Fahlman B.D. Materials Chemistry
Подождите немного. Документ загружается.

Rather than having to use a mold for nanocontact printing, one may also directly
write features onto an appropriate substrate using a molecular ink, via the ultra-fine
tip of an atomic force microscope
[79]
(Figure 4.75). The use of such a “nanofountain
pen” is known as dip-pen nanolithography (DPN), first demonstrated by Mirkin and
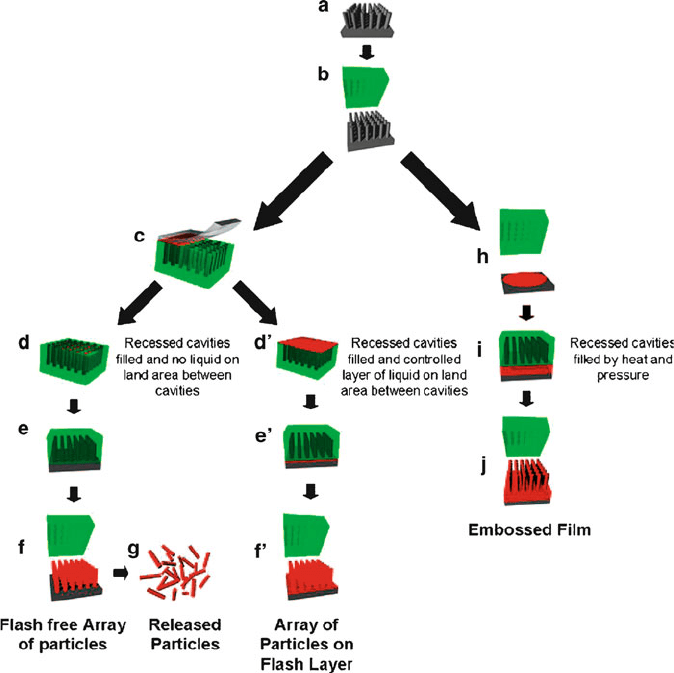
Figure 4.71. Schematic illustration of the particle/pattern replication in nonwetting templates (PRINT)
process and traditional embossing processes. Shown are: (a) silicon master template, (b) mold release
from master template, and (c) mold filling via capillary fill with counter-sheet having a higher surface
energy than the PFPE mold. Depending on the exact nature of the liquid to be molded and the details of the
process, (d) one can fill the cavities only and not wet the land area around the cavities or (d
0
) one can
fill the cavities and have a thin layer of liquid on the land area around the cavities. The thickness of the
layer of connecting flash layer liquid is determined from the principles associated with free meniscus
coating processes with the resulting (e, e
0
) pattern transfer to substrate, (f, f
0
) mold release from array of
isolated features, and (g) dissolution of the harvesting film to yield free particles. As an alternative to
PRINT, one can use PFPEs using traditional embossing processes where pressure and heat are applied
(h, i) to form an embossed film (j) after the mold is removed. Reproduced with permission from Acc.
Chem. Res. 2008, 41, 1685. Copyright 2008 American Chemical Society.
318 4 Semiconductors
coworkers in the late 1990s.
[80]
Though the earliest examples of DPN featured
alkylthiols as the ink onto Au surfaces, there are now an increasingly large number
of other ink/substrate combinations that have been reported for DPN (Table 4.5).
[81]
A general benefit of DPN over other soft lithographic techniques is the ability to
pattern nanostructures (including biological materials) by a single step without
cross-contamination, since the desired chemistry occurs only in a specifically
defined location of the substrate.
The mechanism of ink transport from the AFM tip to substrate is currently an item
of controversy. Recent models suggest that a meniscus forms between the tip and
substrate, which aids in ink transport. As a result, the transport rate is found to
increase concomitantly with the ambient humidity – but only for inks that are
soluble in water. In general, the rate of ink transport is found to decrease signifi-
cantly with increasing contact time, due to the changing surface energy of the
substrate. As you might imagine, there are many factors that govern ink transport
and the final resolution of the printed nanostru cture – tip shape, ink composition/
concentration, substrate surface properties, and ambient conditions. It is clear that
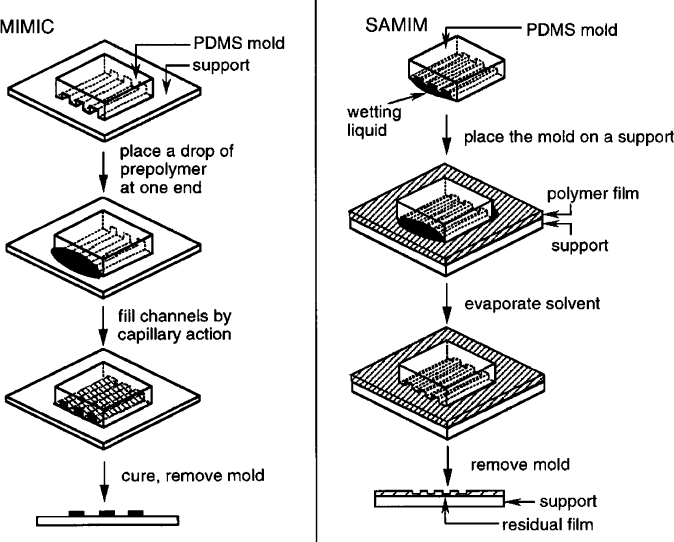
Figure 4.72. Schematic of soft lithographic procedures for (a) replica molding (REM), (b) microtransfer
molding (mTM), (c) micromolding in capillaries (MIMIC), and (d) solvent-assisted micromolding
(SAMIM). Reproduced with permission from Angew. Chem. Int. Ed. 1998, 37, 550. Copyright 1998
Wiley-VCH.
4.2. Silicon-Based Applications 319
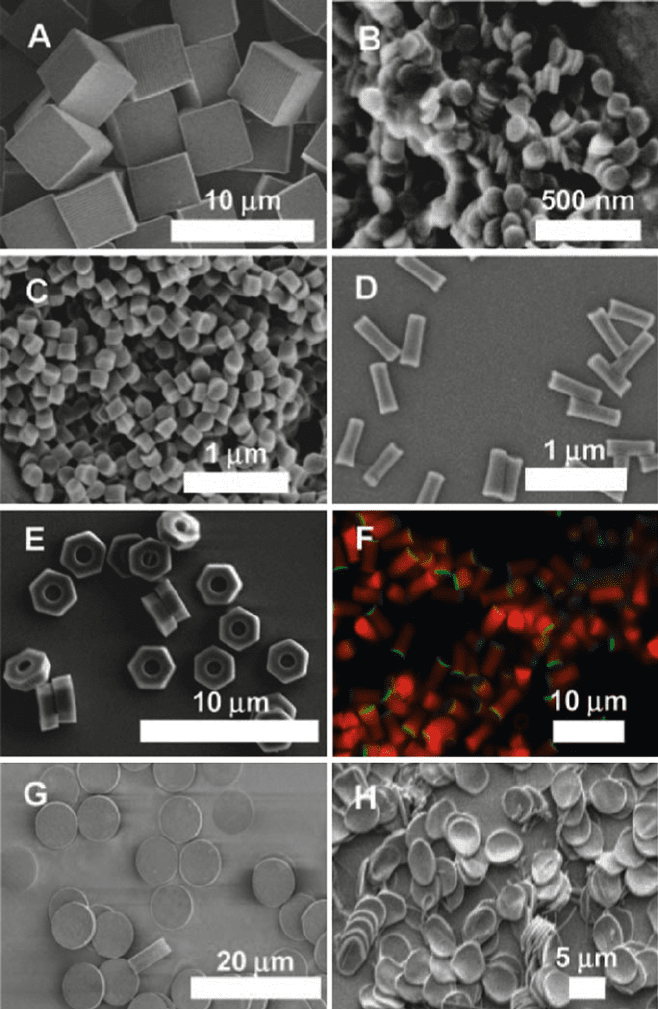
Figure 4.73. PRINT particles varying in size, shape, surface chemistry, and deformability. The particle
composition for all of these particles was approximately the same and included PEG (bulk of the matrix), a
cross-linker, and a linker group for conjugation of stabilizing groups (such as PEG) or targeting ligands
320 4 Semiconductors
the current 15-nm reso lution of DPN may only be improved once a full picture of ink
transport is fully understood.
4.3. LIGHT-EMITTING DIODES: THERE IS LIFE OUTSIDE
OF SIL ICON!
Thus far, we have considered the structure and applications of Si – the most heavily
employed semiconductor. It should be noted that gallium arsenide (GaAs) is also
widely used for FET applications; since GaAs does not form a natural protective
Figure 4.73. Continued (such as peptides, antibodies, etc.): (a) scanning electron micrograph (SEM) of
cube-shaped particles with a cube side length of 5 m m; (b) SEM of cylindrical nanoparticles having
diameter) 110 nm and height) 35 nm; (c) SEM of cylindrical nanoparticles having diameter) 200 nm and
height) 200 nm; (d) SEM of rod-like PRINT particles having diameter) 100 nm and height) 300 nm;
(e) SEM of 3 mm “hex nut” particles; (f) cylindrical PRINT particles containing a covalently attached red
fluorophore that have been functionalized on one face with a generic linker group (green fluorophore) that
will allow the conjugation of targeting peptides, antibodies, and aptamers region-specifically onto the
particle probes; (g, h) particles for mechanobiology studies having approximately the same dimensions as
red blood cells (cylinders with a diameter) 7 mm and a height of 1.7 mm made from (g) a nondeformable,
highly cross-linked hydrogel and (h) lightly cross-linked, deformable hydrogel. Reproduced with
permission from Acc. Chem. Res. 2008, 41, 1685. Copyright 2008 American Chemical Society.
ä
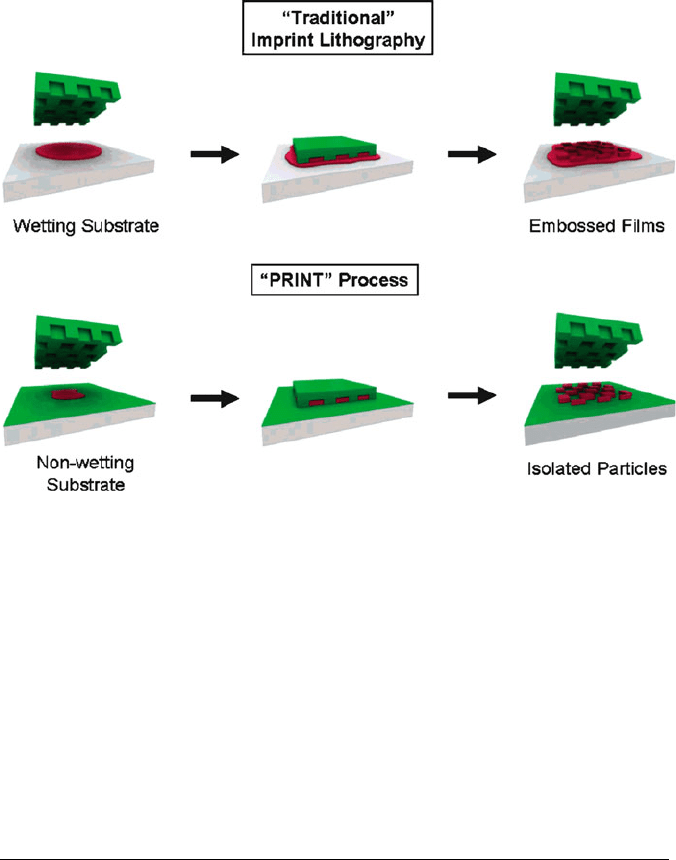
Figure 4.74. Illustration of the PRINT process compared to traditional imprint lithography in which the
affinity of the liquid precursor for the surface results in a scum layer. In PRINT, the nonwetting nature
of fluorinated materials and surfaces (shown in green) confines the liquid precursor inside the features
of the mold, allowing for the generation of isolated particles. Reproduced with permission from J. Am.
Chem. Soc. 2005, 127, 10096. Copyright 2005 American Chemical Society.
4.3. Light-Emitting Diodes: There is Life Outside of Silicon! 321
layer analogous to SiO
2
onto Si, CVD is used to grow films such as GaS for surface
passivation. Many other direct bandgap semiconductors such as II–VI (e.g.,ZnS,
ZnSe) and III–V (e.g., GaN, GaP) are widely used for optoelectronic (light emission)
and photonic (light detection) applications. The most important applications for non-
Si semiconductors are light-emitting diodes (LEDs) and solid-state lasers. Unlike Si-
based devices, the bandgap of compound semiconductors may be significantly
altered by varying the stoichiometry of the composite elements.
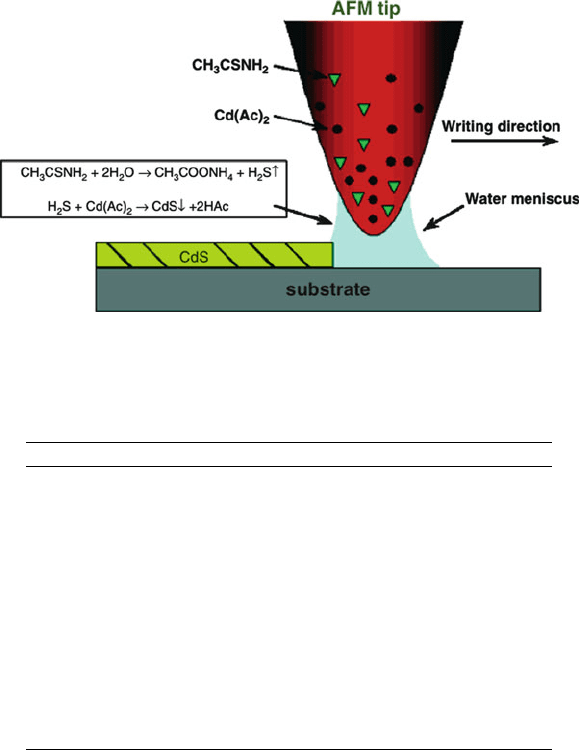
Figure 4.75. Illustration of dip-pen nanolithography, used to write nanoscale features of CdS on mica and
SiO
x
substrates. Reproduced with permission from Ding, L.; Li, Y.; Chu, H.; Li, X.; Liu, J. J. Phys. Chem.
B 2005, 109, 22337. Copyright 2005 American Chemical Society.
Table 4.5. Summary of the Ink-Substrate Combinations Used to Date for DPN
[82]
Molecular ink Substrate
Alkylthiols (e.g., ODT
a
and MHA
b
)Au
Ferrocenylthiols Au
Silazanes SiO
x
, GaAs
Proteins Au, SiO
x
Conjugated polymers SiO
x
DNA Au, SiO
x
Fluorescent dyes SiO
x
Sols SiO
x
Metal salts Si, Ge
Colloidal particles SiO
x
Alkynes Si
Alkoxysilanes SiO
x
ROMP materials SiO
x
Thioacetamide/cadmium acetate
c
SiO
x
, mica
a
1-Octadecanethiol.
b
16-Mercaptohexadecanoic acid, or thiohexadecanoic acid.
c
Ding, L.; Li, Y.; Chu, H.; Li, X.; Liu, J. J. Phys. Chem. B 2005, 109, 22337.
322 4 Semiconductors
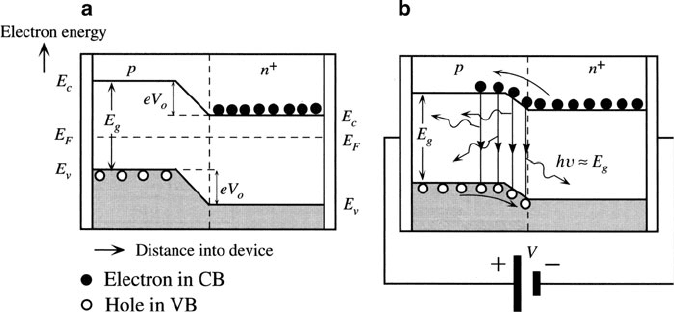
A light-emitting diode is a p–n junction device that is comprised of a direct
bandgap semiconductor(s). The recombination of an electron-hole pair (EHP)
results in photon emission, with energy equivalent to the bandgap, E
g
(Figure 4.76).
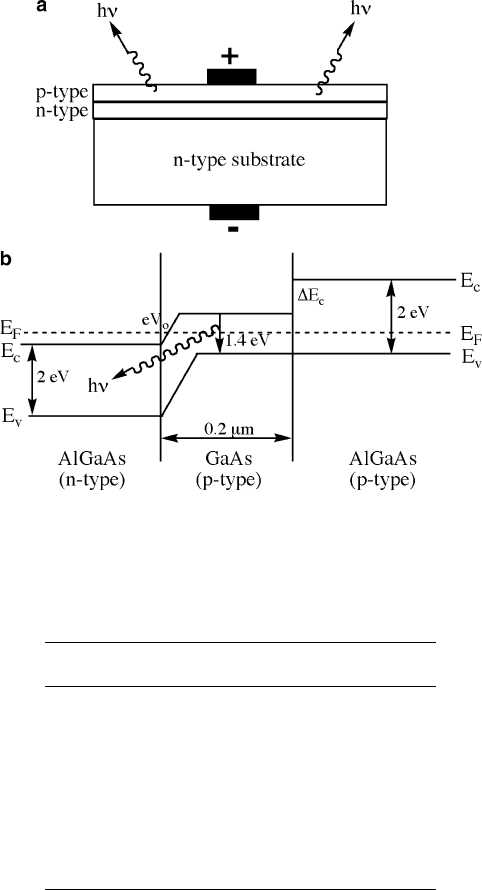
The LED may be structured as a simple multilayer (Figure 4.77a), or as a hetero-
junction that is comprised of two different bandgap semiconductors (Figure 4.77b).
The latter is preferred for high-intensity LED applications, since the emission is
confined to certain regions of the device.
At present, the applications for LEDs have been centere d on commercial elec-
tronic displays such as clock radios, microwave ovens, watche s, etc. However, the
availability of LEDs in a spectrum of colors has opened the floodgates for new
applications. The greatest breakthrough was realized in the 1990s with the discovery
of wide bandgap blue LEDs, making it possible to create any color of light
(Table 4.6). Approximately 10–15% of the traffic lights in the United States have
now been replaced with LED-based lamps. New automobiles also utilize this
lighting for ultrabright brake and turn-signal lighting. The higher initial cost of the
LEDs is quickly recovered due to their greater efficiency in converting electrical
current to light emission relative to incandescent lighting. That is, whereas LEDs
consume 20–1,000 mW, an incandescent bulb of similar brightness consumes ca.
50–150 W. Indeed, once an inexpensive white LED becomes commercially avail-
able, our society will forever change as we shift from our longstanding reliance on
inefficient and short-lived incandescent bulbs. The light emission from LEDs is
classified as a type of luminescence, which is different from incandescence – the
generation of light from a material as a result of its high temperature. Since LEDs
glow as a result of an electrical current, this emission is referred to as electrolumi-
nescence. Two other common types of luminescence include chemoluminescence
Figure 4.76. Band diagram of a p–n junction with a) no bias voltage, and b) with forward bias, V,
resulting in photon emission. Reproduced with permission from Kasap, S. O. Principles of Electronic
Materials and Devices, 3rd ed., McGraw-Hill: New York, 2007. Copyright 2007 The McGraw-Hill
Companies.
4.3. Light-Emitting Diodes: There is Life Outside of Silicon! 323
(induced by a chemical reaction(s); e.g., “glow sticks”), and photoluminescence
(induced through photon excitation; e.g., vaseline glass discussed in Chapter 2).
If the emission is prolonged, lasting long after the stimulation source is removed,
it is known as phosphorescence; otherwise, the short-lived process is termed
fluorescence.
Figure 4.77. Light-emitting diode (LED) structures. Shown are (a) a simple multilayer structure of
epitaxial p- and n-type layers, and (b) a double heterostructure with accompanying band diagram,
illustrating light emission from the p-type region due to confinement between wide-bandgap
surrounding layers.
Table 4.6. Comparison of the Observed Colors of LEDs
Observed color Wavelength of
emission (nm)
Semiconductor
(Infrared) 880 GaAlAs/GaAs
Red 660 GaAlAs/GaAlAs
Red 633 AlGaInP
Orange 612 AlGaInP
Orange 605 GaAsP/GaP
Yellow 585 GaAsP/GaP
Green 555 GaP
Blue 470 GaN/SiC
Ultraviolet 395 InGaN/SiC
White – InGaN/SiC
324 4 Semiconductors

As we saw earlier in this chapter, the wavelength (and color) of light emitted by a
direct bandgap material through electron-hole recombination is influenced by its
bandgap. In order to change the wavelength of emitted radiation, the bandgap
of the semiconducting material utilized to fabricate the LED must be changed.
For instance, gallium arsenide has a bandgap of 1.35 eV (Table 4.7), and emits in
the infrared (ca . 900 nm). In order to decrease the wavelength of emission into the
visible red region (ca. 700 nm), the bandgap must be increased to ca. 1.9 eV. This
may be achieved by mixing GaAs with a material with a larger bandgap, such as GaP
(E
g
¼ 2.35 eV). Hence, LEDs of the chemical composition GaAs
x
P
1x
may be used
to produce bandgaps from 1.4 to 2.3 eV (and varying colors), through adjustment of
the As:P ratio.
The bandgap and concomitant wavelength of light that is emitted from LEDs is
related to the bond strength between atoms in the lattice. For these compounds, as
the bond strength increases, there is more efficient overlap between molecular
orbitals that gives rise to a larger bandgap between bonding and antibonding MOs
(i.e., valence and conduction bands of the infinite lattice, respectively). For a
particular Group 13 metal, as one moves down the Group 15 Period, the bonding
interaction between III–V elements will become weaker through the interaction of
more diffuse atomic orbitals. For instance, the bond strengths of Ga–N and Ga–As
bonds are 98.8 and 50.1 kcal mol
1
, respectively. The larger bandgap for GaN
relative to GaAs translates to a short wavelength (blue color) of emitted light that is
observed.
Most white LEDs employ a semiconductor chip emitting at a short wavelength
(blue), and a wavelength converter that absorbs light from the diode and undergoes
secondary emission at a longer wavelength. Such diodes emit light of two or more
wavelengths, that when combined, appear as white. The most common wavelength
converter materials are termed phosphors (e.g., ZnS – Figure 4.55), which exhibit
luminescence when they absorb energy from another radiation source. Typical LED
phosphors are present as a coating on the outside of the bulb, and are composed of an
inorganic host substance (e.g., yttrium aluminum garnet, YAG) containing an
optically active dopant (e.g., Ce). Use of such a single-crystal phosphor produces
a yellow light, upon combination with blue light gives the appearance of white.
A similar result has recently been produced through use of CdSe nanoparticles (see
Table 4.7. Bandgaps of III–V Semiconductors
Semiconductor Bandgap (eV)
AlN 6.02
AlP 2.45
GaN 3.50
GaP 2.35
GaAs 1.35
GaSb 0.67
InN 1.95
InP 1.27
InAs 0.36
4.3. Light-Emitting Diodes: There is Life Outside of Silicon! 325
Chapter 6).
[83]
White LEDs may also be made by coating near ultraviolet (NUV)
emitting LEDs with a mixture of europium-based red and blue emitting phosphors,
plus green emitting copper- and aluminium-doped zinc sulfide (ZnS:Cu,Al). It is
also possible for LEDs to emit white light without the use of phosphors. For
instance, homo epitaxially grown ZnSe crystals simultaneously emit blue light
from the film, and yellow light from the ZnSe substrate.
Recently, there has been much interest in organic light-emitting diodes (OLEDs).
Flat-panel televisions, cellular phones, and digital cameras are already beginnin g to
employ this technology; it is only a matter of time before the “holy grail” of flexible
display screens and luminous fabric s are produced. OLED displays offer many
benefits relative to standard CRTs and LCDs such as enhanced brightness, lower
power consumption, and wider viewing angles.
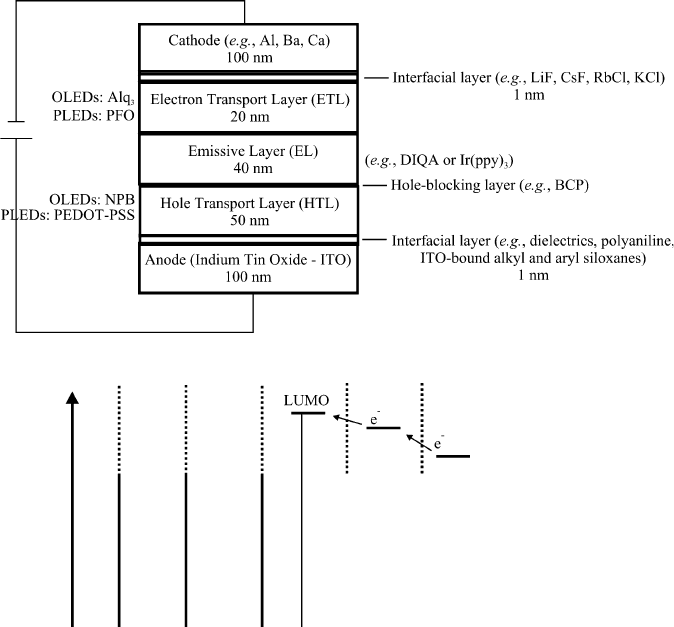
The multilayered structure and electroluminescent mechanism of OLEDs is illu-
strated in Figure 4.78. Depending on whether small organic molecules or long
Figure 4.78. Multilayered structure of OLEDs/PLEDs. Also shown are the relative energy levels for
individual layers; light is emitted as a result of the radiative recombination of electron-hole pairs.
326 4 Semiconductors
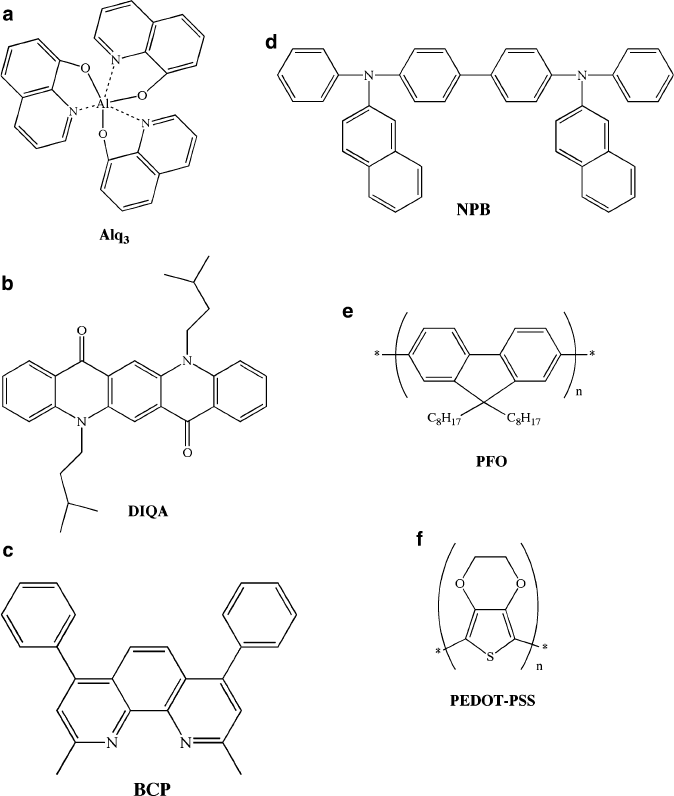
repeating-unit polymers are used (Figure 4.79), the diodes are referred to as OLEDs
or PLEDs, respectively. Under positive current, electrons and holes are injected into
the emissive layer from opposite directions – from the cathode and anode, respec-
tively. The metal cathode is usually an alkaline earth or Al, which readily release a
Figure 4.79. Molecular structures of commonly used OLED/PLED materials. Shown are: (a) Alq
3
(tris
(quinoxalinato)Al (III)) used as an electron-transport material; (b) DIQA (diisoamylquinacridone) used as
an emissive dopant; (c) BCP (2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline) used as an exciton/hole
blocking agent; (d) NPB (1,4-bis(1-napthylphenyl amino)biphenyl); (e) PFO (9,9-dioctylfluorene) used as
an emissive polymer in PLEDs; (f) PEDOT–PSS (poly-3,4-ethylenedioxythiophene–polystyrene
sulfonate) used as a hole transport material in PLEDs.
4.3. Light-Emitting Diodes: There is Life Outside of Silicon! 327
