Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.


metal sublattice presents a connected network for nearest neighbor jumps
of vacancies, whereas the nearest neighbor jumps in NiAl and FeAl are
exclusively the jumps between different sublattices. Since vacancies are
concentrated on the transition-metal sublattices, the sublattice diffusion
mechanism may be proposed for Ni
3
Al, Ti
3
Al, TiAl, and Fe
3
Al from this
general consideration.
We can expect, a priori, that the structural point defects appreciably
affect the self-diffusivity in intermetallic compounds and produce a com-
positional dependence of the diffusivity. From this consideration, several
questions arise: (1) Are the structural vacancies the defects that most
remarkably enhance self-diffusion? (2) To what extent do the anti-structural
atoms affect the diffusivity? (3) How is this effect related to the given
crystalline structure? (4) Does the absence of the connected network for
nearest neighbor jumps of Ni vacancies slow down self-diffusion in NiAl
in comparison with the presence of such a network in the more closed
structure of Ni
3
Al? Discussions in Sections 4.3 through 4.5 aim to provide
insight into the diffusion behavior and the diffusion mechanisms with
respect to the crystalline structure and the type of disorder in the Ni, Ti,
and Fe aluminides.
4.3 Diffusion Mechanisms in Intermetallics
The ordered structure of intermetallic compounds imposes certain
limitations on geometrically possible vacancy-mediated diffusion mecha-
nisms. The most important diffusion mechanisms relevant to the diffu-
sion behavior of the aluminides under consideration are discussed in this
section.
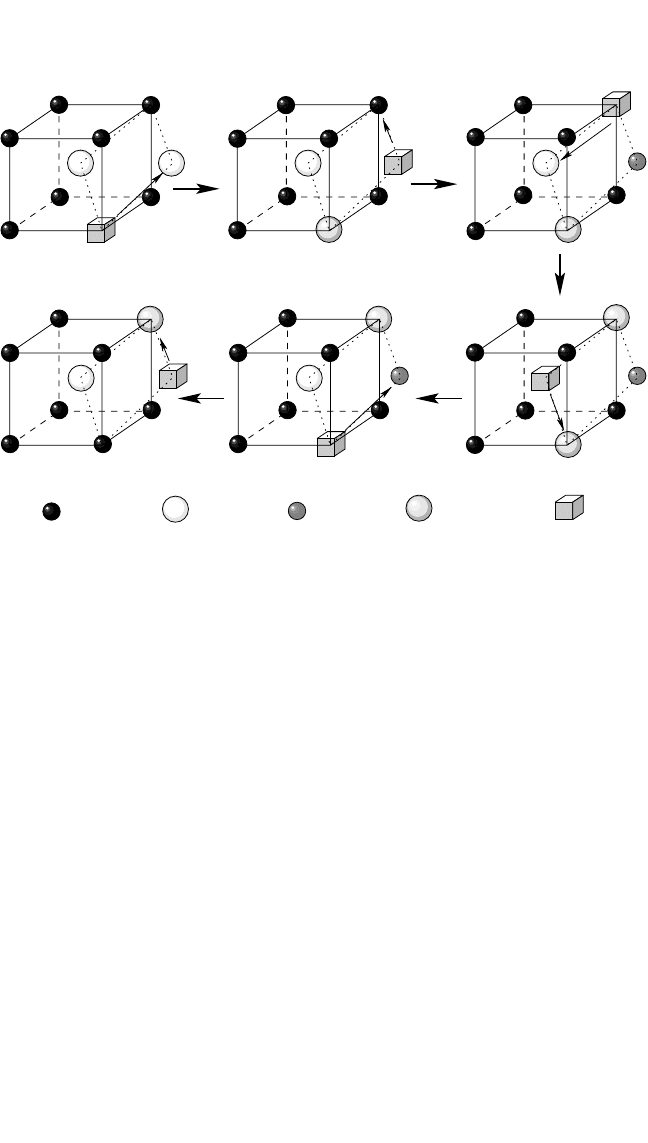
Six-Jump-Cycle Mechanism. This mechanism was originally pro-
posed for B2 compounds;
[10]
later, it was elaborated for other ordered
structures. This mechanism is shown in Fig. 4.4. The correlated jumps of
atomic species during execution of the cycles impose certain limitations
on the quantitites that can be measured in a diffusion experiment. Firstly,
in a highly ordered state, the ratio of tracer diffusivities of both compo-
nents, D
*
A
D
*
B
, can adopt only the values within the interval:
q, (1)
where q was calculated to be 2
[27]
and was later corrected to q 2.034 by
including the correlation effects.
[28]
The ratio of diffusivities of Ag and Mg
in b-AgMg,
[27]
Zn and Au in b-AuZn,
[29]
and Cd and Au in b-AuCd
[30]
fall
D
*
A
D
*
B
1
q
182 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Chapter-04.qxd 11/29/04 6:36 PM Page 182
into these limits. This was considered to be strong support for the six-
jump-cycle mechanism in these compounds.
As the composition deviates from the stoichiometric one, a large
amount of constitutional anti-structure defects appear. Interaction of the
six-jump cycles with these anti-structure atoms remarkably changes the
limits in Eq. (1).
[31]
Thus, in a less ordered state, experimental values of
D
A
D
B
larger than 2 can no longer be considered as an indication that the
six-jump-cycle mechanism does not operate.
Diffusion by the six-jump cycles is a highly correlated process. Thus,
the correlation factor is supposed to be rather small. However, we should
generally distinguish two types of correlations that characterize the six-
jump cycles. Considering the individual cycles as effective vacancy jumps
occurring with the given frequency, we can calculate the resulting corre-
lation factors f
ç
A
and f
ç
B
.
[28]
For B2 NiAl, the Monte Carlo calculations
resulted in f
ç
Ni
0.782 and f
ç
Al
0.860.
[13]
In contrast, the tracer correlation
factors for Ni and Al atoms in that case were calculated to be f
Ni
0.445
and f
Al
0.022, respectively. Note that f
Ni
is not as small as it was usually
anticipated to be for the six-jump-cycle mechanism. This should be taken
into account when interpreting the results of experiments such as the
Mössbauer effect experiments, which allowed the geometry of individual
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 183
2
3
45
1
6
A
B
A
B V
A
B
Figure 4.4 A scheme of the six-jump-cycle mechanism in a B2-compound AB.
Chapter-04.qxd 11/29/04 6:36 PM Page 183

atomic jumps to be established and by which the corresponding correla-
tion factor can be estimated by comparing the local jump rates with the
long-range diffusion data.
[32]
The isotope effect has been measured for both Au and Zn in the B2-
ordered b-AuZn alloys, with E
Au
(and, correspondingly, f
Au
) considerably
larger than E
Zn
(f
Zn
) in Zn-rich alloys.
[33]
(For example, E
Au
0.35 and
E
Zn
0.05 in the Au-51.85 at.% Zn alloy.) This resembles the relation
between f
Ni
and f
Al
in B2-NiAl for the six-jump-cycle mechanism
[13]
and
can be explained by the predominant vacancy concentration in the Au
sublattice and an increased probability of a reverse jump of a Zn atom that
has initiated a six-jump cycle. Thus, the relationship f
A
f
B
in an AB
compound cannot be considered an argument against the six-jump-cycle
mechanism.
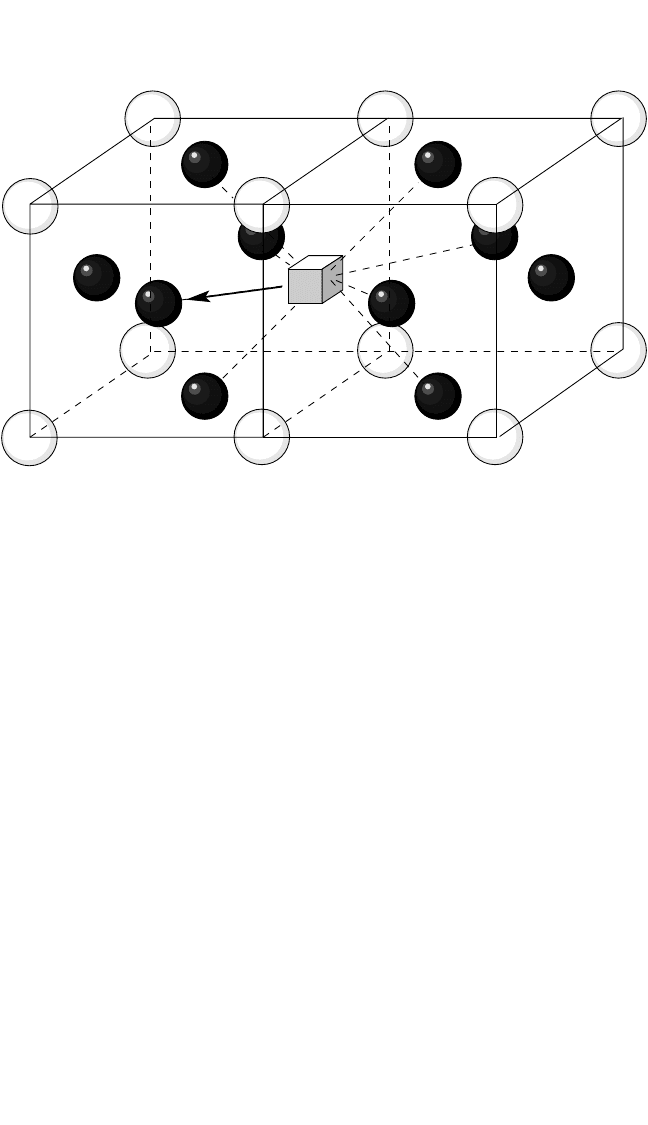
Sublattice Diffusion Mechanism. When one of the components
forms a lattice structure that enables nearest neighbor jumps through
the respective sublattice, random jumps of a vacancy on this sublattice
will not affect the order in the compound. An example of this mecha-
nism for the L1
2
structure is shown in Fig. 4.5. It is important that this
mechanism can dominate diffusion of the minority component as well
as the majority component. In such a case, a minority atom jumps into
the “wrong” sublattice and continues its migration through this sub-
lattice. The sublattice diffusion mechanism has been extensively
analyzed.
[34, 35]
It is obvious that the diffusivities of both components are not cou-
pled by a relation similar to Eq. (1) if the sublattice diffusion mechanism
operates.
The correlation factors for the sublattice diffusion mechanism in the
L1
2
structure of Ni
3
Al have been calculated.
[35]
It was found that
f
Ni
0.689 and f
Al
can be expressed in a usual way via the vacancy Al
atom-exchange frequency w
2
and the vacancy escape frequency H:
[35]
f
Al
. (2)
The expression for H in terms of the modified five-frequency model is
given by Numakura.
[35]
Triple-Defect Diffusion Mechanism. This mechanism was pro-
posed by Stolwijk et al. for the B2 compound CoGa.
[11]
It specifies the
migration of a triple defect, which represents a bounded entity com-
posed of two transition metal vacancies and one transition metal atom
in an antistructural position. The triple-defect mechanism in CoGa was
described to correspond to two nearest neighbor jumps of a Co atom
H
w
2
H
184 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Chapter-04.qxd 11/29/04 6:36 PM Page 184
and one next-nearest neighbor jump of a Ga atom.
[11]
The detailed cal-
culations for NiAl predict that the Al atom performs two nearest neigh-
bor jumps instead of one next-nearest neighbor jump.
[36]
Figure 4.6
shows the triple-defect mechanism with this modification to NiAl,
where an inverse triple defect (2V
Al
Al
Ni
) was found to exist as an
intermediate stage.
[36]
As a result of the indicated sequence of four
jumps of atoms, the triple defect moves, leaving the order in the com-
pound unchanged.
Since a correlated sequence of atomic jumps is involved, the diffusiv-
ities of both components in the perfectly ordered state are coupled by
Eq. (1) with q 13.3.
[11]
The correlation factors are supposed to be small for the triple-defect
diffusion mechanism. They were calculated for NiAl and f
Ni
0.05 at
T 1300 K.
[36]
f
Ni
was found to depend remarkably on temperature, and
the contribution of this temperature dependence to the overall activation
enthalpy of Ni diffusion by the triple-defect mechanism amounts to
17 kJ/mol.
[36]
The triple-defect mechanism is closely related to a divacancy diffu-
sion mechanism, which in its sequence corresponds to the configurations
apprearing after the jumps 1 or 3 in Fig. 4.6.
Antistructure Bridge Mechanism. This mechanism was originally
proposed by Kao and Chang for the B2 structure
[12]
and was later extended
to L1
2
structures.
[37]
The ASB mechanism is schematically presented in
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 185
Figure 4.5 A scheme of the sublattice diffusion mechanism in the L1
2
structure of
an A
3
B compound.
Chapter-04.qxd 11/29/04 6:36 PM Page 185
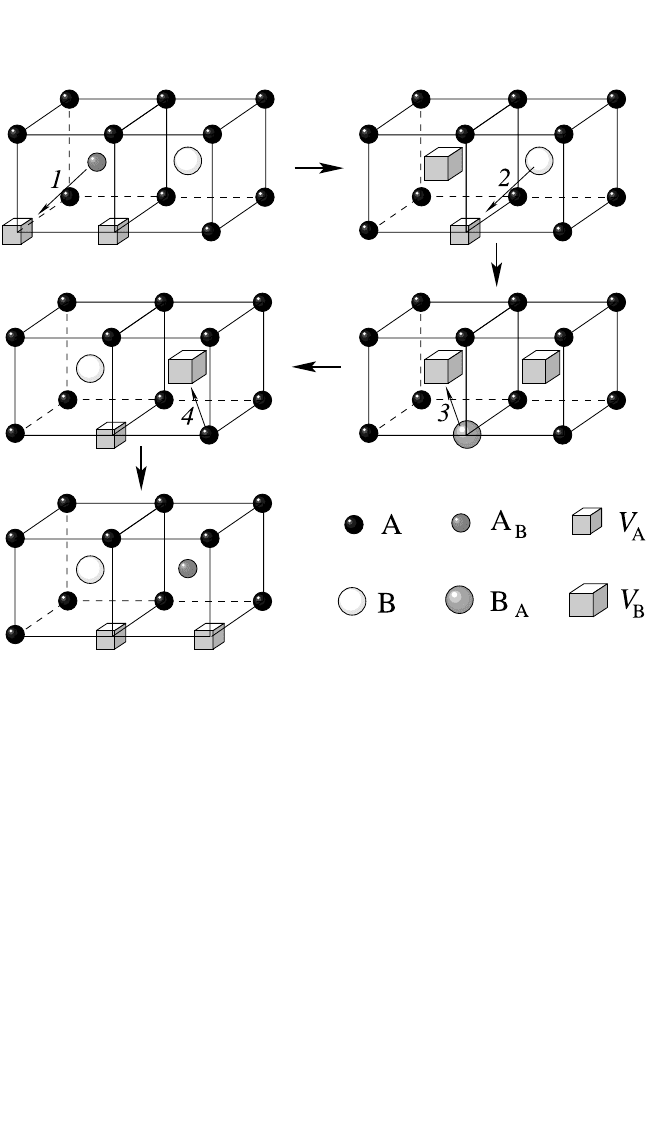
Fig. 4.7(a). As a result of the two indicated jumps, the vacancy and the
antistructure atom effectively exchange their positions. Since the vacancy
can jump up to the fourth or fifth coordination shell from its initial posi-
tion (depending on the lattice structure
[37]
), the resulting large geometrical
factor of the ASB mechanism increases its contribution to the diffusivity.
It is important to note that the contribution of this mechanism has a
percolation effect in the sense that long-range diffusion by the ASB mech-
anism will occur only if the concentration of the antistructure atoms is suf-
ficiently high. A relatively high critical concentration for a B2 structure
was initially estimated from purely geometrical arguments.
[12]
The Monte
Carlo simulation of this process resulted, however, in a smaller value of
the percolation threshold, ∼5%.
[37]
Such an antistructure atom concentra-
tion can indeed exist in intermetallics, and the ASB mechanism becomes
important for explaining the observed diffusion behavior in Ni
aluminides.
[36, 38]
186 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 4.6 A scheme of the triple-defect diffusion mechanism in a B2 structure
AB.The modification of this mechanism that is specific for NiAl
[36]
is shown.
Chapter-04.qxd 11/29/04 6:36 PM Page 186
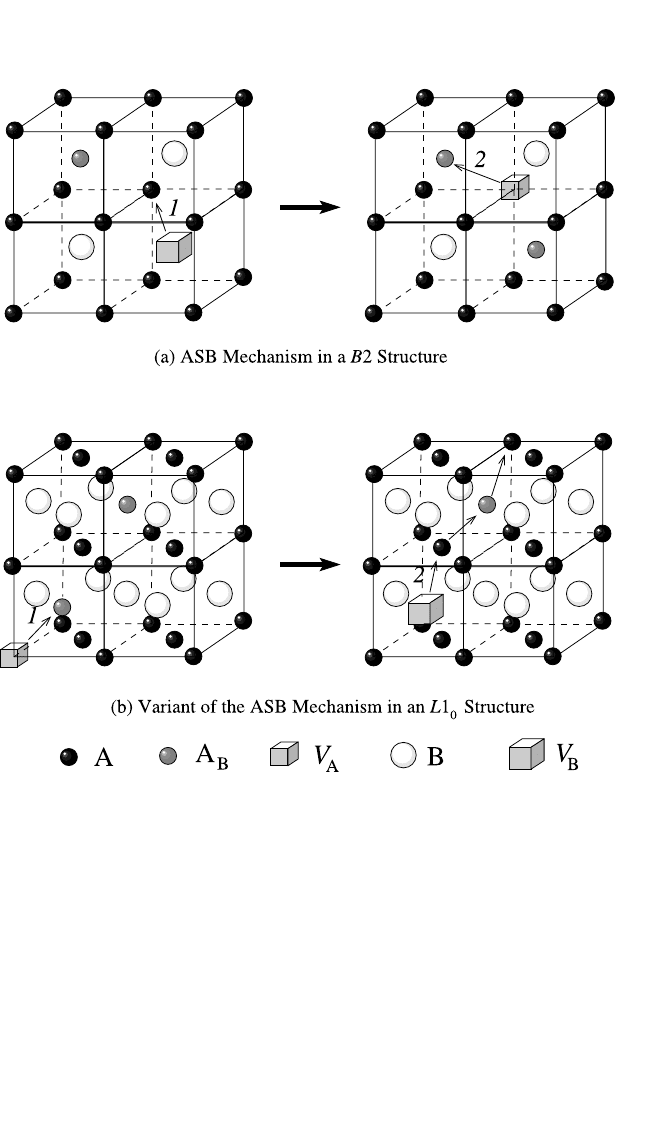
In the L1
0
structure of the phase TiAl, other types of the ASB mecha-
nism are of prime importance.
[7, 8]
One such variant is presented in
Fig. 4.7(b). After the indicated two jumps (1 2), the A vacancy moved
perpendicular to the A atom layers using an antistructure A atom as a
bridge. If a further antistructure atom in a suitable nearest neighbor posi-
tion is available for the vacancy after its second jump, the next ASB
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 187
Figure 4.7 A scheme of the antistructure bridge mechanism in a B2 structure and
a variant of the ASB mechanism in an L1
0
structure. As a result, the vacancy V
B
and the antistructure atom A
B
exchange their initial positions. In the B2 structure
(a), the antistructure atom A
B
can be situated at any B-atom position from 26 unit
cells that neighbor the unit cell with the V
B
vacancy. In (b), the vacancy V
A
moves
along the “bridges” formed by the antistructure atoms A
B
.
Chapter-04.qxd 11/29/04 6:36 PM Page 187

sequence may start, as indicated in Fig. 4.7(b). The Monte Carlo calcula-
tion of the percolation threshold for the long-range diffusion by this vari-
ant of the ASB mechanism yields about 11% of the antistructure atoms as
the critical concentration.
During the ASB sequence of jumps, only one species of atoms moves
(see Fig. 4.7). Therefore, the diffusivities of the two components are not
coupled.
In a strict sense, the genuine ASB mechanism operates only after the
percolation threshold is reached. However, in combination with another
mechanism (usually the sublattice diffusion mechanism), the ASB mech-
anism [for example, jump sequence 1 → 2 in Fig. 4.7(b)] can substantially
contribute to long-range diffusion without any percolation threshold.
[7, 8,39]
We will therefore use the term ASB mechanism in such cases as well,
referring to the specific sequence presented in Fig. 4.7.
Other Diffusion Mechanisms. Several other mechanisms, which may
be relevant in some specific cases, were proposed for ordered intermetal-
lic compounds. The next-nearest neighbor jump mechanism was shown to
correspond to the lowest activation energy of single Ni vacancy migration
in Al-rich NiAl.
[22]
The divacancy mechanism, with both vacancies
belonging to the same sublattice in NiAl, was considered by Divinski
et al.
[40]
After the given sequence of four atomic jumps, the initial order is
completely restored and the divacancy has moved by one step.
4.4 Experimental Results on Bulk Diffusion
in Ordered Aluminides
A common feature of all aluminides is that there are practically no
direct tracer measurements of Al self-diffusion in these compounds. This
problem is related to the very high price and the low specific activity of
the only available
26
Al radioisotope and to the difficulty of avoiding oxi-
dation of this isotope during a diffusion study. The only directly measured
Al-diffusion data
[41]
are considered to be unreliable.
There exist, however, two approaches to overcome this difficulty.
First, by combining interdiffusion and transition metal tracer diffusion
data (D
∼
and D
*
TM
, respectively), the diffusivity of the Al component D
*
Al
in
a binary system can be evaluated by applying the Darken-Manning for-
malism,
[42, 43]
neglecting the volume effects:
D
∼
∼
(N
Al
D
*
TM
N
TM
D
*
Al
)ΦS. (3)
Here, N
TM
and N
Al
are the mole fractions of the transition metal element
and Al in the compound, respectively; Φ is the thermodynamical factor;
188 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Chapter-04.qxd 11/29/04 6:36 PM Page 188

and S is the vacancy wind factor. Φ is determined by independent activity
measurements, and S is usually assumed to be about unity. For the random
alloy model, Manning has shown that:
1 S , (4)
where f
0
is the geometrical correlation factor for the given lattice.
[43]
(Chapter 1 of this book presents a detailed discussion of interdiffusion and
the Darken-Manning equation.)
The applicability of Eq. (3) was experimentally varified for several
compounds.
[44, 45]
Thus we also apply Eq. (3) to interdiffusion in alu-
minides. However, when D
*
Al
is much smaller than D
*
TM
, the determination
of D
*
Al
becomes unreliable due to the experimental uncertainties in the
determination of D
∼
∼
and Φ. This fact should be taken into account when
analyzing interdiffusion data.
A series of theoretical papers derived that the Darken-Manning
approach is applicable to intermetallic compounds with the B2, L1
2
, and
D0
3
lattices at a smaller degree of order.
[46, 47]
Simultaneously, it was found
that the correlated diffusion mechanisms in well-ordered intermetallic
compounds result in values of S that do not necessarily fall into the limits
imposed by Eq. (4). For example, S can be as low as 0.42 for the B2 com-
pounds when only the six-jump-cycle mechanism operates.
[48]
Likewise,
the upper value of S is not bound to f
0
1
in the L1
2
structures.
[49]
Therefore,
each factor has to be thoroughly analyzed when applying Eq. (3) to a
given intermetallic compound.
In an alternative approach, we can use tracers, which substitute Al in
the intermetallic compound (such as Ga, In, or Ge), as a surrogate to the
Al tracer. Both approaches are considered in the present investigation.
4.4.1 Ni
3
Al
4.4.1.1 Self-Diffusion
Ni Diffusion. Ni self-diffusion in Ni
3
Al was intensively investigated
in several experimental studies.
[50–54]
The corresponding Arrhenius
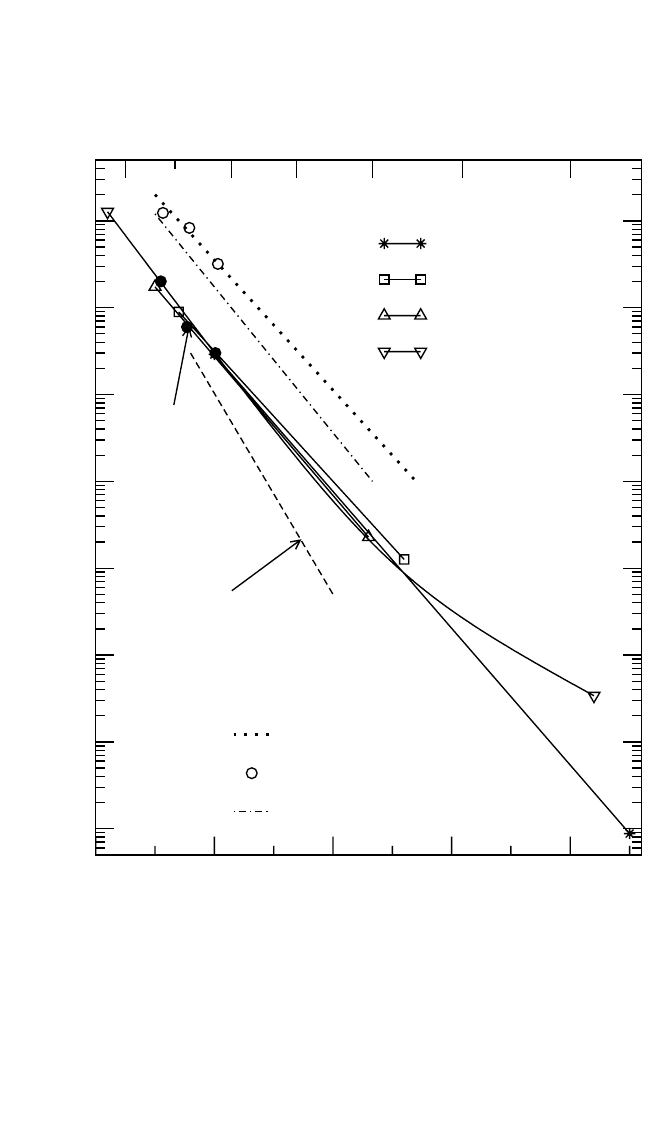
dependencies are presented in Fig. 4.8(a). There is good consistency
between the experimental results for the temperature interval T 1100 K.
At lower temperatures, the results of Hoshino et al.
[51]
show an upward
deviation from the otherwise linear Arrhenius dependence. Note that an
upward deviation was also indicated by Shi et al.,
[52]
although at a somewhat
1
f
0
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 189
Chapter-04.qxd 11/29/04 6:36 PM Page 189
190 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
6
7
8910
T
-1
[10
-4
K
-1
]
10
-20
10
-19
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
D
*
, D [m
2
s
-1
]
Frank et al.
Bronfin et al.
Shi et al.
Hoshino et al.
1400 1300 1200 1100 10001600
T [K]
~
Watanabe et al.
Fujiwara et al.
Ikeda et al.
Ni diffusion:
interdiffusion:
Al (Ikeda et al.)
Al
(Fujiwara
et al.)
(a) Self-Diffusion in Stoichiometric Ni
3
Al
Figure 4.8 Arrhenius plot of self-diffusion and solute diffusion in stoichiometric
Ni
3
Al. In (a), the results of interdiffusion measurements
[57–59]
as well as the calcu-
lated D
*
Al
(dashed line
[57]
and full circles
[59]
) are compared with Ni diffusion.
[50–53]
Chapter-04.qxd 11/29/04 6:36 PM Page 190
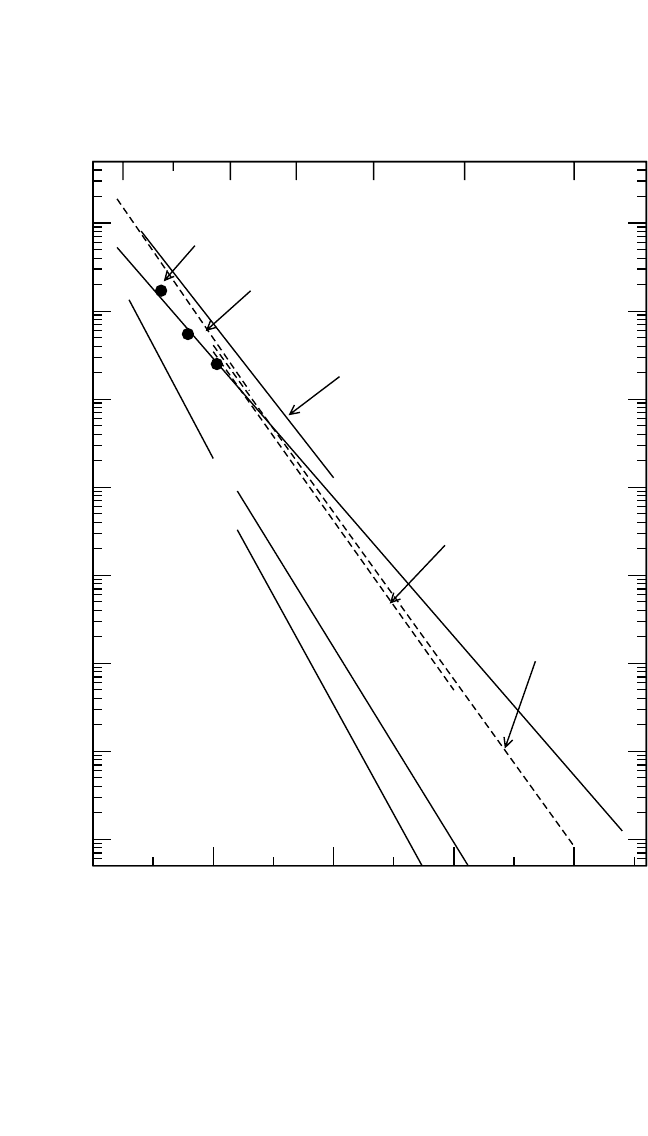
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 191
Figure 4.8(b) Solute diffusion is presented for Ga,
[38]
Ge (Ge1
[38]
and Ge2
[61]
),
Nb,
[63]
Ti,
[63]
Mo,
[61]
and Fe.
[64]
6
7
8910
T
-1
[10
-4
K
-1
]
10
-20
10
-19
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
D
*
[m
2
s
-1
]
1400 1300 1200 1100 10001600
T [K]
Fe
Ni
Nb
Ti
Mo
Al
Ge2
Ga
Ge1
(b) Solute Diffusion in Stoichiometric Ni
3
Al
Chapter-04.qxd 11/29/04 6:36 PM Page 191
