Fahlman B.D. Materials Chemistry
Подождите немного. Документ загружается.

crucible that is heated by a W filament. Temperatures as high as 1,800
C may be
reached inside the chamber, which is enough for some metals or metal salts to
vaporize. Deposition rates for this method are 1–20 A
˚
s
1
. The use of an electron
beam to assist in the precursor evaporation results in temperatures on the order of
3,000
C, being more suited for the deposition of refractory metals/alloys and metal
oxides such as alumina, titania, and zirconia. Since the temperature of the chamber
interior is much higher than the walls, the gas-phase ions/atoms/molecules condense
on the sidewalls as well as the substrate; this may lead to film contamination as the
nonselective coating flakes off the chamber walls.
For high-purity metal or carbon films, an ultrahigh vacuum (UHV) environment
(typically <1 10
6
Torr) must be used during PVD. This is necessary in order to
prevent the gas-phase reaction of metal/carbon atoms with atmospheric gases (e.g.,
H
2
O, O
2
,N
2
) that would prefere ntially form metal oxides, hydroxides, or nitrides
rather than the desired film. If mixed phases such as nitrides or oxides are desired,
purified nitrogen or oxygen may be introduced into the chamber, respectively. For
carbide films, targets o f b oth the metal and carbon are placed together within the
vacuum chamber. As one would expect, since such a high-purity metal and high
vacuum chamber must be used, PVD is relatively quite expensive. Another limita-
tion is the selectivity and confo rmality of the procedure. While PVD wor ks well to
deposit material on surfaces in line-of-sight of the source, nonconformal deposition
of complex or rough surfaces (e.g., fibers) is a critical limitation. This issue becomes
more important for integrated circuits with feature sizes less than 100 nm, often with
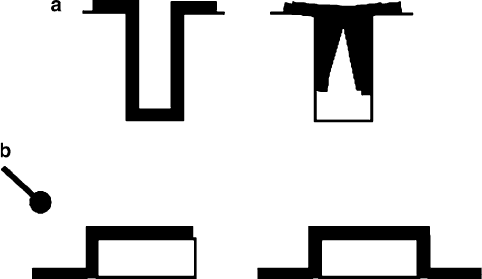
high aspect ratios (height/width ratio of the surface feature, Figure 4.53).
By contrast, CVD is a process in which gaseous precursors are reactively trans-
formed into a thin film, coating or other solid-state material on the surface of a
catalyst or substrate. It should be stressed that CVD is no longer limited to thin film
growth; this method is now the preferred route to generating fiber-optic preforms,
[47]
Figure 4.53. Illustration of conformal thin-film growth for (a) trench filling and (b) step coverage.
The line-of-sight limitation of PVD, relative to a conformal CVD technique, is shown in (b).
298 4 Semiconductors
as well as an increasingly diverse nature of nanostructural architectures, especially
carbon nanotubes (CNTs) that will be detailed in Chapter 6. The CVD procedure is
often denot ed as metal-organic CVD (MOCVD), which more accurately specifi es
the use of an organometallic precursor, containing a central metal and ancillary
ligands.
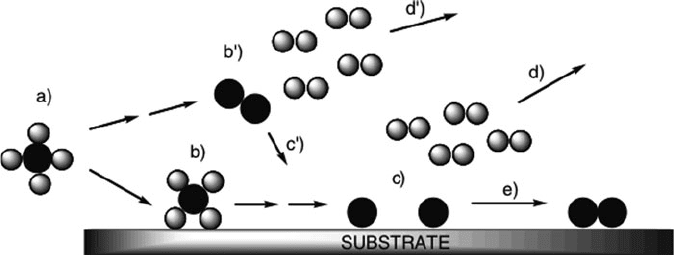
The steps involved in the growth of thin films by CVD are shown in Figure 4.54.
Once the gas-phase precursor molecules enter the deposition zone, (a), they are
physisorbed to the substrate surface through weak van der Waals interactions, (b).
The ancillary ligands are removed through thermolysis, leaving the desired residual
species on the surface, (c), and ligands being removed from the deposition chamber
via the carrier gas flow, (d). Strong covalent interactions are then formed between
the surface and adsorbed species, resulting in chemisorption. The surface-bound
species may migrate along the surface and/or react with other surface species,
eventually nucleating on thermodynamically favored positions, (e) en route toward
thin-film growth. Since film growth occurs through surface migration of intermedi-
ate species, CVD is the method-of-choice for depositions onto irregular surfaces
where conformality is not possibl e using PVD techniques (Figure 4.55).
In addition to the surface governed reactions described above, there are also
gas-phase reactions that may take place between precursor/intermediate molecules.
Although these reactions may also be important in the growth mechanism (e.g., for
plasma-enhanced CVD), significant gas-phase reactions will result in less desirable
impure, granular, non-adhering, and non-conformal films. At relatively high tem-
peratures, the gas-phase precursors may preferentially react with one another rather
than adsorbing to the substrate surface (b
0
,d
0
). The gas-phase nucleation causes
granules to form and fall to the substrate surface due to gravitational forces (c
0
).
Typically, these reactions may be minimized by lowering the deposition tempera-
ture, which will facilitate the surf ace-bound growth route. Though gas-phase growth
is generally not desired for thin-film applications, this technique has also b een
applied for the synthesis of nanopa rticles.
[48]
Figure 4.54. The important steps involved in CVD using the thermolysis of precursor molecules (see text
for details).
4.2. Silicon-Based Applications 299
By definition, CVD is a non-equilibrium process. Although thermodynamics may
provide useful information about the overall energetics of the growth process,
kinetics must be used to provi de information regarding reaction pathways or the
transformation rates of the gaseous precursors. The kine tic description of CVD is
divided into two parts: mass transport and the rates of the specific chemical reactions
involving the precursor and intermediate species. Since the growth rate for CVD
is relatively slow, the differences in forward and reverse reaction rates for interfacial
events are much smaller than the absolute rates themselves.
[49]
Thus, CVD is
frequently treated as a pseudo-equilibrium system, considering only the vapor and
solid immediately adjacent to the interface, the boundary layer.
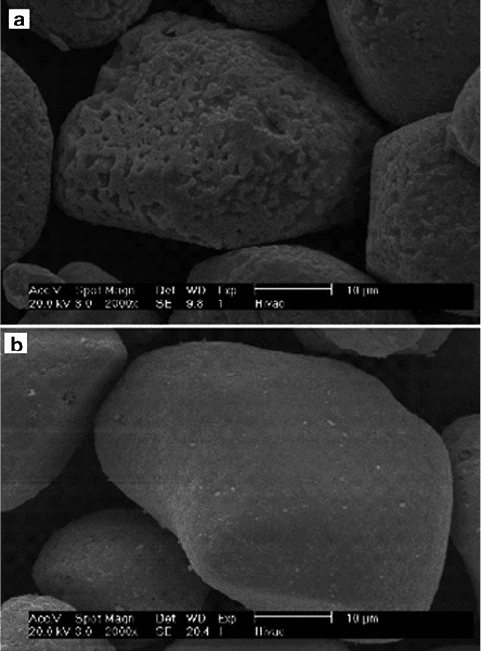
Figure 4.55. SEM images of an electroluminescent phosphor particle, ZnS (used in backlight displays
for cell phones, watches, etc.), before (a) and after (b) the deposition of an aluminum oxide thin film.
This film is a transparent coating that prevents the phosphor particle from undergoing humidity-
accelerated decay. A technique known as fluidized-bed CVD was used, where a carrier gas both
delivered the precursors to a vertically aligned CVD chamber, and dispersed the powdery sample in
order to expose all surface regions to the precursor vapors.
300 4 Semiconductors
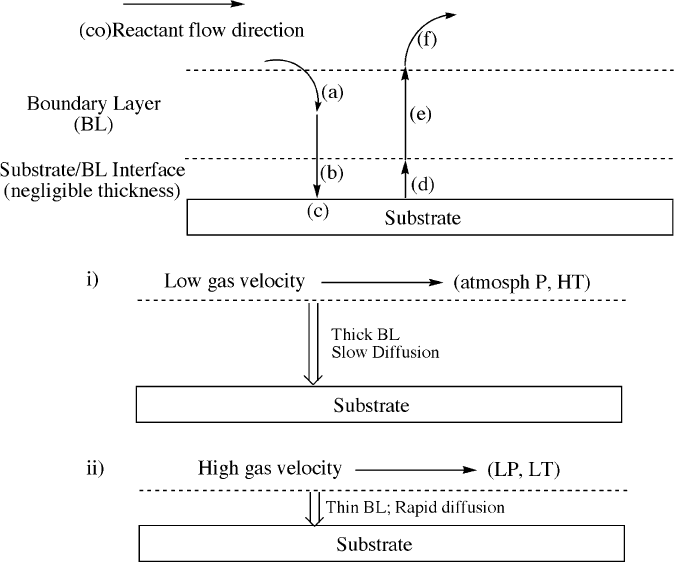
As illustrated in Figure 4.56, the deposition may be controlled by surface-reaction
kinetics (e.g., high gas velocity at low temperatures/pressures), or by diffusion/mass
transport (e.g., low gas velocity at elevated temperatures or pressures (such as
atmospheric)).
[50]
Whereas the deposition rate in the former system is dependent
on the concentration/reactivity of the prec ursor gases, the deposition rate in the latter
is dependent on the diffusion rates of reactants and byproducts. Though the substrate
is often placed horizontally in the CVD chamber, it is more desirable to tilt the
substrate in order to increase the deposition rate and film-thickness homogeneity.
For horizontally-positioned substrates, the velocity of the precursor vapor does
not remain constant across the substrate surface, but will decrease downstream.
Accordingly, the thickness of the boundary layer will increase at downstream
substrate positions, giving rise to depressed thicknesses of the deposited film
Figure 4.56. Schematic of the kinetically-controlled processes involved in MOCVD. When molecular
precursors are introduced into the reaction chamber, they must first diffuse through the boundary layer,
(a). The precursors or reactive intermediates/radicals are then adsorbed onto the substrate surface,
(b), where surface chemical reactions take place, (c). The ancillary ligands and organic residues from
the precursor are then desorbed from the substrate surface, (d), and must diffuse through the BL,
(e), en route from the deposition chamber as gaseous by-products, (f). Also shown are the conditions
that give rise to i) diffusion/mass transport, and ii) surface-reaction controlled kinetics.
4.2. Silicon-Based Applications 301
along the flow direction. By tilting the substrate, the gas velocity over the wafer
surface will increase, leading to a uniform boundary layer thickness. This will also
enhance the residence time of precursor vapors over the substrate surface, leading to
increased growth rates.
At the microscopic level, there are a few key stages of thin-film growth. From
adsorbed monomers and surface migration, the first stage is formation of subcritical
embryos of varying sizes. These particles will further nucleate while taking on more
precursor adsorption, forming a supercritical sized cluster. These clusters will
coalesce into growth islands, which will expose new regions of the substrate that
serve to adsorb additional precursor species . Isolated surface islands eventually
grow together, leaving holes and channels that are filled by adsorbing precursor
molecules to form a continuous thin film.
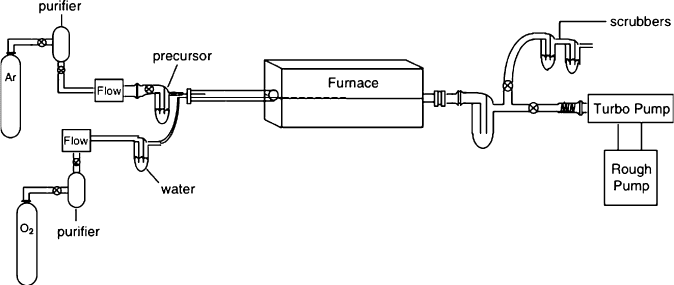
Two general types of reactors are used in CVD proce sses. In a hot-walled reactor
(Figure 4.57), a tube furnace completely surrounds the deposition chamber contain-
ing the substrate. The desired deposition temperature depends on the ther mal
stabilities of the substrate to be coated, and the reactor (i.e., glass (T < 600
C) or
quartz (T < 1,100
C)). Hot-walled reactors are used extensively for laboratory
studies, and also in industry for the CVD of semiconductors and oxides. These
reactors are often preferred d ue to the simplicity of setup, while being able to
maintain a uniform temperature over a large number of substrates. However,
secondary coating of the reactor walls is unavoidable; frequent cleaning of these
reactors is theref ore necessary, as deposits may easily flake off these surfaces and
contaminate the growing film. To prevent such problems, cold-walled reactors may
also be used for a CVD process, wherein only the substrate is heated. This focuses
the surface and gas-phase reactions to a region immediately surrounding the sub-
strate surface. However, this reactor type generally results in narrower deposition
zones and slower growth rates than hot-walled analogues.
Figure 4.57. Schematic of a horizontal hot-walled CVD reactor. Shown is a two-precursor system, where
the water sensitive precursor contacts water vapor directly over the heated substrate.
302 4 Semiconductors
Perhaps more than any other mater ials synthesis technique, semantics becomes a
challenge due to the plethora of acronyms that are used to describe a specific CVD
process. In particular, it is not sufficient to simply cite “CVD” alone in an article
title; a more explicit acronym must be used that states the type of deposition
chamber and precursor decomposition methodology employed. If no prefix is
affixed to CVD, it usually denotes simple thermolysis of a prec ursor within a
standard cold- or hot-wall reac tor. Howeve r, if more energetic sources of energy
are used to degrade the precursor such as laser, plasma, or micro wave plasma, the
acronyms laser-assisted CVD (LACVD), plasma-enhanced CVD (PECVD), and
microwave plasma CVD (MPCVD) are used, respectively. Rather than using high-
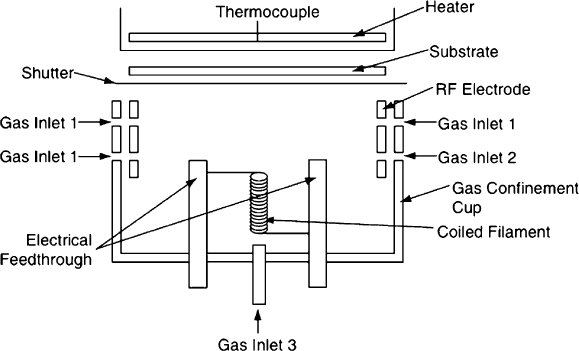
energy plasma sources, a relatively new CVD technique uses a heated filament to
degrade precursor gases and form reactive intermediates. This technique is referred
to as either catalyzed CVD (Cat-CVD) or hot-wire CVD (HWCVD, Figure 4.58).
[51]
The pressure of the reactor must als o be defined, and acro nyms APCVD, LPCVD,
and SCF-CVD are used to denote ambient, in vacuo, and supercritical pressure
conditions within the deposition chamber, respectively. In general, the resultant film
conformality of a CVD process follows the order LPCVD > APCVD, due to the
relative migration rates of intermediate species along the surface of the substrate.
However, it has recently been shown that conformal thin films of metal oxides may
be deposited on nonplanar substrates such as carbon fibers under APCVD, even at
low temperature.
[52]
While plasma-enhanced methods are very useful to lower the substrate tempera-
ture, the as-deposited films are typically less conformal and often contain more
surface impurities than competing methods. In this method, reactive radicals, ions,
and atoms/molecules are formed in the gas phase that interact with the relatively
Figure 4.58. Schematic of a hot-wire CVD system. Reprinted from Povolny, H. S.; Deng, X. Thin Solid
Films 2003, 430, 125. Copyright 2003, with permission from Elsevier.
4.2. Silicon-Based Applications 303

low-temperature substrate to generate a film. Some of the more recent applications
for plasma CVD include growth of cubic boron nitride (c-BN) thin films.
[53]
In recent years, a complementary process known as atomic layer deposition
(ALD) has been widely utilized.
[54]
In contrast to CVD, ALD features the sequential
exposure of a substrate to two or more precursors. The precursor vapors are pulsed
into the reactor one at a time, bein g separated by inert-gas purges or evacu ation
(Figure 4.59). This establishes user-controlled deposition cycles, each comprising
surface saturation by one precursor, followed by the self-limiting surface reaction of
a second precursor. Consequently, deposition is unaffected by varying vaporization
rates of solid precursors – especially problematic for CVD. Further, ALD is gener-
ally carried out at lower temperatures than CVD since co-reactants are highly
reactive toward each other (e.g., trimethylaluminum and water vapor). In contrast,
using the same co-reactants for CVD results in hard-to-control gas-phase reac-
tions, even at low temperatures.
[55]
Since film growth occurs sub-monolayer at a
time, ALD allows for an unprecedented control over the resultant film thickness,
conformality, homogeneity, and stoichiometry. This strategy will become most
useful for the deposition o f the thin high-k gate oxide layers (e.g., HfO
2
, ZrO
2
)
required for next-generation CMOS devices.
Ideally, the film thickness (i.e., growth rate) will be uniform across the entire
substrate surface. The flux of gas molecules that impinge on a substrate surface may
be expressed as Eq. 17:
J=
P
ffiffiffiffiffiffiffiffiffiffiffiffiffiffi
2pmkT
p
ð17Þ
where: J is the rate at which gas-phase molecules hit a surface (in units m
2
s
1
); m
is the mass of a gas molecule; k is the Bolt zmann’s constant (1.38 10
23
JK
1
);
T is the temperature in K. Hence, the growth mechanism of ALD is unaffected by
the presence of an inert carrier gas, as long as it exhibits a purity level of at least the
parts-per-billion (ppb) level.
In theory, ALD growth proceeds by one atomic layer per cycle; however, due to
steric hindrances and limited number of reactive surface sites, the growth rate per
cycle is a fraction of a monolayer (ML) thickness – typically less than 0.9 ML.
Substrate
B layer
A
Pulse
B
Pulse
A
Pulse
B
Pulse
A layer
Purge Purge Purge
Figure 4.59. Illustration of atomic layer deposition (ALD).
304 4 Semiconductors
The sticking coefficient,S
c
, is often used to describe the fraction of incident
molecules that adsorb upon the substrate surface. In general, S
c
is dependent upon
the degree of coverage, temperature, and crystal structure/reactivity of the substrate
surface. It should be noted that S
c
is generally lower for CVD/ALD relative to PVD
techniques. Consequently, the lower S
c
results in being able to coat complex
topographies (conformal growth), as well as selected areas of the substrate.
A number of optimization runs must be performed in order to achi eve self-limited
growth, known as the ALD window. If the thickness is greater at the inlet end, it may
be due to insufficient purging between pulses, or too low a deposition temperature
that results in condensation of the precursor (i.e., physisorption is occurring rather
than chemisorption). If the thickness increases toward the outlet end, it is usually a
sign of too high a deposition temperature that is causing surf ace decomposition of
the precursor. If the purge/evacuati on cycles are too long, the precursor may be
desorbed from the substrate. This would result in film thickness that decreases
along the inlet-outlet direction. Finally, if the growth rate is too slow, the system
is activation-e nergy limited; that is, the temperature must be increased in order to
facilitate a suitable level of precursor reactivity.
Oftentimes, the deposition mechanisms between ALD and CVD are quite
different, even for identical precursor combinations. In particular, it is well known
that thin-film growth by CVD is heavily influenced by side reactions (Figure 4.60)–
not as problematic for ALD (Figure 4.61). A CVD process often generates reaction
products such as CO, RH, or HCl that may be preferentially adsorbed onto the
substrate surface. This will cause film-growth termination unless the competitive
adsorbents are removed through purging with inert gas or introduction of a reducing
gas such as H
2
.
The chemical nature of the prec ursor represents the most critical component of
a CVD/ALD process. Generally speaking, the choice of a particular precursor is
governed by the relative stabilities of the precursor and substrate, as well as the
volatility, cost, and hazards of the precursor. The coordination sphere of ligands
surrounding the central metal is extremely important; the organic ligands in these
precursors may lead to contamination of the films if they are not completely
removed through a combination of pyrolysis, reduc tion, or oxidation processes.
Some precursors pose a high risk when being used. For example, Ni(CO)
4
has
a very high toxicity, Al(Me)
3
is pyrophoric, B
2
H
6
is explosive, and chloride-
containing species are corrosive. In general, organometallic precursors pose lower
hazards than hydrides and halides, but are much more costly.
Although it was once essential that volatile precursors be used, this is no longer a
synthetic limitation. Within the last decade, the gas/liquid properties of supercritical
fluids (e.g.,CO
2
) have been used to solvate certain precursors, facilitating their
use for CVD.
[56]
Two variations of this tech nique may be used; supercritical fluid
transport (SFT), using the fluid as an aerosol-like delivery vehicle, or in situ thin-
film growth within a high pressure reactor, known as supercritical fluid deposition
(SFD). In these methods the precursor must be soluble in CO
2
, which is analogous
in solvating ability to hexane with an enhanced fluorophilic character.
4.2. Silicon-Based Applications 305

Figure 4.60. Illustration of chemical vapor deposition (CVD) of an aluminum film from Al(O
i
Pr)
3
,
showing the influence of temperature (decomposition route) on the resultant film purity.
306 4 Semiconductors
A CVD process may involve the use of either single or mixed precursors. The best
precursor is a molecule that has sufficient volatility (or CO
2
solubility), and contains
labile ligands that will leave no organic residue behind during its surface-catalyzed
decomposition. In general, volatile liquids and oils are attractive CVD precursors due
to the relative ease of vapor transport through simple carrier-gas bubbling, vaporiza-
tion, or direct liquid injection (DLI) techniques (Figure 4.62). However, low-melting
powders with high volatilities and low decomposition temperatures are regarded as the
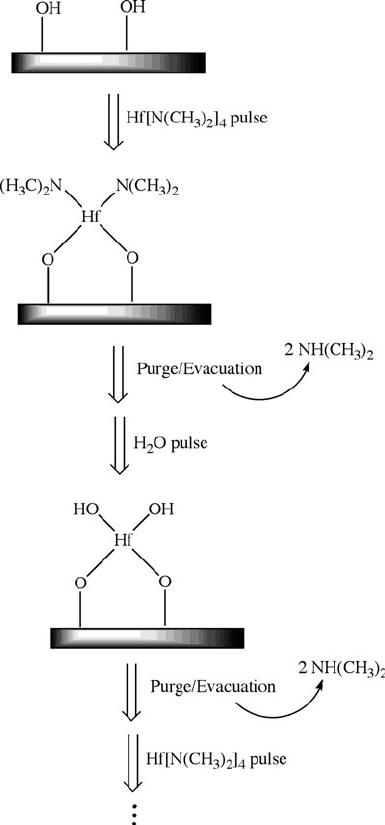
Figure 4.61. Stepwise scheme for the atomic layer deposition (ALD) of a HfO
2
thin film from Hf
(NMe
2
)
4
/H
2
O sequential pulsing.
4.2. Silicon-Based Applications 307
