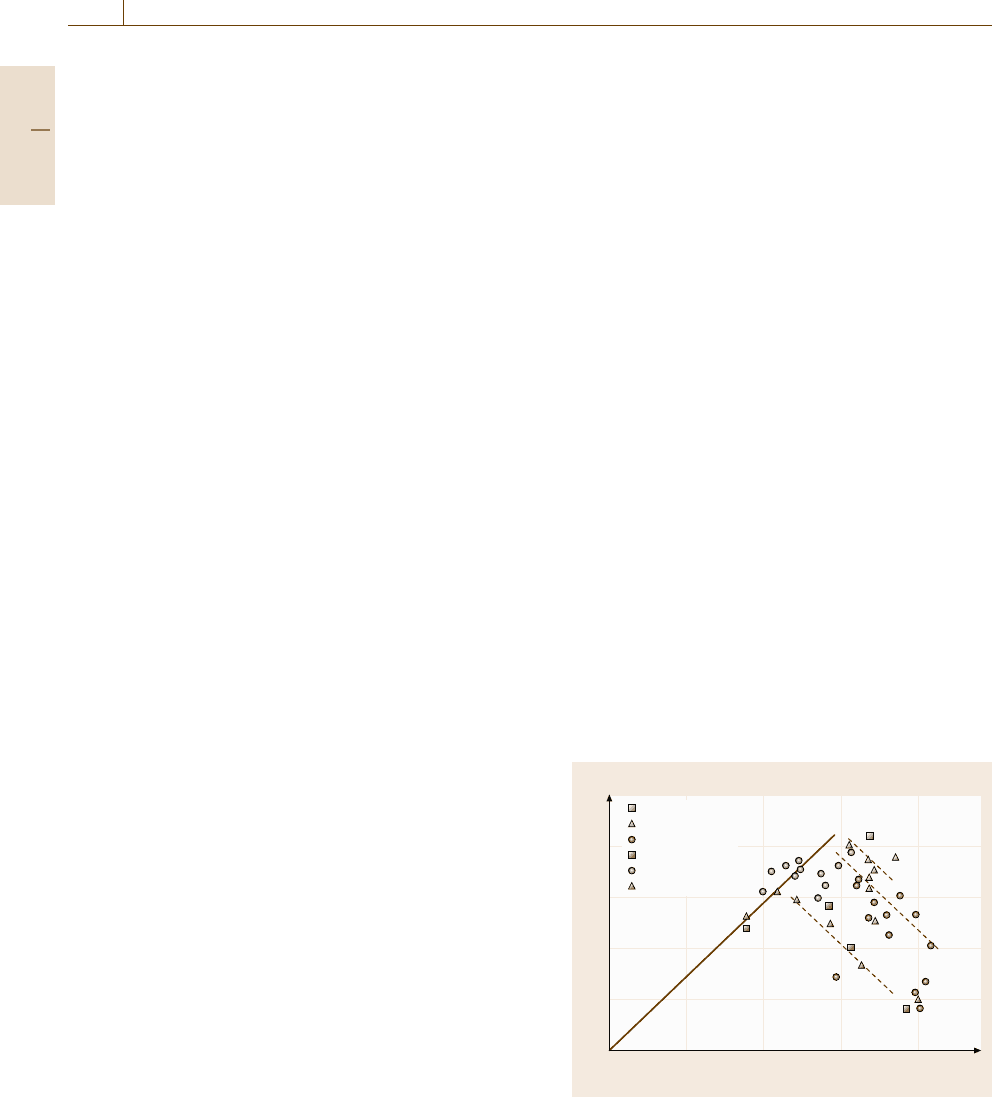
404 Part C Materials Properties Measurement
slip systems in the vicinity of a grain boundary due to
high local internal stresses. The amount of strengthening
is given by the Bailey–Hirsch relation (7.72). Because
the increase in the dislocation density is proportional to
the grain diameter d, the resultant equation exhibits the
same form as (7.75). Another interpretation for grain-
refinement hardening is that newdislocations are emitted
from a grain boundary in the neighboring grain to relax
high local internal stresses. As (7.75) was first proposed
by Hall and Petch [7.221–223] based on experimental
data, it is called the Hall–Petch relation. In fact, sev-
eral phenomena must be overlapping in this mechanism,
but the increase in strength can be expressed roughly
by (7.75).
Duplex Structure (Coarse Multiphase) Hardening
When the grain diameter of the second phase is compa-
rable to that of the matrix, the mechanism for dispersion
hardening such as the Orowan bypass model is no longer
applicable. Plastic flow takes place preferentially in the
soft phase, resulting in stress partitioning between the
constituents, known as phase stress or averaged internal
stress. Such strengthening is similar to that for composite
materials. Many engineering materials such as ferrite–
pearlite steel, dual-phase steels, α −β Cu-Zn alloys etc.,
belong to this category. Although micromechanics mod-
els have been developed to express the deformation of
a multiphase material, flow stress is described roughly
by a kind of mixture rule. In the case of a two-phase alloy,
σ =σ
1
(1 − f )+σ
2
f , (7.76)
where σ
1
, σ
2
, f refer to the strength of the matrix, the
strength of the second phase and the volume fraction of
the second phase, respectively.
Usually the above strengthening mechanisms are su-
perposed. Hence, a suitable superposition rule has to be
used for real engineering materials. This may be simply
described by
σ =
n
i=1
σ
N
i
. (7.77)
In the case of superposition of strong and weak obsta-
cles, the value of N is believed to be nearly unity but is
less than unity in other cases where both obstacles are
strong.
7.4.5 Environmental Effects
The environment where a material is used influences
its strength. Such influences include chemical reactions
such as corrosion and radiation damage in a nuclear
furnace. Here, two examples are explained because the
social impact of these fracture is serious: hydrogen em-
brittlement and stress corrosion cracking.
Hydrogen-Induced Embrittlement
The most difficult barrier to the use of high-strength
steels is hydrogen embrittlement, or so-called delayed
cracking. Hydrogen atoms invade not only during the
processing of products but also during service. Cur-
rent topics in this area include the interaction of mobile
dislocation and hydrogen atoms and the diffusion of hy-
drogen atoms within materials. The fracture mechanism
is, however, not yet clear.
Figure 7.65 shows the fracture strength after 100 h
in water for various kinds of steels [7.224]. The frac-
ture strength is plotted as a function of the tensile
strength obtained using the conventional tension test.
It is clear the fracture strength increases with in-
creasing tensile strength up to approximately 1.0GPa.
However, steels in the 1.5 GPa (tensile strength) class
exhibit a large scatter in their fracture strength; some
remain strong while others can drop below 1.0GPa.
Therefore, the strengthening in a mild environment
is not necessarily valid under different environmen-
tal conditions. The occurrence of fracture depends
strongly on the microstructure, i. e., strengthening
mechanism.
Another fracture mechanism caused by hydrogen
atoms is hydrogen attack which is observed for ma-
terials subjected to high temperatures in chemical
Fracture stress (GPa)
2.5
2
1.5
1
0.5
0
0 0.5 1 1.5 2
Tensile strength (GPa)
Piano wire
NiCrCoMo
18Ni marage
04C-SiMnCrMo
SCM22
SCM4
Fig. 7.65 Hydrogen-induced embrittlement observed in
various kinds of steels. (Fracture stress at 100 h in water for
a notched specimen with a stress concentration factor of 10
versus tensile strength)
Part C 7.4
Mechanical Properties 7.4 Strength 405
atmospheres such as that in an oil plant. In the case of
carbon steel, the invading hydrogen atoms react with ce-
mentite particles to produce methane gas, resulting in
the formation of blisters that lead to rupture. The crit-
ical condition for hydrogen attack is summarized by
the so-called Nelson diagram, in which the dangerous
conditions are summarized in terms of temperature and
partial pressure of hydrogen.
Stress Corrosion Cracking
Stress corrosion cracking (SCC) is observed in many
alloys (not in pure metals) under certain combined
conditions of stress, chemical environment and mi-
crostructure, as shown in Fig. 7.66a. For instance, SCC
has been found for Al alloys in air and sea water, Mg
alloys in sea water, Cu alloys in water or ammonium at-
mosphere, carbon steels in NaOH solution, austenitic
stainless steels in hot water, and so on. External or
a)
b)
Stress
Material
Chemical
condition
Crack propagating rate log (da/dt)
K
C
2nd region
1st region
3rd region
Stress intensity factor K
I
SCC
K
ISCC
Fig. 7.66a,b Stress corrosion cracking: (a) influential fac-
tors of stress, microstructure and chemical environment for
stress corrosion cracking and (b) crack-propagation rate as
a function of stress-intensity factor
residual tensile stress always plays an important role in
fracture. Stress accelerates local corrosion (anode so-
lution) and amplifies the stress at crack tips, leading
to fracture. SCC fracture occurs along either certain
crystal planes in grains or grain boundaries. Macro-
scopic crack propagation is summarized by using the
stress intensity factor K, as shown in Fig. 7.66b. The
threshold K is defined as K
ISCC
, which is dependent on
the materials’s microstructure. In general, the stronger
the yield strength, the lower the value of K
ISCC
,so
that the balance between strength and toughness is
important.
When an austenitic stainless steel is exposed to
a high-temperature atmosphere, Cr carbide precipitates
along the grain boundaries, leading to the formation
of a low-Cr zone. Corrosion then concentrates in such
low-Cr zone in the vicinity of the grain boundary and
cracks are initiated along the grain boundary, influenced
by local corrosion and local stress concentration. The
concentration of carbon is, therefore, extremely reduced
in steel-making for this use; in a nuclear reactor, it is
known that stress corrosion cracking is a key problem
for safe operation. Thus, extensive investigations have
been made on SCC for austenitic stainless steels. It
is also known that radiation damage accelerates SCC
cracking. However, recent results have indicated that
stress corrosion cracking on shroud plates in a nuclear
reactor starts in the interior of grains at the surface,
although the reason for this is not clear.
7.4.6 Interface Strength:
Adhesion Measurement Methods
Most engineering materials consist of multiple phases.
For instance, inclusions or precipitates are usually intro-
duced into engineering materials, whether intentionally
or not. Some of these are harmful to the material proper-
ties but others are useful for improving properties such
as material strength. A typical example is a composite
material. In the case of electronics packaging, interfaces
between different materials are very important. In par-
ticular, for nanosized devices, performance is strongly
dependent on the situation of interfaces. To examine the
strength of an interface experimentally, several meth-
ods are employed depending on the requirements. The
tests are not easy and require careful analysis, some-
times with the help of FEM calculations. The strength
of an interface is theoretically predicted by means of
the molecular dynamics method, which is helpful for
understanding the mechanism as well as interface de-
sign.
Part C 7.4
406 Part C Materials Properties Measurement
Tensile Strength
The fracture strength perpendicular to the interface is
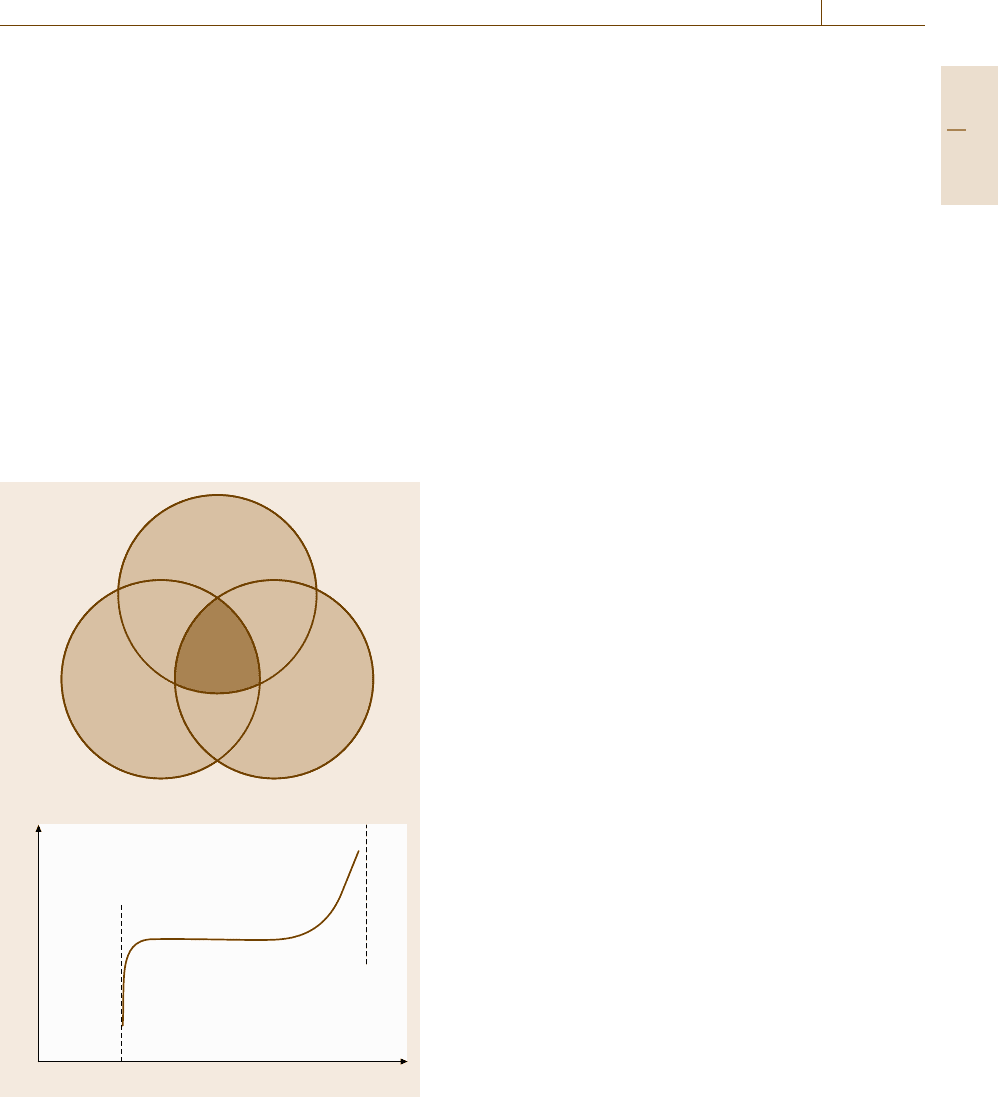
tested in a similar method to tension test. Figure 7.67
shows the tension test used to measure the interface
strength [7.225]. Here, one should note the inclination
of the interface with respect to the tensile direction.
If the interface is inclined, even at an angle less than
1
◦
, the shear stress component appears to influence the
fracture mode. The normal to the interface should be
carefully adjusted to be parallel to the tensile direction.
This test is quite simple and easy to apply to some artifi-
Grip Interface
Grip
Fig. 7.67 Tension test to measure the normal separation at
the interface
a)
b)
c)
d)
Interface
Interface
Interface
Interface
Load
Load
Load
Load
Load
Load
Load
Load
Load
Load
Fig. 7.68a–d Bending test to evaluate cleaving stress at
the interface:
(a) compact tension test, (b) three-point bend-
ing loading parallel to the interface, (c) three-point bending
loading perpendicular to the interface and
(d) peel-off test
cially bonded parts but is not easy to apply to multiphase
alloys.
Cleavage Strength (Bending)
Similar to fracture toughness testing, compact tension
testing, using a specimen shown in Fig. 7.68a, is em-
ployed to examine the cleaving features of the interface
where the fracture mode is tensile [7.225]. Simpler
methods based on three- or four-point bending are
showninFig.7.68b and c. The strength for the deco-
hesion can be evaluated by these tests, while Fig. 7.68d
shows the peeling test. Some modifications of these
tests are also used.
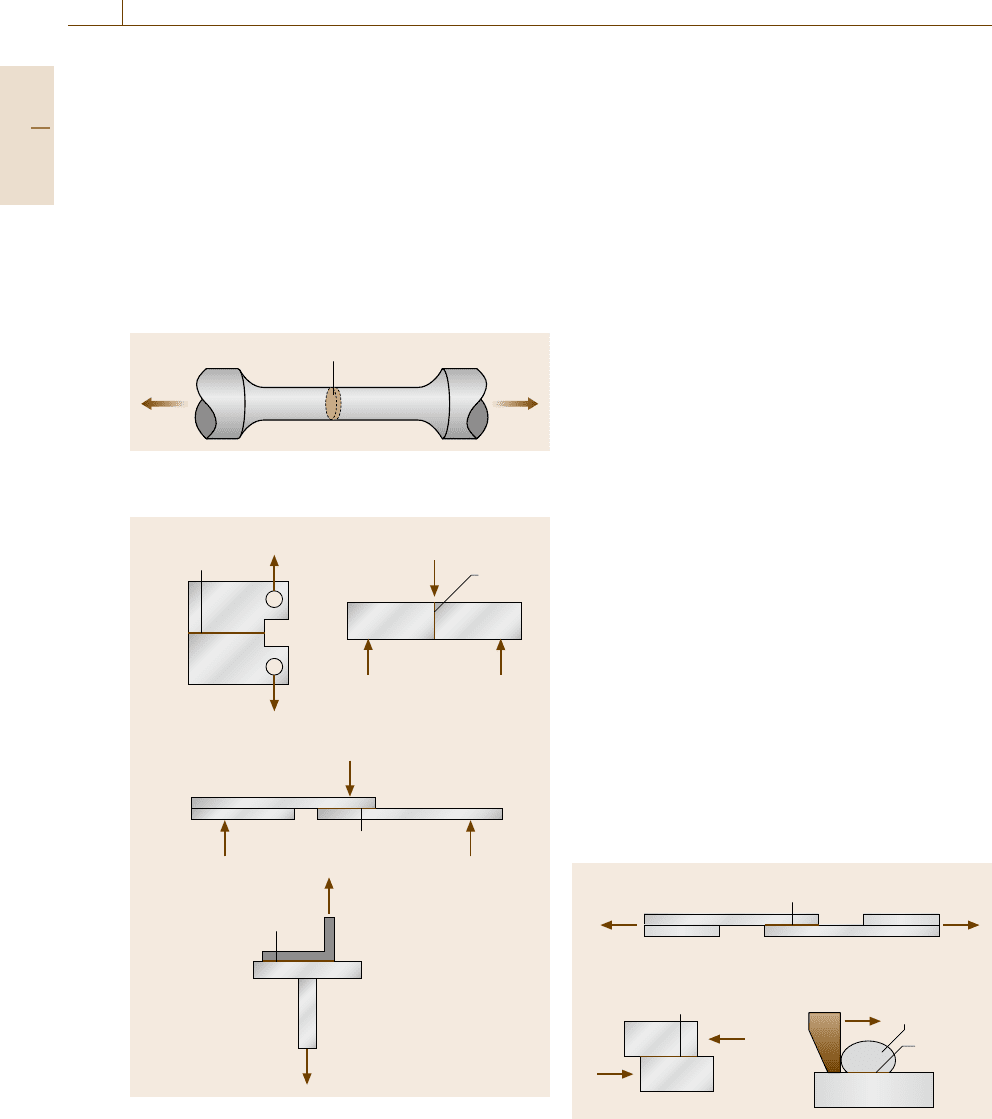
Shear Strength (Adhesive Strength)
The evaluation of shear strength at the interface, which
is a key issue for composite strengthening and several
mechanically bonded parts, is an interesting measure-
ment. To measure the adhesive shear strength, the most
popular test is shown in Fig. 7.69a[7.225]. The thick-
ness of the grip is the same as that of the specimen
containing the interface, so that a bending moment
can be avoided. Figure 7.69b exhibits the compression-
type shearing test, which is frequently employed to
evaluate the strength of wire bonding in electric de-
vices. As shown in Fig. 7.69c, a special tool is prepared
to push the bonded wire to measure the decohesion
stress [7.226].
The interface strength between inclusions or pre-
cipitated particles and the matrix in engineering ma-
terials cannot be measured directly. In the case of
fiber-reinforced materials, methods to measure the shear
strength between the reinforcement and the matrix based
on pulling or pushing the fiber have been attempted. To
realize such a test, a small jig to grip the fiber or a small
a)
c)b)
Grip
Grip
Load
Load
Load
Load
Interface
Tool
Bonded wire
Interface
Interface
Device
Load
Fig. 7.69a–c Shearing tests: (a) tension-type test for
shearing the interface, (b) compression-type test, (c) shear-
ing test for wire bonding
Part C 7.4
Mechanical Properties 7.4 Strength 407
a)
b)
c)
d)
Pull
out
Push
down
Matrix
Fiber
Fiber
Matrix
d
Lc/2
Lc/2
Lc/2
Lc/2
Fig. 7.70a–d Shear strength of interface in a composite material: (a) model of short fiber-reinforced composite, (b) shear-
stress distribution under the applied stress when the matrix is yielded. (c) tensile-stress distribution corresponding to (b)
and (d) pull-out and push-down tests to evaluate the shear strength of the interface
indenter with a tiny tip must be prepared. The schematic
testing arrangement is illustrated in Fig. 7.70 [7.227].
The simple shear-lag model for composite strengthen-
ing is shown in Fig. 7.70a. When the matrix yields under
the external tensile stress, the load transfer from the ma-
trix to a fiber, i. e., the shear stress τ
c
, can be roughly
described as in Fig. 7.70b. Within a limited length L
c
,
shear stress is generated and the integrated tensile stress
inside the fiber is shown in (b). If the fiber is short,
a pull-out phenomenon occurs, but if the fiber is long
enough, the tensile stress reaches the fracture strength
σ
f
. Hence, in order to use the strength of fiber efficiently,
the length should be larger than σ
f
d/(2τ
c
), where d is the
fiber diameter. This is called the critical length Lc
max
.
When a fiber is shorter than Lc
max
, fracture does not take
place. Figure 7.70d presents mechanical tests to mea-
sure the decohesion shear strength at the interface. If
the length of the fiber is shorter than Lc
max
, it can be
pulled out or pushed down and the interface strength
can be evaluated from the relevant load–displacement
curve. However, in most cases, the fiber fractures during
pulling, and suitable modeling has to be considered to es-
timate the shear stress. When a brittle phase is formed at
the interface, fracture occurs easily and the cracking of
the brittle layer plays the part of a notch in reducing the
fiber strength.
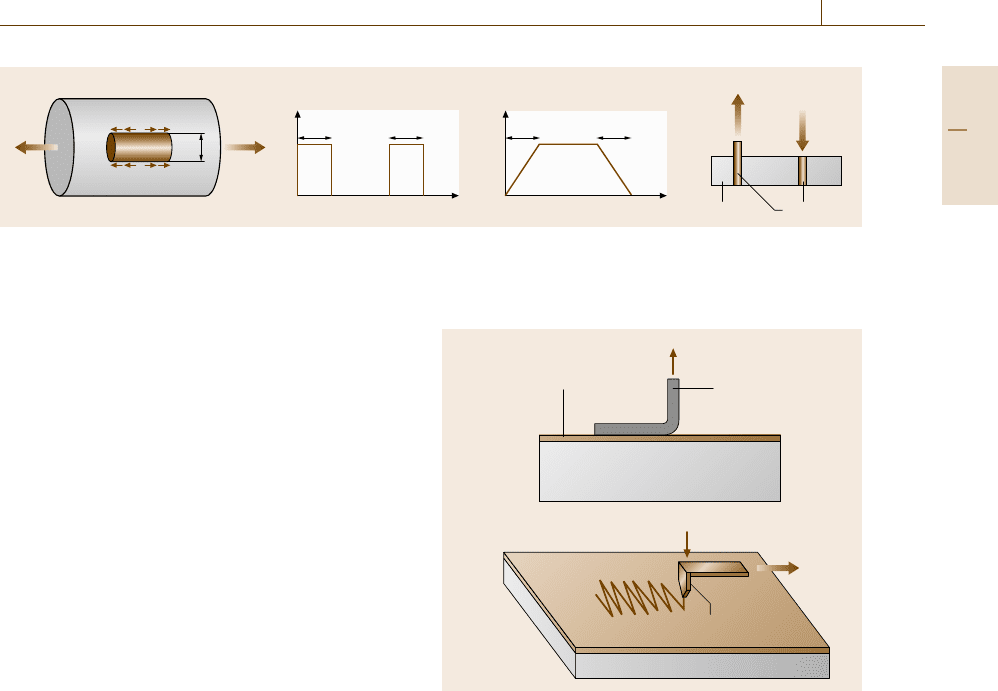
Scratch Test for Thin-Film Coatings
The decohesion strength of thin or thick films is fre-
quently of concern for surface modification. Various
surface treatment such as chemical vapor deposition
(CVD), physical vapor deposition (PVD) etc. have been
developed. For rough testing to evaluate the interface
strength, adhesive tape is stuck onto the film and pulled
quickly, as shown in Fig. 7.71a. If the interface strength
Load
Thin film
Adhesive
tape
Substrate
Interface
Load
Scanning
Indenter
Film
a)
b)
Fig. 7.71a,b Tests to evaluate the strength of interface be-
tween the deposited film and substrate: (a) peel-off test
using adhesive tape and (b) scratching test for thin film on
a substrate to examine decohesion behavior, where a di-
amond indenter with a tip radius of several μmisused
is weak, the film can be removed easily. However, this
test is not sufficient to evaluate the reliability of bonding
and hence more quantitative tests are used. Figure 7.71b
shows a schematic illustration of such a test [7.226].
An indenter is pressed into the film and pulled along to
scratch a zigzag route, during which the applied stress
is increased gradually. After this test, the position where
separation starts can be determined by observation with
a scanning electron microscope (SEM) and the applied
stress at that point can then be found. Sometimes, the
test is performed inside a SEM.
Part C 7.4
