Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.


212 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
of S that would formally satisfy Eq. (3):
S
D
∼
D
(
∼
Ni)
. (8)
Although such calculations neglect the contribution of D
∼
(Al)
, a rough esti-
mate is obtained.
[85]
In Fig. 4.15(b), this hypothetical value of S is plotted
against composition.
On the other hand, the vacancy wind factor S was calculated by the
Monte Carlo approach for a number of possible diffusion mechanisms in
NiAl.
[85]
The results for the next-nearest neighbor jump mechanism and
the triple-defect mechanism, for example, are presented in Fig. 4.15(b).
The unusually small values of S deduced from Eq. (8) are qualitatively
reproduced by the triple-defect diffusion mechanism in NiAl. In the per-
fectly ordered B2 structure of NiAl, the triple-defect mechanism would
result in S 0 at stoichiometry. It is the existence of thermal defects that
gives rise to S 0 in such conditions.
[85]
Correspondingly, S is temperature-
dependent in Ni
50
Al
50
and also contributes to the activation enthalpy of
interdiffusion to about 70 kJ/mol.
[85]
In the nonstoichiometric alloys, the
triple-defect mechanism gives rise to normal values of the vacancy wind
factor S ≈ 1.
In summary, the estimate of S [Eq. (8)] supports the conclusion about
the dominance of the triple-defect diffusion mechanism, at least in near-
stoichiometric NiAl alloys.
4.4.4.2 Solute Diffusion
Indium diffusion in NiAl was measured as an Al-substituting
solute.
[86, 87]
In Fig. 4.14(a), the compositional dependence of the In diffu-
sivity D
*
In
measured at T 1523 K and calculated for T 1273 K from
the corresponding Arrhenius plots
[87]
are compared with the Ni tracer dif-
fusion results of Frank et al.
[36]
Note that In diffusion is faster than Ni at
higher temperatures in Ni-rich compositions, and that this relation is
reversed at lower temperatures. However, the absolute values of D
*
In
and
D
*
Ni
remain similar. This indirectly supports the application of Eq. (8) as a
rough estimate of S in NiAl. In Al-rich compounds, however, In diffuses
remarkably faster than Ni at all studied temperatures; the difference
amounts to an order of magnitude. Such behavior does not contradict the
triple-defect diffusion mechanism in near-stoichiometric compositions.
The abrupt increase of D
*
In
in Al-rich compositions can be related to the
corresponding type of ASB mechanism that involves Ni vacancies and Al
antistructure atoms. The appearance of Al atoms in antistructure positions
in Al-rich compositions of NiAl was already indicated.
[18, 23]
Chapter-04.qxd 11/29/04 6:36 PM Page 212

DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 213
This diffusion behavior in NiAl is somehow paradoxical: Because Ni
atoms in antistructure positions are necessary for long-range diffusion,
and they are only supplied by thermally created triple defects, a large
amount of structural Ni vacancies does not enhance Ni self-diffusion. It
does, however, facilitate diffusion of the Al component.
Co
[88, 89]
and Pt diffusion
[90]
in NiAl was studied, revealing a pro-
nounced compositional dependence with a minimum value near the stoi-
chiometric composition. Simultaneously, the activation enthalpies Q
Co
and
Q
Pt
have a maximum near the stoichiometry. The observed diffusion
behavior of Co and Pt favors the triple-defect diffusion mechanism, espe-
cially in compositions around stoichiometric NiAl.
[89, 90]
The Arrhenius parameters of self-diffusion and solute diffusion in
some compositions of NiAl alloys are presented in Table 4.4.
4.4.5 Fe-Al System
4.4.5.1 Self-Diffusion
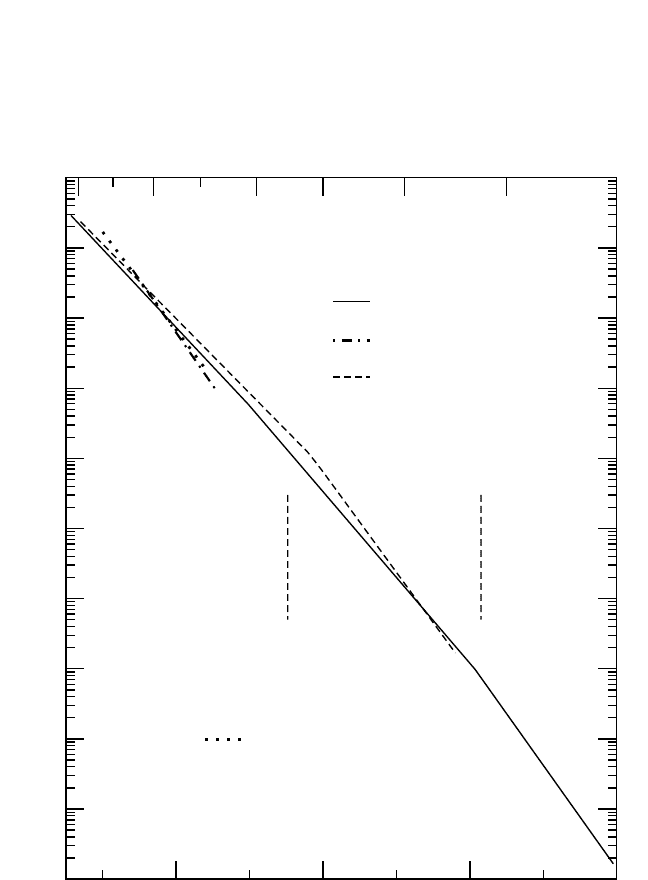
Fe Diffusion. Tracer measurements of self-diffusion in the Fe-Al sys-
tem were carried out in several investigations.
[41, 91, 92]
The results for FeAl
alloys with compositions of approximately Fe
3
Al, Fe
2
Al, and FeAl are
presented in Fig. 4.16.
According to Fig. 4.1(c), the phase diagram, Fe
3
Al reveals sequen-
tially ordered A2, B2, and D0
3
structures with decreasing temperature.
Thus, the temperature dependence of diffusion in Fe
3
Al may elucidate the
effect of order on diffusion. Fe self-diffusion was measured in a very
extended temperature interval that comprises all three possible structures
in Fe
3
Al
[92]
[Fig. 4.16(a)]. The increase of the degree of order resulted in
an increase in the activation enthalpy:
Q
Fe
(A2)
Q
Fe
(B2)
Q
Fe
(D0
3
)
. (9)
The Arrhenius parameters of diffusion in FeAl alloys are summarized
in Table 4.5.
The effect of the A2 B2 transition on Fe self-diffusion was studied
in a Fe
3
Al alloy with a slightly different composition (corresponding to a
slightly different A2 B2 transition temperature).
[91]
It is observed that a
more perfect order results in a larger activation enthalpy for Fe diffu-
sion. This effect was analyzed
[91]
in terms of Girifalco’s model,
[93]
which
predicts:
D
v
D
0
exp
. (10)
Q (1 g S
2
)
RT
Chapter-04.qxd 11/29/04 6:36 PM Page 213
8101214
T
-1
[10
-4
K
-1
]
10
-21
10
-20
10
-19
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
10
-11
D
*
[m
2
s
-1
]
Eggersmann et al.
Larikov et al.
Tôkei et al.
1500 1300 1100 1000
900
800
T [K]
Larikov et al.
Fe in Fe
3
Al:
Al in Fe
3
Al:
A2
B2
D0
3
(a) Fe
3
Al
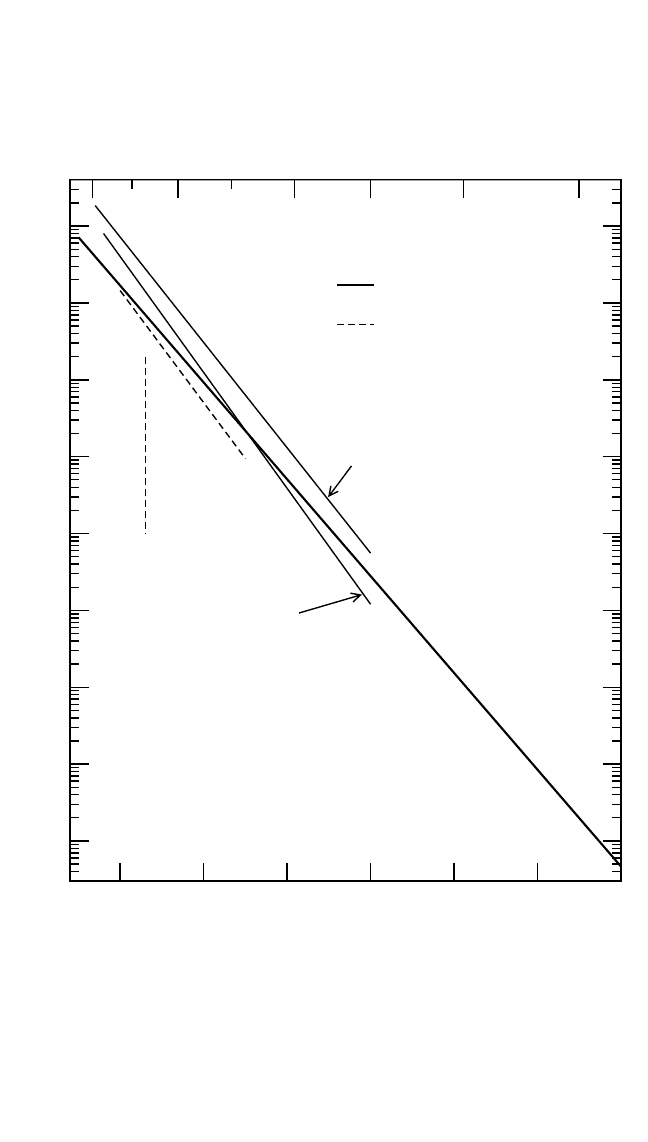
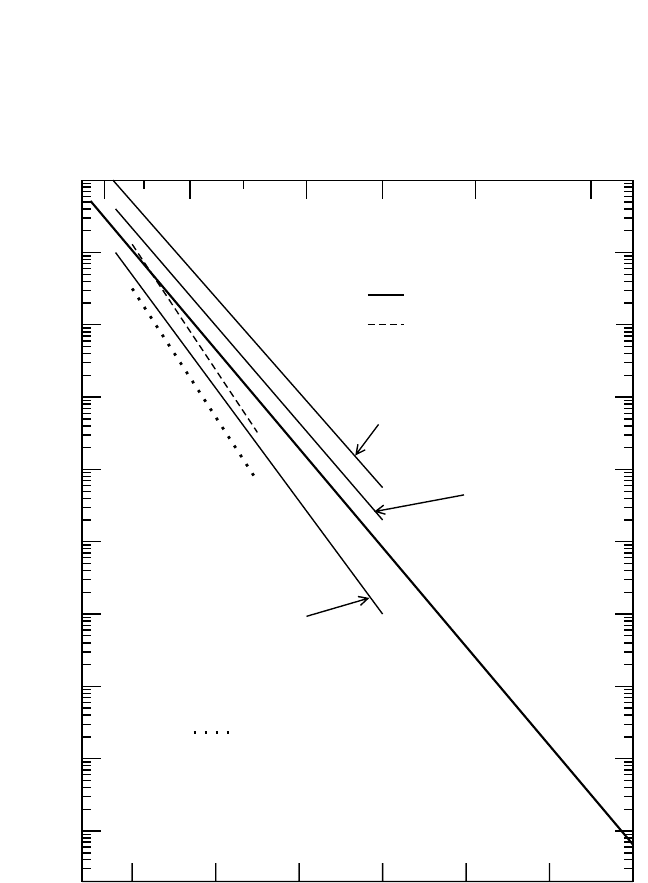
Figure 4.16 Self-diffusion
[41, 91, 92]
and interdiffusion
[99]
in Fe
3
Al, Fe
2
Al, and FeAl.
In (c), Al(1) and Al(2) refer to Al tracer diffusion data estimated from the Darken-
Manning equation with the thermodynamic factor calculated according to two dif-
ferent theoretical models.
[99]
214 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Chapter-04.qxd 11/29/04 6:36 PM Page 214
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 215
7
891011 12 13
T
-1
[10
-4
K
-1
]
10
-19
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
10
-11
D
*
, D [m
2
s
-1
]
Eggersmann et al.
Larikov et al.
1500 1300 1100 1000
900
800
T [K]
~
D (Salamon et al.)
Al
~
Fe in Fe
67
Al
33
:
B2A2
(b) Fe
2
Al
Figure 4.16 (Continued)
Chapter-04.qxd 11/29/04 6:36 PM Page 215
216 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
7
891011 12 13
T
-1
[10
-4
K
-1
]
10
-20
10
-19
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
10
-11
D
*
, D [m
2
s
-1
]
Eggersmann et al.
Larikov et al.
1500 1300 1100 1000
900
800
T [K]
~
Larikov et al.
D (Salamon et al.)
Al(1)
~
Al(2)
Fe in Fe
52
Al
48
:
Al in Fe
52
Al
48
:
(c) FeAl
Figure 4.16 (Continued)
Chapter-04.qxd 11/30/04 3:28 PM Page 216
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 217
Here, D
v
is the bulk diffusivity, D
0
and Q are the Arrhenius parameters of
diffusion in the disordered state, S is the long-range-order parameter, and
g is a constant. The value of g was found to be g 0.1.
[91]
In such an
approach, the change in the correlation factor below the transition tem-
perature is neglected, an effect that was shown later to be very impor-
tant.
[94]
If we simply approach Tôkei et al. data
[91]
by two independent
Arrhenius dependencies for the A2 and B2 regions, we arrive at the values
3.2 10
5
and 1.4 10
1
m
2
/sec, and 204 and 274 kJ/mol, for D
0
and Q,
respectively.
Fe self-diffusion in Fe
2
Al and FeAl was investigated almost exclu-
sively in the B2 phase region. In both cases, perfectly linear Arrhenius
dependencies were obtained [Fig. 4.16(b) and (c)].
[92]
Since only two
points lie in the A2 phase region for the Fe
2
Al composition,
[92]
it was not
possible to detect the effect of order on diffusion reliably. However, a rela-
tion similar to Eq. (9) can be expected. Fe diffusion was studied by
Larikov et al.
[41]
in much more restricted temperature intervals. It was
found that the absolute values of the diffusivity are within the same order
of magnitude. However, the deduced activation enthalpies are consider-
ably higher than those in the recent investigation.
[92]
Table 4.5. Arrhenius Parameters of Self-Diffusion and Solute Diffusion in
FeAl
Approximate D
0
Q
Composition Tracer Structure (m
2
s
1
) (kJ/mol) Ref.
Fe
3
Al Fe A2 8.1 10
5
217 92
Fe A2 3.2 10
5
204 91
Fe B2 3.8 10
4
232 92
Fe D0 3.3 10
1
278 92
In A2/B2 1.9 10
4
214 92
Zn A2/B2 1.8 10
4
221 92
Ni A2 4.7 10
4
240 91
Ni A2/B2 2.9 10
1
310 102
Co A2/B2 5.4 10
2
290 102
Cr A2/B2 8.6 10
4
233 102
Mn A2/B2 1.1 10
3
240 102
Fe
2
Al Fe A2/B2 1.1 10
3
241 92
In A2/B2 2.1 10
3
239 92
Zn A2/B2 4.3 10
3
240 92
FeAl Fe B2 4.1 10
3
262 92
In B2 5.4 10
3
257 92
Zn B2 1.2 10
2
251 92
Chapter-04.qxd 11/29/04 6:36 PM Page 217

218 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
A systematic change of the absolute values of the Fe diffusivity and
the corresponding activation enthalpies with increasing Al content in the
B2 phase region was observed:
[92]
D
Fe
(FeAl)
D
Fe
(Fe
2
Al)
D
Fe
(Fe
3
Al)
(11)
and
Q
Fe
(FeAl)
Q
Fe
(Fe
2
Al)
Q
Fe
(Fe
3
Al)
. (12)
The absolute vacancy concentration was measured in FeAl alloys by
means of differential dilatometry and positron annihilation techniques.
[95]
These results suggest that the B2 phase field has to be split into several
regions: B2, B2(l), and B2(h). [These are shown in Fig. 4.1(c).] It was
concluded that single vacancies are the main vacancy-type defects in the
B2 region, whereas triple defects are the main defects in the B2(l) region
and additional divacancies are produced in the B2(h) region.
[95]
These
changes in the defect behavior are not manifested in the self-diffusion
behavior. We can relate this to some specific mechanism of diffusion in
B2 FeAl. Note that in NiAl, the large concentration of structural vacancies
does not increase the Ni diffusivity. This was explained by the triple-
defect diffusion mechanism. A similar effect may exist in FeAl, if the
triple-defect mechanism dominates self-diffusion.
The dominating diffusion mechanism in FeAl is unclear.
[92]
Large val-
ues of the activation volume of Fe self-diffusion have been reported,
[96]
which indicates a possible effect from a composed defect as a diffusion
vehicle. Results of Mössbauer spectroscopy and quasielastic neutron scat-
tering measurements were interpreted in terms of nearest neighbor jumps
of Fe atoms.
[32, 97]
However, the type of diffusion vehicle could not be
identified in such measurements.
Summarizing the data of the differential dilatometry study,
[95]
we can
conclude that single vacancies are the main defects in all possible structures
of the Fe
3
Al alloy. Formally, the Fe sublattice diffusion mechanism can be
suggested for Fe diffusion in the D0
3
structure [Fig. 4.2(e)]. Since the for-
mation enthalpy of Fe vacancies on the a sublattice is smaller than that on
the g sublattice,
[25, 26]
the jumps a → g are likely to be the rate-determining
step, which results in a relatively high activation enthalpy of Fe diffusion
in this structure (Table 4.5). The decrease of the activation enthalpy Q
Fe
of
Fe diffusion in the B2 and then in the A2 structure in comparison with the
D0
3
structure is likely to be related to a relative easiness for a Fe atom to
explore the g and then the b sublattice at higher temperatures.
Al Diffusion.
26
Al diffusion was measured for several FeAl alloys
[41]
[Fig. 4.16(a) and (c)]. The main result was that the Al and Fe diffusivities
Chapter-04.qxd 11/29/04 6:36 PM Page 218

DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 219
are of the same order of magnitude. However, since the measured activa-
tion enthalpy of Fe self-diffusion
[41]
is remarkably larger than that
observed in other studies [Fig. 4.16(a) and (c)], these tracer data of
26
Al
diffusion are suspect.
Interdiffusion in the Fe–Al system was investigated in several stud-
ies.
[98, 99]
From the measured value of the Kirkendall shift, corrected for
volume contraction, it was concluded that the ratio of intrinsic diffusivi-
ties D
*
Al
D
*
Fe
is always larger than unity. It is about 1.2 to 1.5 in B2 FeAl
and about 1.8 in the A2 phase reagion for a composition roughly corre-
sponding to Fe
3
Al.
[95]
Interdiffusion was studied using diffusion couples
between Fe
50
Al
50
and Fe
70
Al
30
.
[99]
Aweak dependence of D
∼
on the compo-
sition was stated, with no significant influence of the A2 B2 transi-
tion.
[99]
The results for compositions of approximately Fe
2
Al and FeAl are
presented in Fig. 4.16(b) and (c), respectively. Using the thermodynamic
factors Φ calculated from the x-ray scattering data,
[100]
and assuming
S 1 for the vacancy wind factor, the Al tracer diffusivity was estimated
for these two compositions
[99]
[Fig. 4.16(b) and (c)]. In conclusion, both
components diffuse in Fe
2
Al with very similar rates. In FeAl, this situa-
tion remains less clear, since no experimental data for the thermodynamic
factor are available for such compositions. Different theoretical models
for the calculation of the thermodynamic factor result in Al diffusivities
that differ by a factor of 5 to 10
[99]
[see Fig. 4.16(c)]. At present, the uncer-
tainty in the Al diffusivity does not allow definite conclusions about the
relevant diffusion mechanism to be drawn.
4.4.5.2 Solute Diffusion
Recent comprehensive information on solute diffusion in Fe
3
Al is pre-
sented in Fig. 4.17(a). A pronounced effect of the A2 B2 transition on
Ni diffusion in Fe
3
Al was determined.
[101]
The high-pressure measure-
ments indicated that the diffusion process is controlled by single-vacancy
motion in both structures.
[101]
The slower Ni diffusion and the larger acti-
vation enthalpy with respect to that of Fe self-diffusion were explained by
a predominant Ni solubility on the a sublattice [Fig. 4.2(e)]. Diffusion of
In and Zn as Al-substituting solutes was studied.
[92]
Both solutes diffuse
faster than Fe [Fig. 4.17(a)]. The A2 B2 transition does not practically
influence diffusion of Al substitutes, whereas some small effect from the
B2 D0
3
transition is indicated in the case of In diffusion [Fig. 4.17(a)].
Recently, diffusion of several transition metals was studied in Fe
3
Al
in the temperature interval corresponding to the A2 and B2 structures.
[102]
An effect of the A2 B2 transition remained within limits of the experi-
mental uncertainties. The activation enthalpies and frequency factors of
Chapter-04.qxd 11/29/04 6:36 PM Page 219
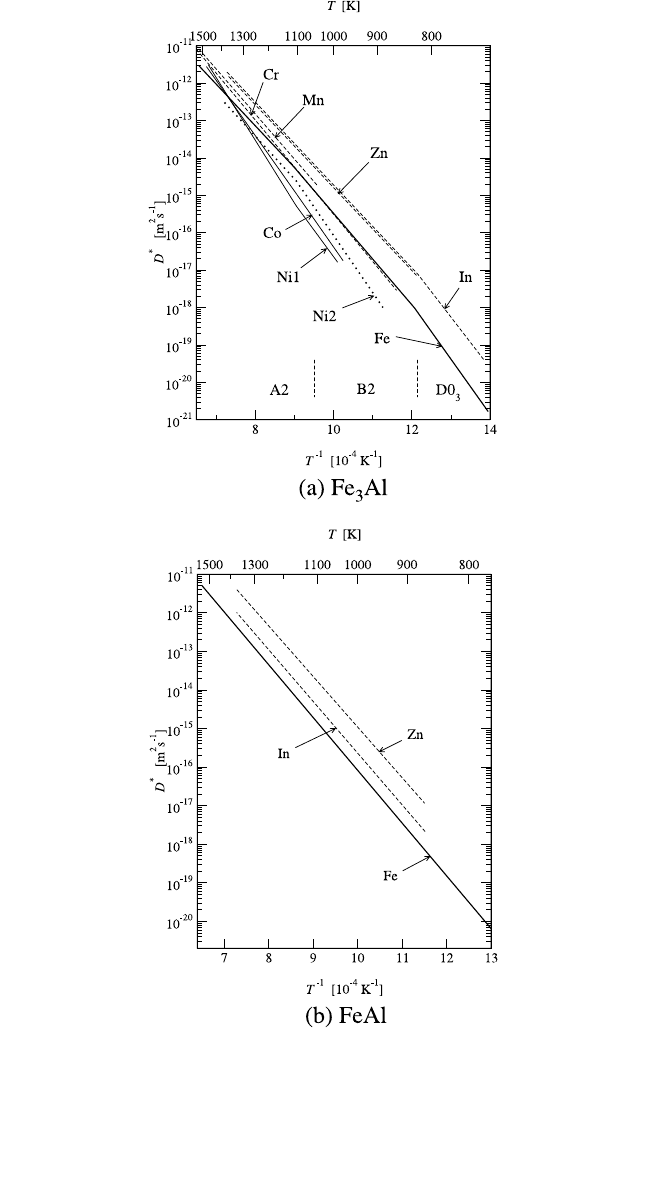
220
Figure 4.17 Solute diffusion of In and Zn
[92]
and of Ni, Co, Cr, and Mn
[102]
in Fe
3
Al
and FeAl. In (a), Ni1 and Ni2 represent Ni diffusion in Fe
3
Al measured by Peteline
et al.
[102]
and Tôkei et al.,
[101]
respectively.
Chapter-04.qxd 11/29/04 6:36 PM Page 220

the combined data for the A2B2 region are listed in Table 4.5. The diffu-
sion behavior of the transition metal atoms can be correlated with the pref-
erential solubility of these atoms on different sublattices.
[102]
Co and Ni,
which reveal preferential solubility on the a sublattice,
[103]
are slow dif-
fusers in Fe
3
Al, whereas Cr and Mn, which occupy mainly Al sites,
[103,104]
diffuse slightly faster than Fe.
4.5 Discussion of Lattice Diffusion in
Intermetallics
Section 4.4 analyzed self-diffusion in Ni, Ti, and Fe aluminides with
different structures. The systematic investigations demonstrate that the
antistructure-atom systems, that is, the systems with the antistructure-
defect type of disorder on both sides of the stoichiometry (Ni
3
Al, Ti
3
Al,
and TiAl), reveal only a weak compositional dependence of self-diffusivity.
In these systems, the effect of the compositional antistructure defects is
only marginal and is within experimental error. The analysis shows that
there are a number of reasons for such behavior: (1) The transition-metal
sublattice forms a connected network for intrasublattice nearest neighbor
jumps of atoms, which does not change the state of the order in the com-
pound [Fig. 4.2(a)–(d)]. (2) The vacancy concentration on the transition-
metal sublattice is larger by several orders of magnitude than that on the
Al sublattice [Fig. 4.3(a) and (b)]. (3) The sublattice diffusion mechanism
produces the dominant contribution in these compounds. Note that in
TiAl, the third argument is relevant only at temperatures below 1470 K,
where a linear Arrhenius dependence is observed [Fig. 4.11(a)].
Al diffusion in Ni
3
Al and Ti
3
Al, calculated from the interdiffusion
data, reveals a slight but clear increase of D
Al
with increasing Al content
on the Al-rich side of the compositions. This is related to the transition
metal sublattice diffusion mechanism for Al atoms and the appearance of
compositional Al antistructure atoms in addition to the thermal ones in
these compositions.
The most prominent compositional dependence of the activation enthalpy
Q of self-diffusion in Ni and Ti aluminides is observed in the phase NiAl
exhibiting triple defects. The most remarkable change in Q, however, occurs
on the Ni-rich side in NiAl, while the formation of the Ni structural vacancies
does not practically affect Q [see Fig. 4.14(b)]. The latter behavior is
explained by the triple-defect diffusion mechanism producing a dominant
contribution in the Al-rich, stoichiometric, and slightly Ni-rich compositions
of NiAl. The distinct decrease of Qat larger Ni content (above 54 at.% Ni) is
explained by an additional contribution of the ASB mechanism, which oper-
ates in NiAl with the percolation threshold at about 55 at.% Ni.
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 221
Chapter-04.qxd 11/29/04 6:36 PM Page 221
