Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.

Ch_03.qxd 11/29/04 6:08 PM Page 172

4 Bulk and Grain Boundary Diffusion
in Intermetallic Compounds
Christian Herzig and Sergiy Divinski
Institut für Materialphysik
Universität Münster, Münster, Germany
4.1 Introduction
According to a simple definition,
[1]
the intermetallics are compounds
of two and more metals with structures that differ from those of the con-
stituent metals. The intermetallic compounds exist in a variety of lattice
structures: from the simplest, such as B2 (NiAl, FeAl) and L1
0
(TiAl,
CuAu), to very sophisticated configurations, such as quasicrystalline
i-AlCuFe. The short- and long-range order are important phenomena of
the intermetallic compounds that notably affect their mechanical and
physical properties.
[2]
Thus, sublattices and preferential site occupation
are important terms that describe microscopic features of the atomic dis-
tribution in these compounds. Intermetallic compounds are widely used in
different technological applications and still have an enormous potential
for future applications.
[3]
The development of intermetallic compounds for application as struc-
tural materials inevitably requires the knowledge of the relevant bulk and
grain boundary (GB) self-diffusion and solute diffusion data. During the
last decade, defect and diffusion phenomena in intermetallics have
attracted much attention, not only from the point of view of accumulating
experimental data, but also with the purpose of gaining a deeper insight
into the underlying microscopic diffusion mechanisms.
Recently, general overviews on diffusion in intermetallic compounds
were published.
[4, 5]
The diffusion behavior of some technologically
important compounds was also comprehensively analyzed, namely, the
L1
2
-structures in the Ni–Al, Ni–Ge, and Ni–Ga systems,
[6]
in the Ti–Al
system,
[7, 8]
and in the Fe–Al system.
[9]
Diffusion in pure materials is mostly mediated by the nearly random
motion of vacancies. Due to the ordered structure of intermetallic com-
pounds and the different probabilities of finding a vacancy on a particular
sublattice, diffusion in intermetallics occurs through more sophisticated
jump sequencies involving several atoms. It is very important to realize
that even in a completely ordered intermetallic compound at stoichio-
metric composition, a given amount of thermal defects (vacancies and
Chapter-04.qxd 11/29/04 6:36 PM Page 173

anti-structure atoms) exists at the given temperature T 0. This implies
that the order parameter is not really unity, which has an important con-
sequence: While the given state of order has to be maintained on average,
local deviations are possible. Thus, the relevance of a specific diffusion
mechanism in an intermetallic compound as a well-defined sequence of
atom jumps becomes vague, especially for compounds and compositions
with a large deviation from the perfectly ordered state.
It is still attractive, however, to classify the vacancy motion in terms
of particular diffusion mechanisms that maintain the order locally. Several
mechanisms of this kind, such as the six-jump-cycle mechanism,
[10]
the
triple-defect mechanism,
[11]
and the antistructure bridge (ASB) mecha-
nism,
[12]
were suggested for ordered structures. The concept of particular
diffusion mechanisms is attractive not only because it provides a simpli-
fied physical picture of a complex diffusion behavior, but also because it
allows calculation of effective activation energies and entropies for these
processes (energies and entropies of defect formation, migration, and
binding, as well as correlation effects), as has recently been done, e.g.,
for the six-jump-cycle diffusion mechanism in NiAl.
[13]
Using elaborate
approaches, such as ab initio calculations or molecular static calculations
with embedded-atom (EAM) potentials, we can compare the theoretical
predictions with experimental data and select the most plausible diffusion
mechanism. At present, however, large-scale molecular dynamic calcula-
tions including a large number of isolated vacancies are still impossible.
This chapter focuses on diffusion in ordered binary aluminides of Ni,
Ti, and Fe. In these cases, a lot of new, reliable experimental data exist,
and the diffusion mechanisms are already elaborated in some detail. These
aluminides form different structures, such as B2 (NiAl, FeAl), L1
2
(Ni
3
Al), D0
19
(a
2
-Ti
3
Al), L1
0
(g-TiAl), and D0
3
(Fe
3
Al). Some fundamen-
tal insight into the interdependence of diffusion behavior and diffusion
mechanisms on structure and ordering are provided. The impact of order,
thermal and structural defects, and composition are discussed.
4.2 Crystal Structures and Point Defects
in Ni,Ti, and Fe Aluminides
Phase diagrams for the Ni–Al,
[14]
Ti–Al,
[15, 16]
and Fe–Al
[17]
systems are
shown in Fig. 4.1. Self-diffusion and solute diffusion in ordered aluminides
with XAl and X
3
Al compositions are reviewed here, with X Ni, Ti, or
Fe. These ordered phases exist in wide compositional ranges of the
corresponding phase diagrams. Whereas the TiAl phase field extends pre-
dominantly on the Al-rich side of the stoichiometric composition and FeAl
on the corresponding Fe-rich side, NiAl can accommodate a remarkable
174 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Chapter-04.qxd 11/29/04 6:36 PM Page 174
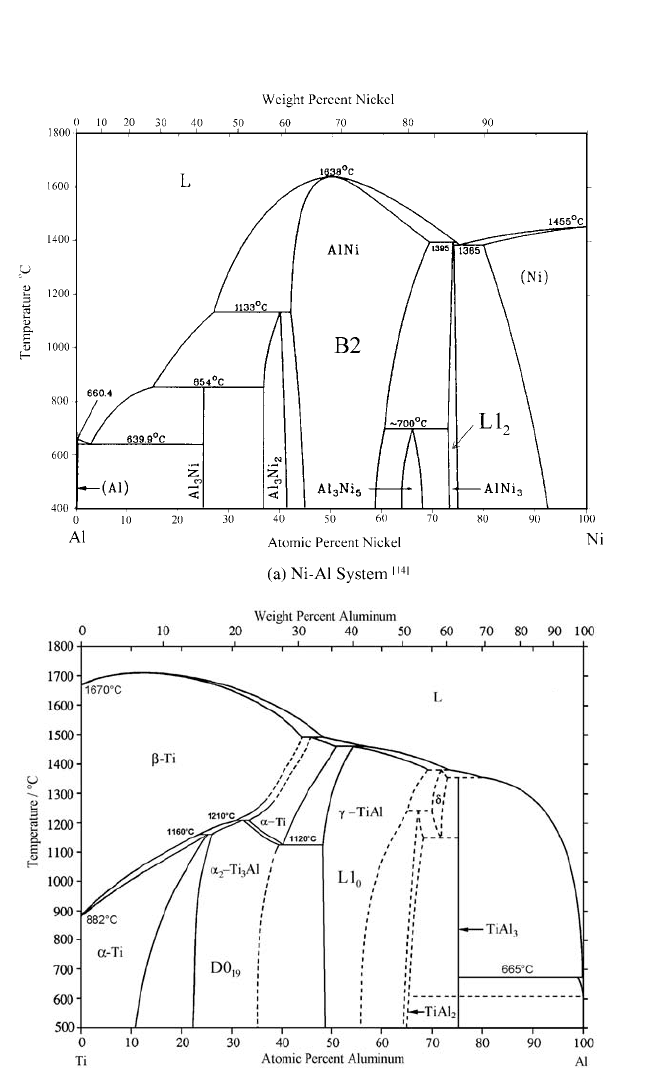
175
(b) Ti-Al System
[15,16]
Figure 4.1 Phase diagrams of Ni-Al, Ti-Al, and Fe-Al. (continued)
Chapter-04.qxd 11/29/04 6:36 PM Page 175
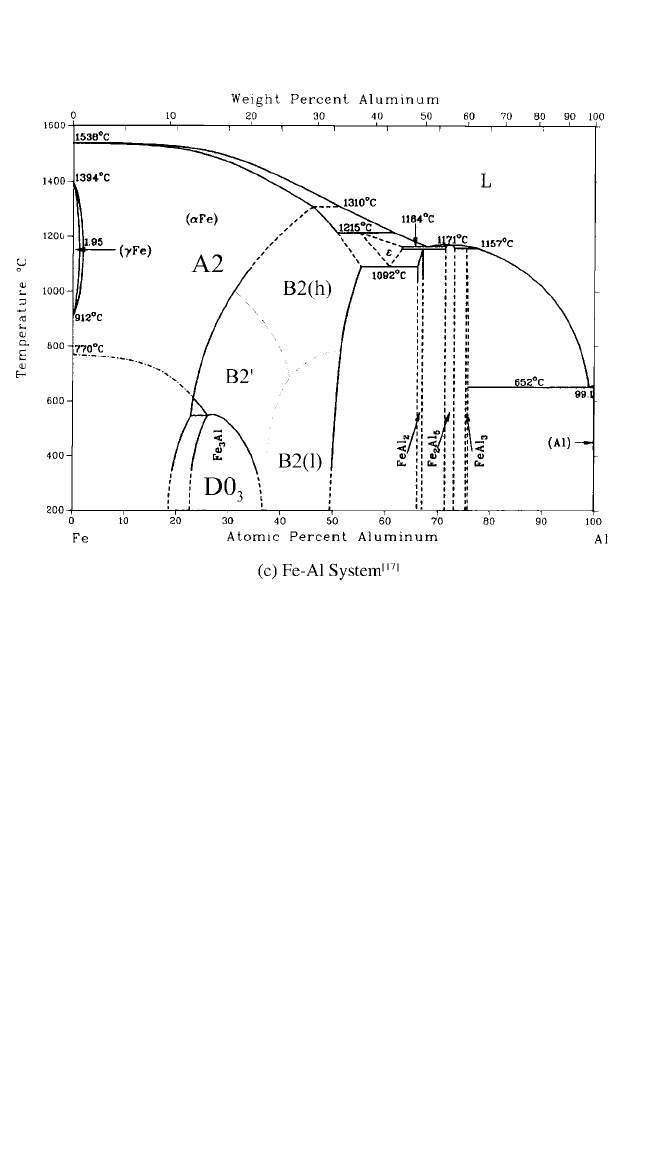
excess of both Al and Ni atoms (Fig. 4.1). The Ni
3
Al phase field also exists
on both sides of the stoichiometric composition, but in a narrower compo-
sitional interval. In contrast, both Ti
3
Al and Fe
3
Al exist as Al-rich ordered
phases in limited temperature intervals. Thus, diffusion studies, especially
in the two nickel aluminides, are very promising from a fundamental point
of view for explaining the influence of composition and defect structure.
In Fig. 4.2, the ideally ordered crystalline structures of Ni, Ti, and Fe
aluminides are schematically presented, that is, the structures at zero tem-
perature and at perfect stoichiometric compositions. As the temperature
increases and/or the composition deviates from stoichiometry, point
defects are inevitably generated. Four types of point defects can be intro-
duced in two-atomic intermetallics AB; namely, the vacancies on both sub-
lattices, V
A
and V
B
, and the atoms on unlike sublattices A
B
and B
A
(the anti-
structure atoms). Both structural (constitutional) and thermal point defects
exist in an off-stoichiometric intermetallic compound. In a strict definition,
the structural defects are those defects that remain in thermal equilibrium
in the intermetallic compound, even at T 0 in its maximally ordered
state,
[18]
to accommodate the deviation from the stoichiometric composi-
tion. The difference between the real concentration of defects at T 0 and
the concentration of the structural defects presents the concentration of
thermal defects. In such a definition, the concentration of thermal defects
176 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 4.1 (Continued)
Chapter-04.qxd 11/29/04 6:36 PM Page 176
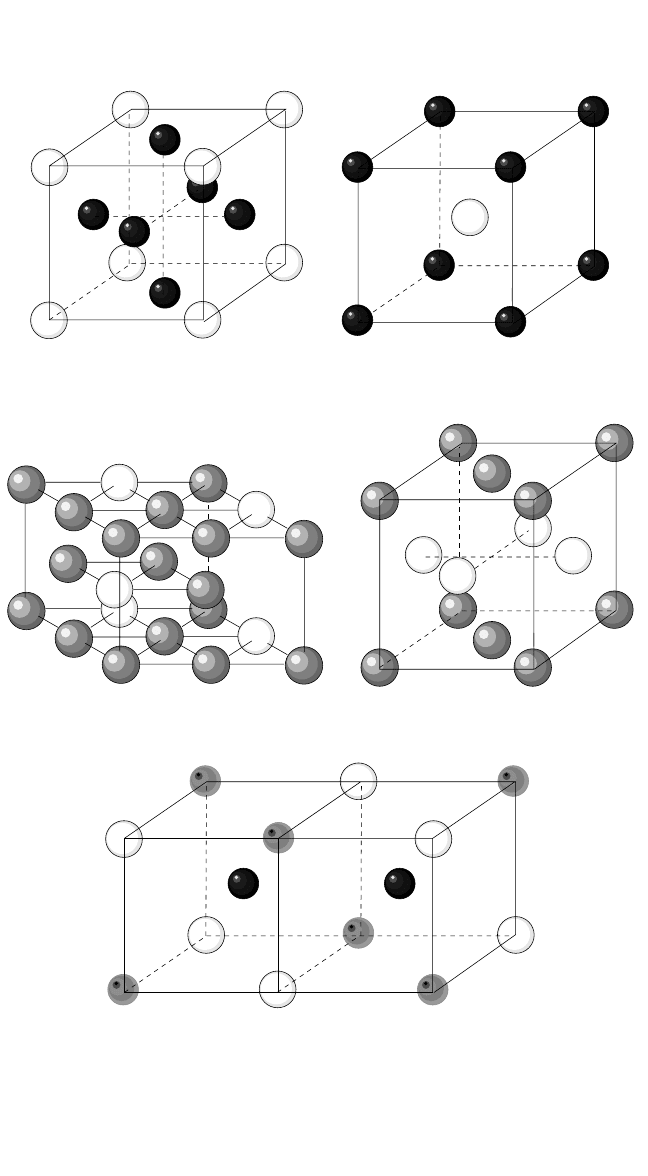
177
(a) Ni
3
Al
Figure 4.2 Lattice structures of the aluminides: Ni
3
Al, NiAl, Ti
3
Al, TiAl, and Fe
3
Al.
Ni, Ti, and Al atoms are represented by black, grey, and white spheres, respec-
tively. In the D0
3
structure of Fe
3
Al (e), black and grey spheres distinguish the two
sublattices for Fe atoms.
(b) NiAl
(c) Ti
3
Al
(d) TiAl
(e) Fe
3
Al
Chapter-04.qxd 11/29/04 6:36 PM Page 177
can even be negative. The Al-rich phase NiAl seems to present such an
example.
[18]
Afurther difference between the structural and thermal defects
stems from the fact that one type of structural point defect is generally suf-
ficient to accommodate the deviation from the stoichiometry, whereas at
least two types of thermal point defects have to be simultaneously created
to satisfy the mass-balance conditions (to preserve the given composition,
that is, the given ratio between the constitutional elements).
Moreover, the point defects do not have to be uniformly distributed
over the different sublattices in an ordered intermetallic compound; this
was established experimentally
[19–21]
and by theoretical analysis.
[22, 23]
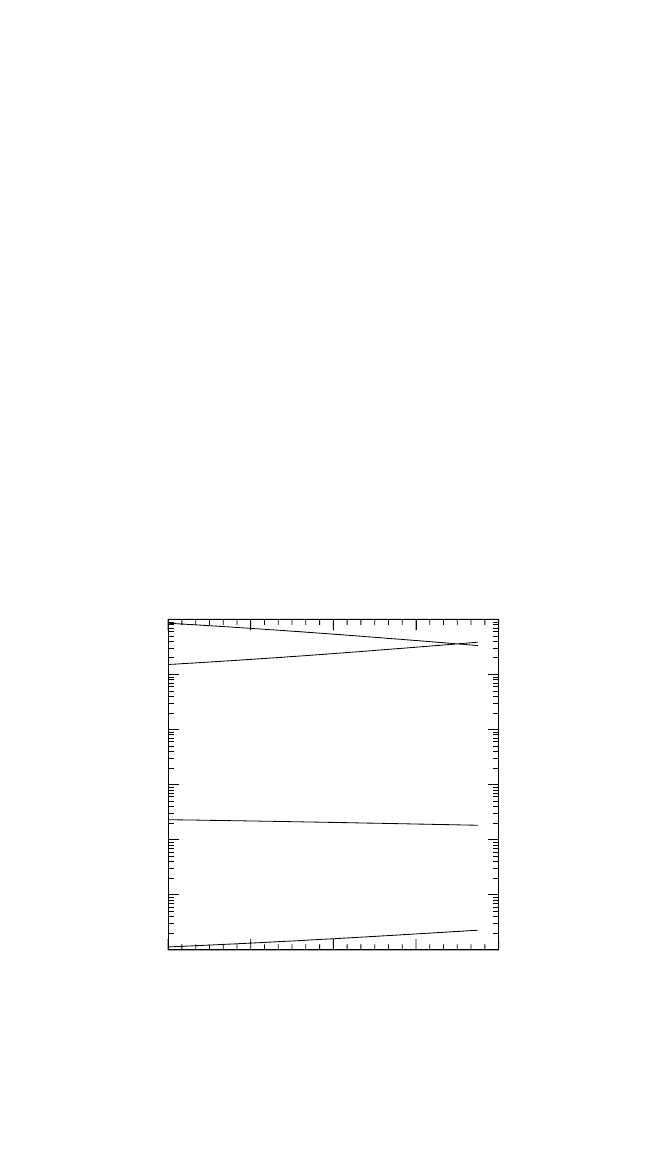
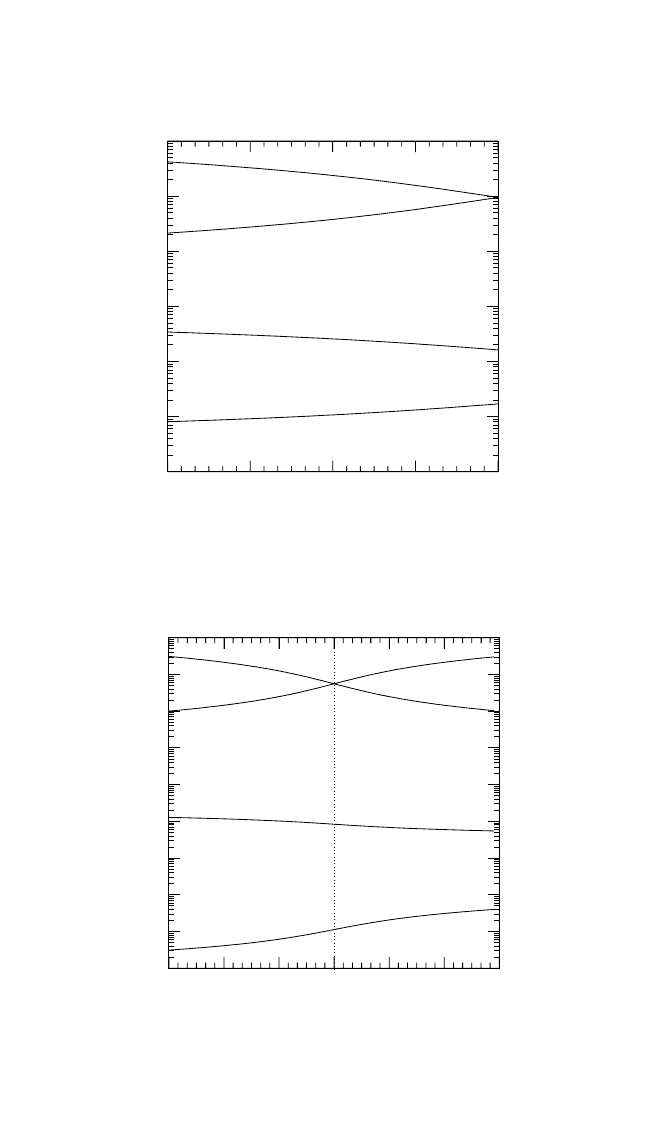
In Fig. 4.3, the concentrations of different defects in the intermetallic
compounds under consideration are compared at T 0.75T
m
. This tem-
perature corresponds to T 1252 K for Ni
3
Al, 1434 K for NiAl, 1457 K
for Ti
3
Al, 1294 K for TiAl, and 1195 K for FeAl. T
m
is the melting tem-
perature of the stoichiometric composition of the given compound.
The defect concentrations were calculated according to the chemical-
reaction approach.
[8]
The concentration of point defects depends on the
formation energies of all four types of defects because, in an intermetallic
compound, point defects are created in a correlated way to preserve the
178 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
68
70 72 74
76
c
Ti
[at.%]
10
-7
10
-6
10
-5
10
-4
10
-3
10
-2
10
-1
Defect concentration
32 30 28 26
24
c
Al
[at.%]
V
Ti
V
Al
Ti
Al
Al
Ti
Ti
3
Al(a)
Figure 4.3 Vacancy and antistructure atom concentrations on transition metal and
Al sublattices in Ti
3
Al, TiAl, Ni
3
Al, NiAl, and FeAl as functions of the composition at
T 0.7T
m
.
Chapter-04.qxd 11/29/04 6:36 PM Page 178
179
46
47 48 49
50
c
Ti
[at.%]
10
-7
10
-6
10
-5
10
-4
10
-3
10
-2
10
-1
Defect concentration
54 52 50
c
Al
[at.%]
V
Ti
V
Al
Ti
Al
Al
Ti
(b) TiAl
72 73 74
75 76
77
78
c
Ni
[at.%]
10
-10
10
-9
10
-8
10
-7
10
-6
10
-5
10
-4
10
-3
10
-2
10
-1
Defect concentration
28 27 26 25
24
23
22
c
Al
[at.%]
V
Ni
V
Al
Ni
Al
Al
Ni
Ni
3
Al
(c)
Figure 4.3 (Continued)
Chapter-04.qxd 11/29/04 6:36 PM Page 179
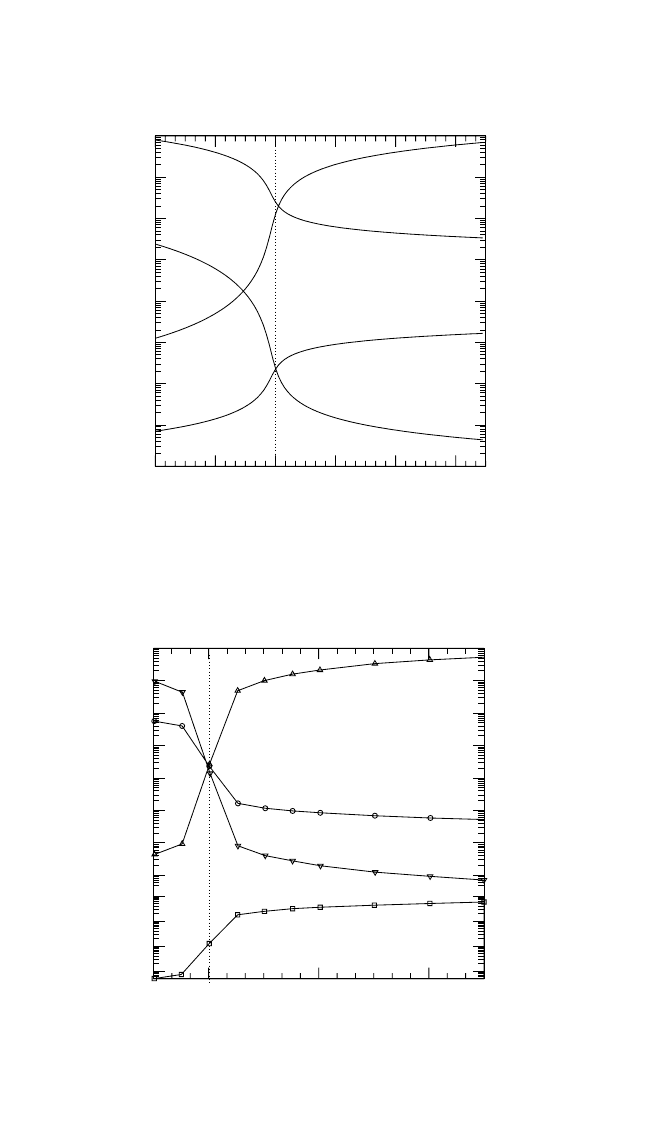
180
50 52 54
c
Fe
[at.%]
10
-17
10
-16
10
-15
10
-14
50 48 46
c
Al
[at.%]
10
-8
10
-7
10
-6
10
-5
10
-4
10
-3
10
-2
10
-1
Defect concentation
V
Fe
V
Al
Fe
Al
Al
Fe
=
=
(e) FeAl
46
48
50 52 54 56
c
Ni
[at.%]
10
-9
10
-8
10
-7
10
-6
10
-5
10
-4
10
-3
10
-2
10
-1
Defect concentration
54 52 50 48 46
44
c
Al
[at.%]
V
Ni
V
Al
Ni
Al
Al
Ni
(d) NiAl
Figure 4.3 (Continued)
Chapter-04.qxd 11/29/04 6:36 PM Page 180

DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 181
given composition. The defect formation energies for Ti
3
Al and TiAl have
been calculated
[7, 8]
and are discussed in this chapter. The EAM potentials
developed by Voter and Chen
[24]
for Ni
3
Al, and the potentials of Mishin
and Farkas
[22]
for NiAl, have been applied to Ni aluminides. The results of
ab initio calculations of defect formation energies by Mayer et al.
[25]
were
used for B2-FeAl. Formation entropy effects were neglected.
Figures 4.3(a) through (e) demonstrate a few important features of
defect behavior. Both the Ti aluminides, Fig. 4.3(a) and (b), and Ni
3
Al,
Fig. 4.3(c), obviously belong to the anti-structure-defect type of inter-
metallic compounds, since anti-structure atoms are predominantly gener-
ated to accommodate the deviation from the stoichiometry. In contrast,
NiAl reveals a triple-defect type of point defect disorder, and constitu-
tional Ni vacancies exist in NiAl on the Al-rich side, as shown in
Fig. 4.3(d). Moreover, the Ni vacancy concentration is very large on the
Ni-rich side, for example, C
V
Ni
10
4
at T 0.75T
m
. In the other inter-
metallics under consideration, the vacancies are also mainly concentrated
on the transition-metal sublattice, and their concentration amounts to
about 10
6
to 10
5
at T 0.75T
m
. These are also the typical vacancy con-
centrations in close-packed pure metals at the same reduced temperature.
The vacancy concentration on the Al sublattice is remarkably smaller,
especially in B2-FeAl, as shown in Fig. 4.3(e).
According to Mayer and co-workers,
[25, 26]
B2-FeAl is neither a com-
pound with pure anti-site disorder nor a compound with pure triple-defect
disorder. FeAl demonstrates a hybrid behavior in which the relation
between the Fe vacancy concentration and that of the anti-structure atoms
depends crucially on temperature.
The concentration of Ti anti-structure atoms in the Ti aluminides is
generally larger than that of Ni anti-structure atoms in the Ni aluminides
of the same composition (Fig. 4.3). This corresponds to a higher degree of
thermal disorder inherent in Ti aluminides at similar reduced tempera-
tures. These features play a decisive role in the analysis of the respective
self-diffusion behavior.
The important question is how the particular crystal structure of the
given intermetallic compound can affect the self-diffusion properties. It is
generally accepted that self-diffusion in closed-packed structures occurs
via nearest neighbor jumps of vacancies. Since random vacancy jumps
between different sublattices would generally produce disorder, and since
there is a strong tendency to accomplish a reverse, ordering jump after a
given disordering jump, the correlated jumps of vacancies will clearly
play a decisive role in the long-range diffusion process.
Comparing the structures in Fig. 4.2, we can observe an important
feature that largely determines the self-diffusion properties of these com-
pounds. It is obvious that in Ni
3
Al, Ti
3
Al, TiAl, and Fe
3
Al, the transition
Chapter-04.qxd 11/29/04 6:36 PM Page 181
