Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.

202 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 4.11 (Continued)
6
7
89
T
-1
[10
-4
K
-1
]
10
-20
10
-19
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
D
*
[m
2
s
-1
]
1500 1400 1300 1200 11001600
T [K]
Cr (Herzig et al, 2001)
Fe
Nb
Cr (Lee et al,1993)
Co
Zr
Ni
Ti
(c) Solute Diffusion in TiAl
Chapter-04.qxd 11/30/04 3:28 PM Page 202
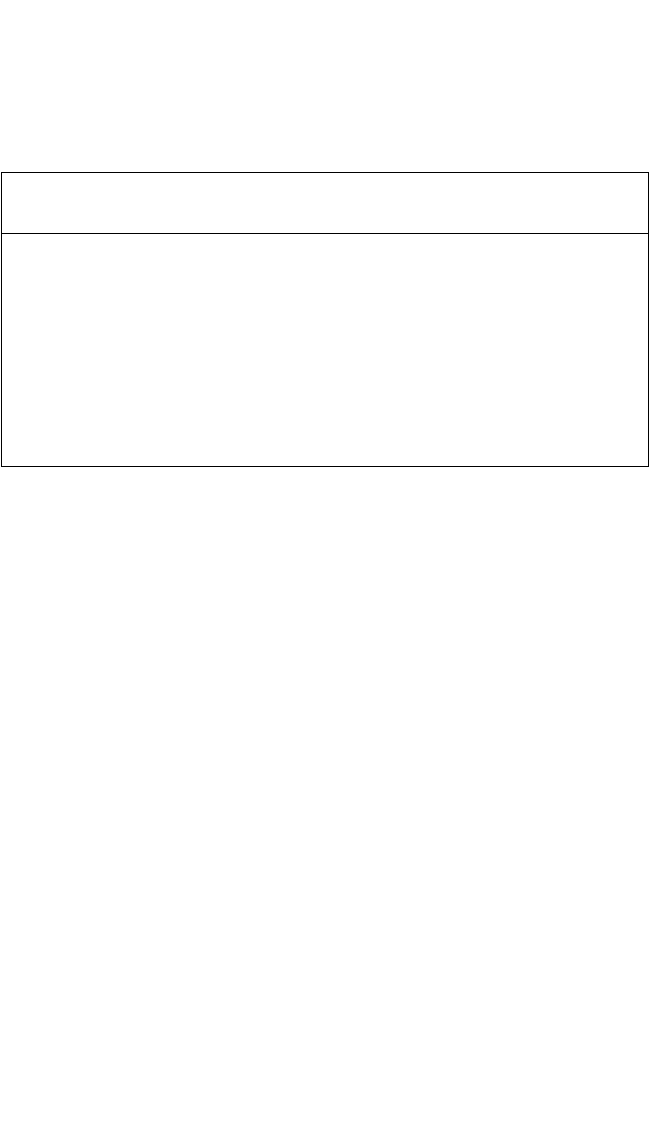
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 203
for the ASB mechanism inevitably has to be reached to produce a long-
range diffusional contribution from this mechanism, as shown by Divinski
and Larikov.
[37]
Note that in TiAl we need to distinguish between different
types of ASB mechanisms that start from either a Ti or an Al vacancy.
[8]
TiAl has the layered L1
0
structure that, generally, should result in an
anisotropy of self-diffusion. Diffusion measurements were carried out on
polycrystalline samples that concealed the anisotropy effects.
[7]
The
anisotropy of Ti self-diffusion in single-crystalline TiAl alloys was
recently studied;
[71]
results are presented in Fig. 4.11(a). As can be
expected, Ti diffusion along Ti layers in the L1
0
structure is faster than
perpendicular to this direction. The geometrical consideration of the L1
0
structure [see Fig. 4.2(d)] suggests that the Ti-sublattice diffusion mecha-
nism should result in a strong anisotropic contribution, whereas the ASB
mechanism, which occurs by intersublattice jumps of the Ti atoms, corre-
sponds to an almost isotropic mass transport. As the temperature
increases, the contribution of the ASB mechanism increases and the
anisotropy of Ti diffusion decreases.
[71]
This increase in the diffusional
contribution of the ASB mechanism with increasing temperature was
already manifested in the nonlinear Arrhenius dependence of Ti self-
diffusion.
[7]
The relevant Arrhenius parameters are listed in Table 4.3.
Al Diffusion. Interdiffusion in g-TiAl was measured using single-
phase diffusion couples.
[72]
These data were used to deduce the Al
Table 4.3. Arrhenius Parameters of Self-Diffusion and Solute Diffusion in
TiAl
*
D
0
Q
Tracer (m
2
s
1
) (kJ/mol) Ref.
Ti 3.2 10
6
261 7
Al
**
2.1 10
2
358 7
Ga 4.4 10
5
293 68
Co 1.1 10
3
318 74
Cr 4.4 10
3
350 73
Fe 2.7 10
6
252 39
Ni 1.8 10
2
340 39
Nb 1.5 10
4
324 39
Zr 6.4 10
3
348 39
Cr 5.2 10
3
332 39
*
Activation enthalpies of Ti And Fe diffusion were calculated by the fit of experimental points
below T 1470 K.
**
Calculation from the Darken-Manning equation
Chapter-04.qxd 11/29/04 6:36 PM Page 203

204 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
diffusion coefficients D
*
Al
.
[7]
The corresponding Arrhenius dependencies
are presented in Fig. 4.11(a). Using the thermodynamic factor Φ calcu-
lated by the CALPHAD method
[67]
and applying the Darken-Manning
equation [Eq. (3)] with S 1.14, the Al tracer diffusion coefficients were
deduced.
[7]
The resulting activation enthalpy of Al diffusion is fairly large,
Q
Al
358 kJ/mol.
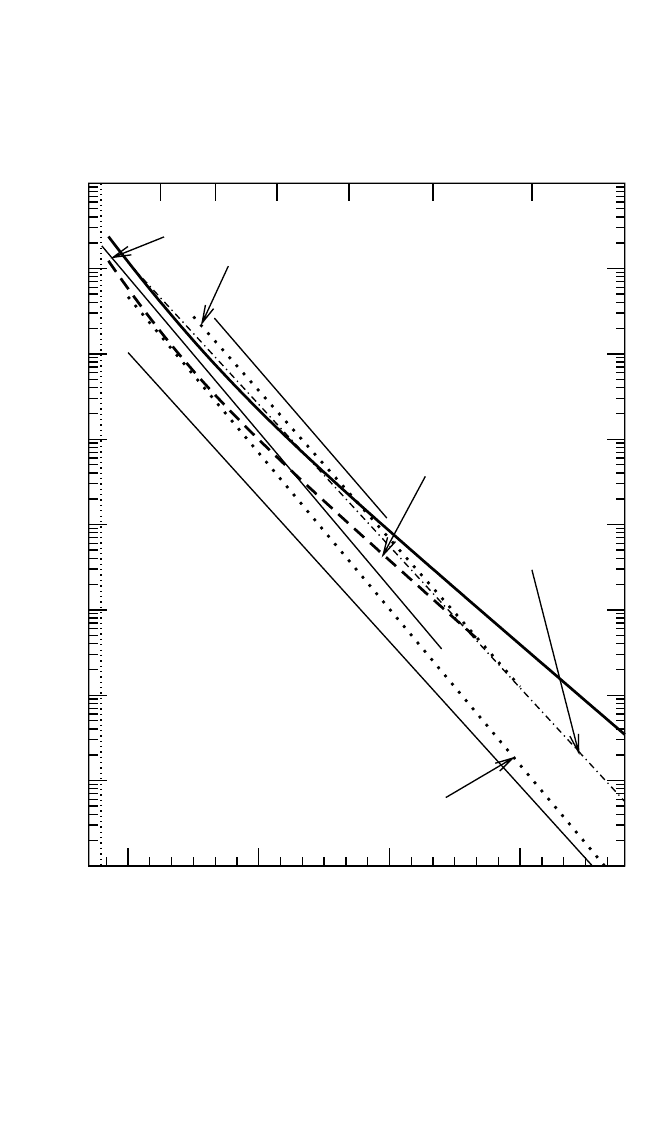
4.4.3.2 Solute Diffusion
Al diffusion in TiAl was examined using Ga as an Al substitute.
[68]
The corresponding diffusion data are presented in Fig. 4.11(b). It is
observed that D
*
Ga
D
*
Ti
in the whole temperature interval of the investi-
gations, whereas D
*
Al
turns out to be smaller than D
*
Ti
only at lower tem-
peratures. However, the absolute values of D
*
Al
are very similar to the Ga
diffusivity. The observed difference in Q
Al
and Q
Ga
was related to the
uncertainty in the determination of D
*
Al
from the interdiffusion data via the
Darken relation.
[68]
Since Ga occupies exclusively the Al sublattice in
TiAl,
[69]
the diffusion process of Ga atoms consists of jumps from the Al
to the Ti sublattice, migration through it, and reverse jumps on the Al sub-
lattice.
[68]
The difference between Q
Ga
and Q
Ti
in the low-temperature
interval, where the Ti-sublattice diffusion mechanism dominates for Ti
atoms, is therefore mainly attributed to the formation enthalpy of Ga
atoms as antistructure atoms on the Ti sublattice.
Results of tracer solute diffusion in TiAl are also available for
Cr,
[39, 73]
Co,
[74]
Nb,
[39]
Zr,
[39]
Fe,
[39]
and Ni.
[39]
These data are plotted in
Fig. 4.11(c). Diffusion of the solutes studied deviates by less than one
order of magnitude from Ti self-diffusion. This behavior corresponds to
the substitutional character of these solutes and to the vacancy mecha-
nism of diffusion.
Nb preferentially occupies Ti sites and is a slow diffuser in TiAl. The
Ti-sublattice diffusion mechanism was suggested for Nb atoms,
[39]
and the
slow diffusivity is most likely related to a certain repulsive vacancy-Nb
atom interaction.
Fe diffusion in TiAl presents a very interesting case because it demon-
strates a strong nonlinear Arrhenius dependence [see Fig. 4.11(c)]. The
magnitude of the Fe diffusion coefficients is similar to that of Ti self-
diffusion. Moreover, the curvatures of the Ti and Fe Arrhenius plots and
the activation enthalpies in the lower temperature intervals are also quite
similar. The behavior of Fe diffusivity was explained
[39]
in relation to the
equal preference of Fe atoms to occupy Ti or Al sites in TiAl.
[69]
In such a
case, Fe atoms located on the Ti sublattice mainly dominate Fe diffusion at
lower temperatures, and the ASB mechanism, which becomes progressively
Chapter-04.qxd 11/29/04 6:36 PM Page 204

DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 205
important at higher temperatures, produces a curvature of the Arrhenius
dependence.
[39]
Similarly to Fe, the Ni diffusivity in TiAl is close to the Ti self-
diffusivity in the temperature range investigated. This finding contrasts
with their diffusion behavior in Ti
3
Al. When changing from a-Ti to
a
2
-Ti
3
Al and, finally, to g-TiAl, the mechanism of Fe and Ni diffusion
obviously changes from interstitial to dissociative, and then to substi-
tutional diffusion. This change is accompanied by a remarkable
decrease in the diffusion rates: While Fe and Ni mobilities are seven to
eight orders of magnitude faster than Ti in a-Ti,
[75, 77]
they exceed Ti
self-diffusion in a
2
-Ti
3
Al only by two to four orders of magnitude
[Fig. 4.10(b), and all of them diffuse with similar magnitude in g-TiAl
(Fig. 4.12). This behavior was explained by the different type of atomic
arrangements forming interstitial sites in the materials under consider-
ation.
[39]
In the D0
19
structure of Ti
3
Al, there are two different intersti-
tial sites: One of them is built up exclusively by Ti atoms; the second
type is formed by two Al and four Ti atoms. In the L1
0
structure of TiAl,
all interstitial sites have both Al and Ti atoms as environment. The
observed diffusion behavior suggests that the presence of Al atoms
strongly decreases the interstitial solubility of Fe and Ni atoms at such
positions.
[39]
4.4.4 NiAl
4.4.4.1 Self-Diffusion
Ni Diffusion. The B2 structure of NiAl is very interesting from the
theoretical point of view, since all nearest neighbor jumps are the jumps
between different sublattices in this structure [see Fig. 4.2(b)]. Unlike the
other transition-metal-rich Ni and Ti aluminides, NiAl exhibits a triple-
defect type of disorder. [The triple defects (two Ni vacancies and one Ni
antistructure atom) reveal the lowest formation energy between the point
defects, the thermal formation of which does not change the composition.]
Moreover, the structural Ni vacancies, which are available in NiAl on the
Al-rich side of compositions, are generally thought to affect significantly
Ni self-diffusion in this compound.
[78, 79]
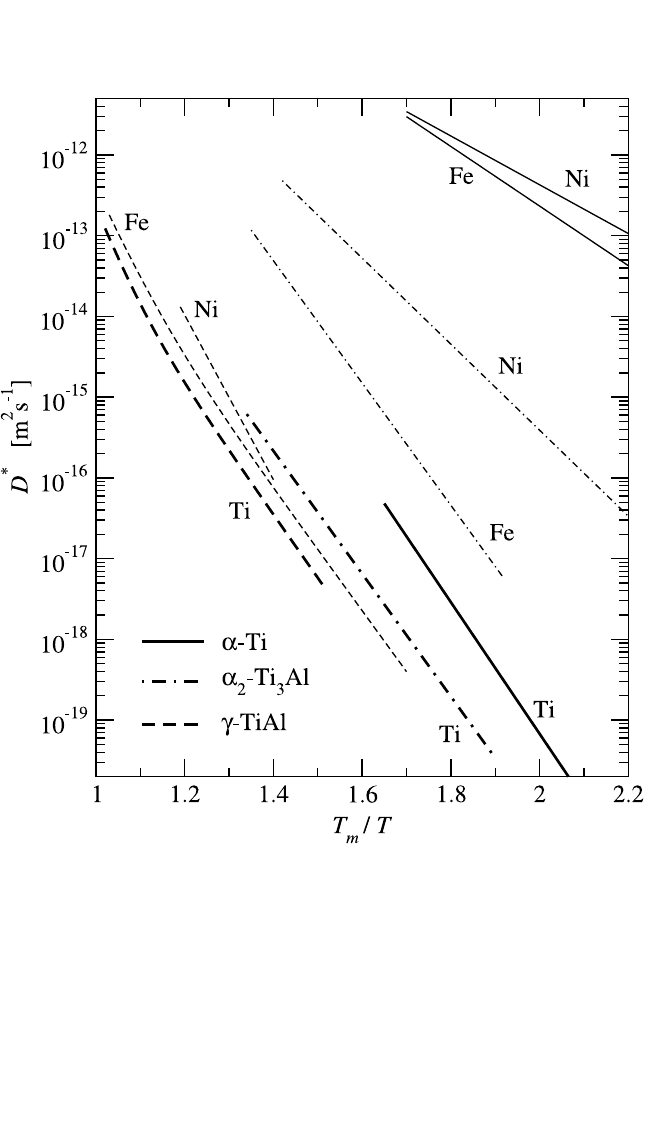
Ni diffusion in NiAl was measured on polycrystalline NiAl alloys
[78]
and on single crystals.
[36]
These results are presented in Fig. 4.13. Note that
at T 1500 K, an upward deviation from the otherwise straight Arrhenius
dependencies was observed in all compositions.
[36]
(This is not indicated in
Fig. 4.13 to avoid overloading of the figure. This curvature is analyzed
below.) A qualitatively very different diffusion behavior was observed in
Chapter-04.qxd 11/29/04 6:36 PM Page 205
206 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
these two investigations, especially in Al-rich compositions. First, Ni dif-
fusivities D
*
Ni
measured by Frank et al.
[36]
are generally smaller by a factor
of two to five than those measured by Hancock and McDonnell.
[78]
Second,
a deep minimum in D
*
Ni
at the stoichiometric composition was postu-
lated,
[78]
whereas similar values of D
*
Ni
in Al-rich and stoichiometric NiAl
alloys were measured by Frank et al.
[36]
[see Fig. 4.14(a)]. Finally, a small
activation enthalpy of Ni diffusion in Al-rich compositions was determined
Figure 4.12 Comparison of Fe and Ni diffusion with Ti self-diffusion in a-Ti,
[75–77]
a
2
-Ti
3
Al,
[65, 70]
and g-TiAl.
[7, 39]
T
m
is the melting temperature of corresponding material.
Chapter-04.qxd 12/2/04 11:51 AM Page 206
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 207
by Hancock and McDonnell to be about 180 kJ/mol,
[78]
whereas Frank
et al. obtained a value of about 290 kJ/mol.
[36]
The activation enthalpy Q of Ni self-diffusion in NiAl calculated by
the Arrhenius fit in the interval 1050 K T 1500 K
[36]
is given in
Figure 4.13 The Arrhenius diagram of Ni diffusion in different NiAl alloys (in at.% Ni).
The results of Hancock and McDonnell
[78]
(dashed lines) and Frank et al.
[36]
(solid
lines) are compared.
Chapter-04.qxd 11/29/04 6:36 PM Page 207
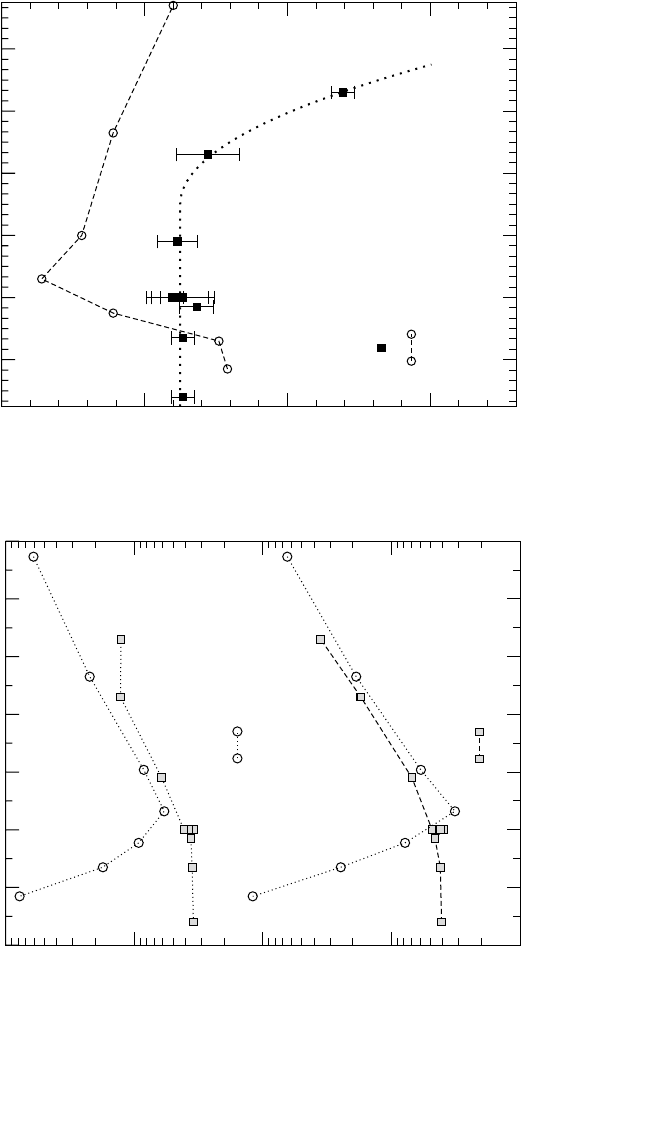
46
48
50 52 54 56 58 60
Composition (at.% Ni)
10
-17
10
-16
10
-15
10
-14
10
-13
D
*
(m
2
s
-1
)
Ni (Frank et al.)
54 52 50 48 46
44 42
40
Composition (at.% Al)
In (Minamino et al.)
1523 K
1273 K
(a) Diffusion Coefficients
48
50 52 54 56 58
Composition (at.% Ni)
200
250
300
350
Q (kJ/mol)
Ni (Frank et al.)
In (Minamino et al.)
52 50 48 46
44 42
Composition (at.% Al)
b)
(b) Activation Enthalpies
Figure 4.14 Composition dependence of the diffusion coefficients and activation enthalpies of Ni
[36]
and In
[87]
diffusion in NiAl.
208
Chapter-04.qxd 11/29/04 6:36 PM Page 208
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 209
Fig. 4.14(b) as a function of composition. Astonishingly, Q results in the
constant value of about 290 kJ/mol at compositions lower than 53 at.% Ni,
but substantially decreases in alloys with larger Ni concentrations
approaching the value of 230 kJ/mol in Ni
56.6
Al
43.4
. The Arrhenius param-
eters of Ni self-diffusion in representative Al-rich, stoichiometric, and
Ni-rich NiAl alloys are summarized in Table 4.4.
Hancock and McDonnell’s results
[78]
agree qualitatively well with
recent calculations of the activation enthalpy of Ni diffusion by next-
nearest neighbor jumps of Ni vacancies over the Ni sublattice.
[22]
In the
case of Al-rich compositions, where constitutional Ni vacancies exist, the
activation enthalpy Q
Ni
should be equal to the migration enthalpy of Ni
vacancies, which was calculated to about 200 kJ/mol.
[22]
It was shown that
along with the small activation enthalpy, the next-nearest neighbor jumps
of Ni atoms entail a large and negative migration entropy, which decreases
remarkably the contribution of this mechanism at elevated tempera-
tures.
[36]
Moreover, the next-nearest neighbor jumps of Ni atoms would
produce a continuous increase of D
*
Ni
with increasing Al content on the Al-
rich side, which was not observed in the recent experiments on single-
crystalline NiAl alloys.
The difference between results
[36, 78]
was proposed
[36]
to stem from the
difference in the thermal equilibration procedure of the samples and/or
from short-circuit diffusion, which, according to our recent experiments
on grain boundary diffusion of Ni in polycrystalline NiAl samples,
[80]
could have affected Hancock and McDonnell’s low-temperature
results.
[78]
Table 4.4. Arrhenius Parameters of Self-Diffusion and Solute Diffusion in
NiAl
Composition D
0
Q
Tracer at.% Ni (m
2
s
1
) (kJ/mol) Ref.
Ni 46.8 2.3 10
5
287 36
50.0 3.5 10
5
290 36
51.8 4.8 10
5
288 36
54.6 4.4 10
5
278 36
56.6 1.0 10
6
231 36
In 47.7 1.6 10
4
271 87
49.5 4.6 10
4
311 87
50.6 2.0 10
3
336 87
52.0 9.8 10
4
322 87
55.3 1.1 10
3
311 87
Chapter-04.qxd 11/29/04 6:36 PM Page 209

210 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Possible diffusion mechanisms in NiAl were analyzed
[36, 40]
for
dependence on the composition. The results suggest that the triple-defect
mechanism, which is characterized by a constant activation enthalpy of
about 300 kJ/mol independent of the composition (Fig. 4.6), most likely
is the dominating diffusion process in NiAl.
[36]
This result agrees perfectly
with the recent experiments [see Fig. 4.14(b)]. The observed increase of
D
*
Ni
and the decrease of Q
Ni
at compositions with larger Ni content corre-
spond to the activation of the ASB mechanism after reaching the percola-
tion threshold at about 55.5 at.% Ni.
[36]
These results do not exclude an
additional and strong contribution of next-nearest neighbor jumps of Ni
atoms at lower temperatures in Al-rich compositions expected by Mishin
and Farkas.
[22]
The relatively small activation enthalpy would favor such
a diffusion process.
[22]
The upward deviation of D
*
Ni
at T 1500 K from the otherwise linear
Arrhenius dependence reported by Frank et al.
[36]
correlates with recent
results of differential dilatometry measurements in NiAl alloys,
[81]
which
reveal a nonlinearity in the Arrhenius plot of the vacancy concentration in
the high temperature range.
Al Diffusion. At present, there exist no directly measured Al tracer
diffusion data in NiAl. However, interdiffusion in NiAl was investi-
gated.
[82, 83]
A substantial compositional dependence of the interdiffusion
coefficient D
∼
was deduced,
[83]
characterized by a deep minimum around
the stoichiometric composition, [see Fig. 4.15(a)]. Using the thermodynamic
data of Steiner and Komarek
[84]
and the Darken-Manning equation, the
contribution of Ni tracer diffusion to the interdiffusion coefficient can be
estimated, rewriting Eq. (3) as:
D
∼
D
∼
(Ni)
D
∼
(Al)
, (6)
where
D
∼
(Ni)
N
Al
D
*
Ni
Φ S and D
∼
(Al)
N
Ni
D
*
Al
Φ S. (7)
Assuming S 1, the results of this estimation of D
∼
(Ni)
are also presented
in Fig. 4.15(a). A paradoxical difference between the measured inter-
diffusion coefficient D
∼
and D
∼
(Ni)
is observed, especially around stoichio-
metric NiAl. D
∼
is by about two orders of magnitude smaller than D
∼
(Ni)
in Ni
50
Al
50
. Any contribution of the Al tracer diffusion, D
∼
(Al)
, further
increases this difference.
As already noted, the value of the vacancy wind factor S may not fall
into the limits predicted by Manning [Eq. (4)], especially in well-ordered
compounds. Bearing this in mind, we can tentatively estimate the values
Chapter-04.qxd 11/29/04 6:36 PM Page 210
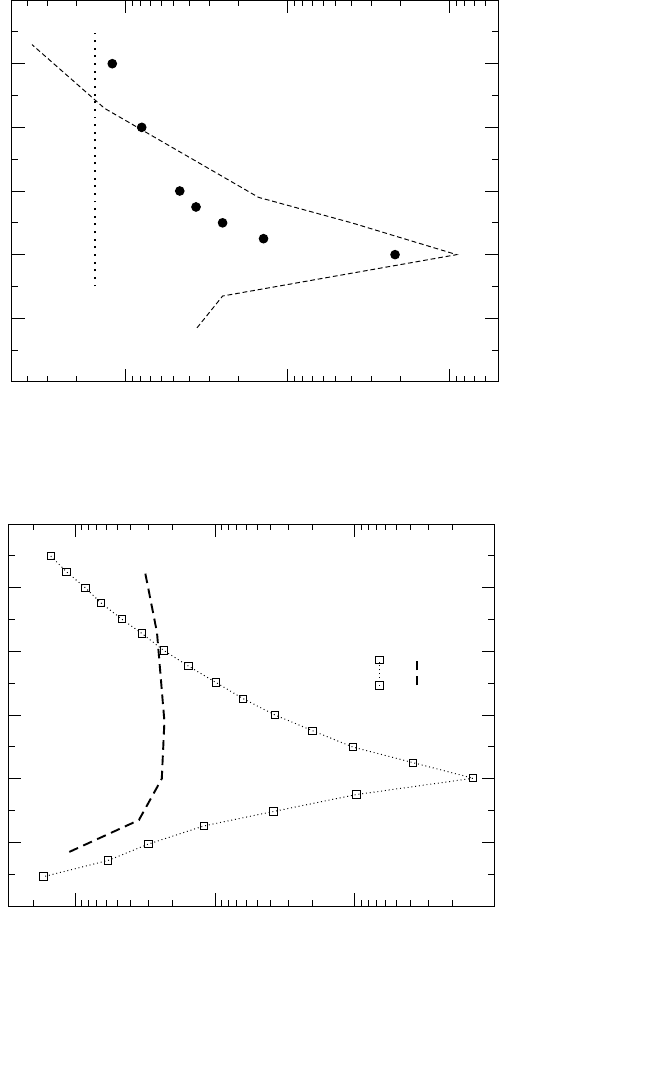
211
46
48
50 52 54 56 58
Composition (at.% Ni)
10
-17
10
-16
10
-15
10
-14
~
D (m
2
s
-1
)
Kim et al.
D
(Ni)
54 52 50 48 46
44 42
Composition (at.% Al)
T = 1273 K
~
(a) Interdiffusion in NiAl Alloys
Figure 4.15 Comparison of experimentally measured
[83]
and calculated [by Eq.(7)] interdiffusion coefficients in NiAl (a). In (b), the
vacancy wind factor S estimated from Eq. (8) (dashed line) is compared with the values of S calculated for the triple-defect diffu-
sion mechanism
[85]
(full circles) and for the next-nearest neighbor jumps of Ni atoms (dotted line).
46
48
50 52 54 56 58
Composition (at.% Ni)
10
-2
10
-1
10
0
S
54 52 50 48 46
44 42
Composition (at.% Al)
T = 1273 K
(b) Vacancy Wind Factors in NiAl Alloys
Chapter-04.qxd 11/30/04 3:28 PM Page 211
