Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

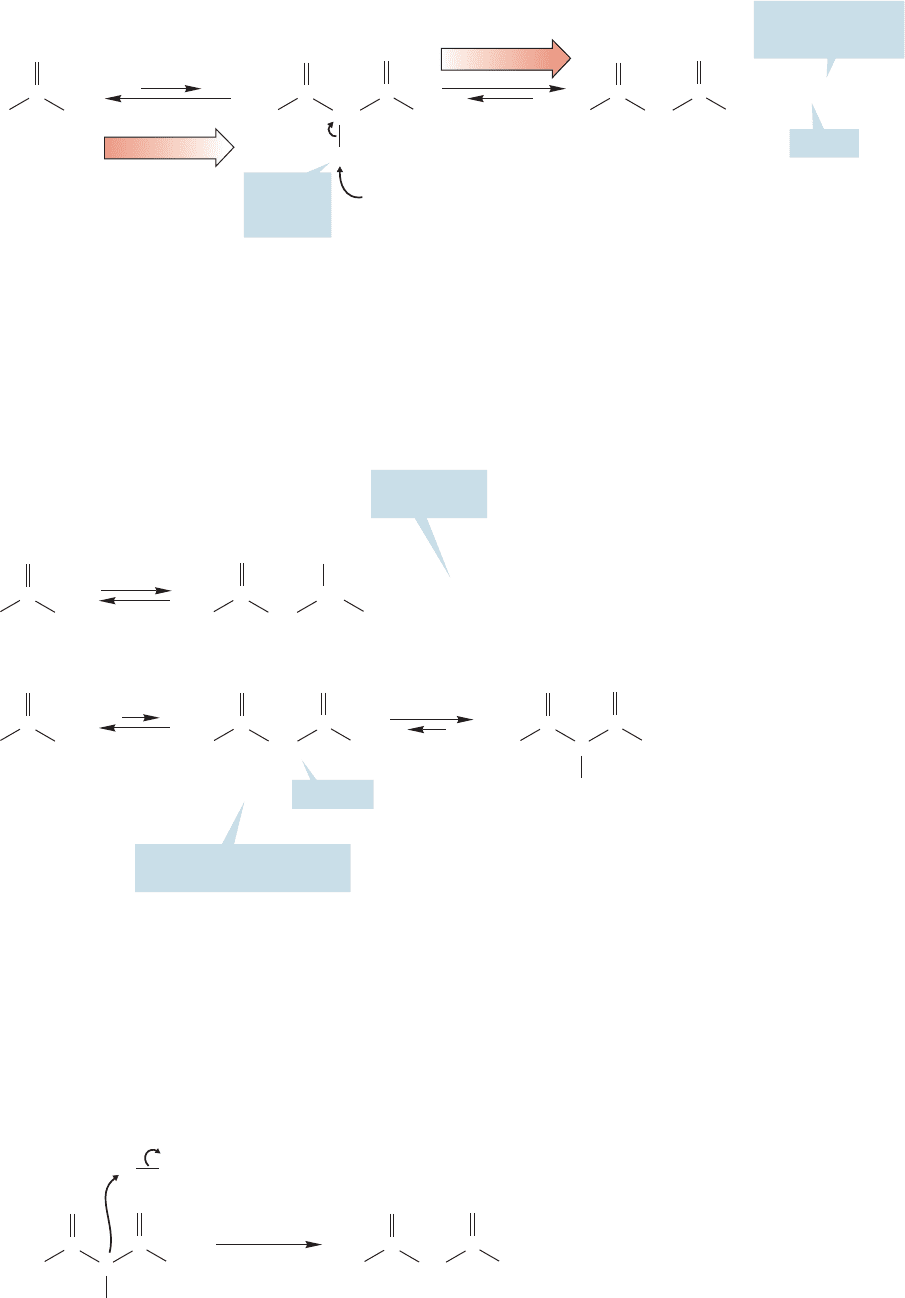
19.8 Addition of Acid Derivatives to the Position: The Claisen Condensation 989
␣
the condensation step is removal of one of the doubly α hydrogens to give the highly
resonance-stabilized salt of the β-keto ester (Fig. 19.102).
Note resonance
stabilization
..
..
O
RO
2
..
..
C
CH
3
–
..
..
OR
..
–
..
..
OR
..
..
..
HOR
..
..
HOR+
..
..
O
RO
..
..
C
CH
H
CH
3
O
..
..
C
endothermic
exothermic
..
..
..
O
RO
..
..
C
CH CH
3
O
..
..
C
–
(–) (–)
pK
a
~ 11
Doubly
hydrogen
pK
a
~17
..
..
..
–
Catalyst (RO ) is
destroyed!
FIGURE 19.102 Alkoxide is destroyed
in the last step of the Claisen
condensation as a doubly α proton is
removed to give a stable anion.
If the doubly α proton is removed, all is well thermodynamically; now the product
is more stable than the starting material, but the catalyst for the reaction has been destroyed!
A Claisen condensation using a catalytic amount of base must fail, because it can only
generate an amount of product equal to the original amount of base.The simple reme-
dy is to use a full equivalent of alkoxide, not a catalytic amount. A thermodynamically
favorable Claisen condensation is not run with catalytic base, but requires a full equiv-
alent of base.Be certain you are clear as to the reason why the aldol condensation requires
only a catalytic amount of base but the Claisen needs a full equivalent (Fig. 19.103).
..
..
OR
..
–
..
..
O
RO
2
..
..
C
CH
3
–
..
..
OR
..
..
..
..
..
HOR
..
..
HOR+
O
RO
..
..
C
CH
2
CH
3
O
..
..
C
..
..
..
O
RO
..
..
C
C
H
CH
3
O
..
..
C
–
(–) (–)
Doubly
Removes doubly proton;
the catalyst is destroyed
Claisen
O
H
2
..
..
C
CH
3
Aldol
–
..
..
OH
..
..
H
2
O
..
..
OH+
O
H
..
..
..
C
CH
2
CH
3
..
..
CH
–
..
OH
Can carry out
another aldol
FIGURE 19.103 The aldol
condensation can be carried out using
a catalytic amount of base because
the final protonation step regenerates
a molecule of hydroxide. In the last
step of the Claisen condensation,
the alkoxide ion is consumed by
removing the doubly α proton.
Because we are left with a carbanion after the Claisen condensation, acidification
of the reaction mixture is required in order to form the neutral β-keto ester. What
prevents this product,thermodynamically unstable with respect to the starting ester mol-
ecules, from re-forming the starting esters? Certainly the Claisen condensation is
reversible.But the β-keto ester that is formed in the basic conditions is much more like-
ly to form the resonance-stabilized enolate than go back to starting material.And once
the stable enolate is formed it is not likely to react with alkoxide,a nucleophile nucleo-
phile reaction. And after acidification there is no base present! Without the base, there
can be no reverse Claisen condensation, and the product ester is obtained (Fig. 19.104).
OH
2
H
..
..
..
H
3
O
..
+
+
H
2
O
..
..
H
2
O
+
..
..
..
O
RO
..
..
C
C
H
CH
3
O
..
..
C
protonation
–
..
..
O
RO
..
..
C
CH
3
CH
2
O
..
..
C
No base present!
-Keto ester
FIGURE 19.104 Acidification of the
Claisen condensation produces a
β-keto ester in the absence of base.
PROBLEM 19.32 Draw the mechanism for the Claisen condensation of ethyl phenyl-
acetate using the appropriate base. Include the acidification process at the end.
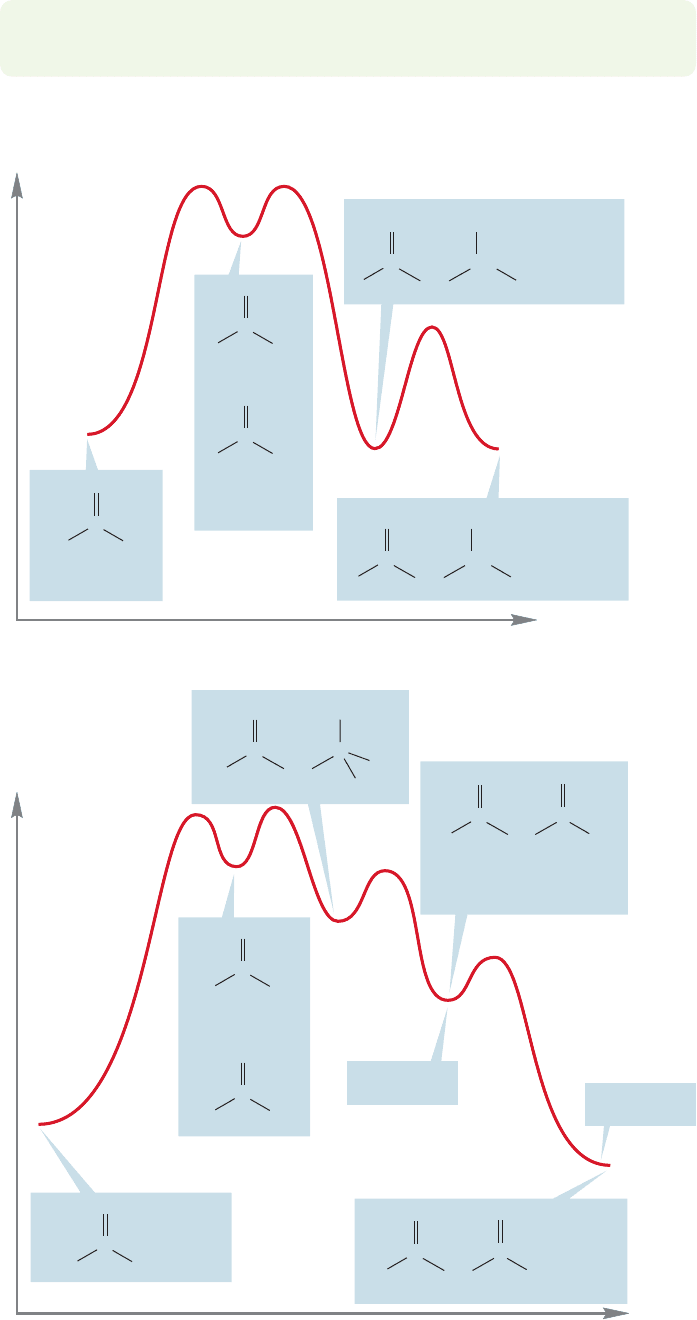
Figure 19.105 shows Energy versus Reaction progress diagrams for the aldol and
Claisen condensations.
Energy
..
..
H
2
O
..
..
H
2
O
O
H
..
..
C2
CH
3
O
H
..
..
C
CH
2
–
..
–
–
..
..
OH
..
+
+
O
H
..
..
C
Reaction progress
The aldol condensation for acetaldehyde is approximately thermoneutral
CH
3
+
..
O
H
..
..
C
CH
3
O
..
..
CH
+
–
..
..
HO
..
Energy
O
RO
..
..
C2
CH
3
O
..
..
C
CH
2
–
..
+
O
..
..
C
Reaction progress
CH
3
+
RO
..
..
RO
..
..
C
O
..
..
O
..
..
C
CH
3
+
–
..
..
RO
..
–
..
..
RO
..
RO
..
..
C
Endothermic
to here
Exothermic
to here
O
..
..
O
..
..
C
CH
3
CH
+
–
..
..
HOR
RO
..
..
..
–
..
O
..
..
C
CH
3
O
..
..
C
RO
..
..
OR
..
..
CH
2
CH
2
–
..
O
H
..
..
C
CH
3
O
..
..
CH
CH
2
CH
2
FIGURE 19.105 Energy versus
Reaction progress diagrams for the
aldol and Claisen condensations.
990 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
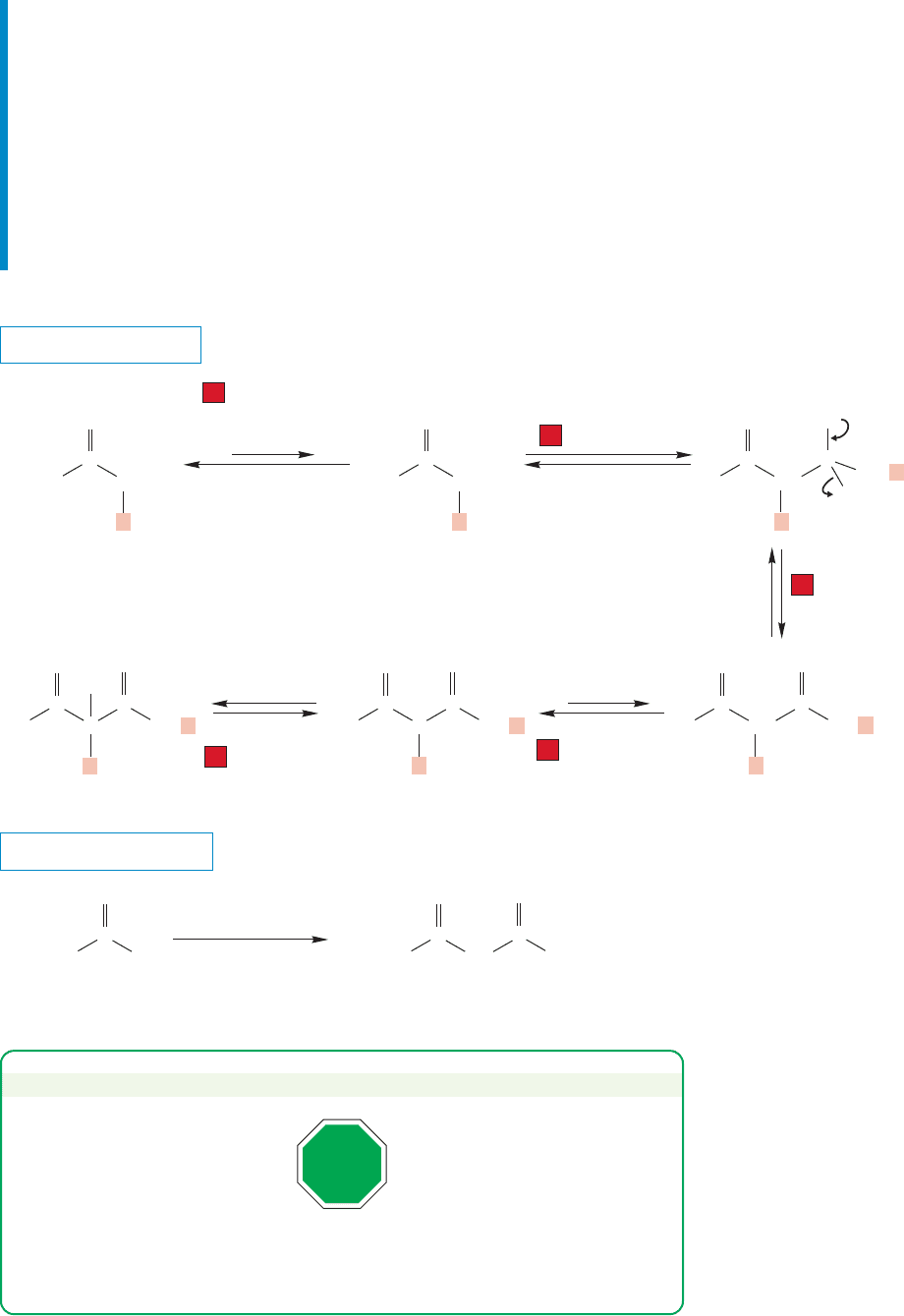
PROBLEM SOLVING
Summary
A full equivalent of base makes the Claisen condensation a practical source of
acetoacetic esters (β-keto esters).The five steps of the process are summarized in
Figure 19.106:
1. Enolate formation.
2. The condensation itself (addition).
3. Loss of alkoxide from the tetrahedral intermediate (elimination).
4. Removal of the doubly α hydrogen.
5. Acidification to produce the β-keto ester.
19.8 Addition of Acid Derivatives to the Position: The Claisen Condensation 991
␣
THE GENERAL CASE
A SPECIFIC EXAMPLE
Enolate
formation
1
Loss of
alkoxide
3
Condensation
2
Removal of
“doubly ”
hydrogen
4
Acidification
5
2. Acidification
1.
8 h, 78 ⬚C
..
..
H
3
O
..
+
H
2
O
..
..
O
RO
..
..
C
CH
2
O
RO
..
..
C
CH
R
R
+
..
..
O
RO
..
..
OR
..
..
C
CH
CH
2
R
O
..
..
..
C
..
–
..
–
–
(–)
(–)(–)
..
..
O
RO
..
..
C
CH CH
2
R
O
..
..
C
R
RR
R
..
..
O
RO
..
..
C
CCH
2
R
O
..
..
C
..
..
O
RO
..
..
C
C
H
CH
2
R
O
..
..
C
+
–
O
..
..
C
CH
3
CH
2
(75%)
O
..
..
C
..
..
..
..
O
CH
3
CH
2
OCH
3
CH
2
O
..
..
CH
3
CH
2
ONa
..
..
..
C
CH
3
–
..
..
RO
..
–
..
..
RO
..
FIGURE 19.106 The Claisen
condensation.
“All” β-keto esters are Claisen products or products of closely related reactions.
Every time you see a β-keto ester, think—“What Claisen condensation would
have formed it?”
GO
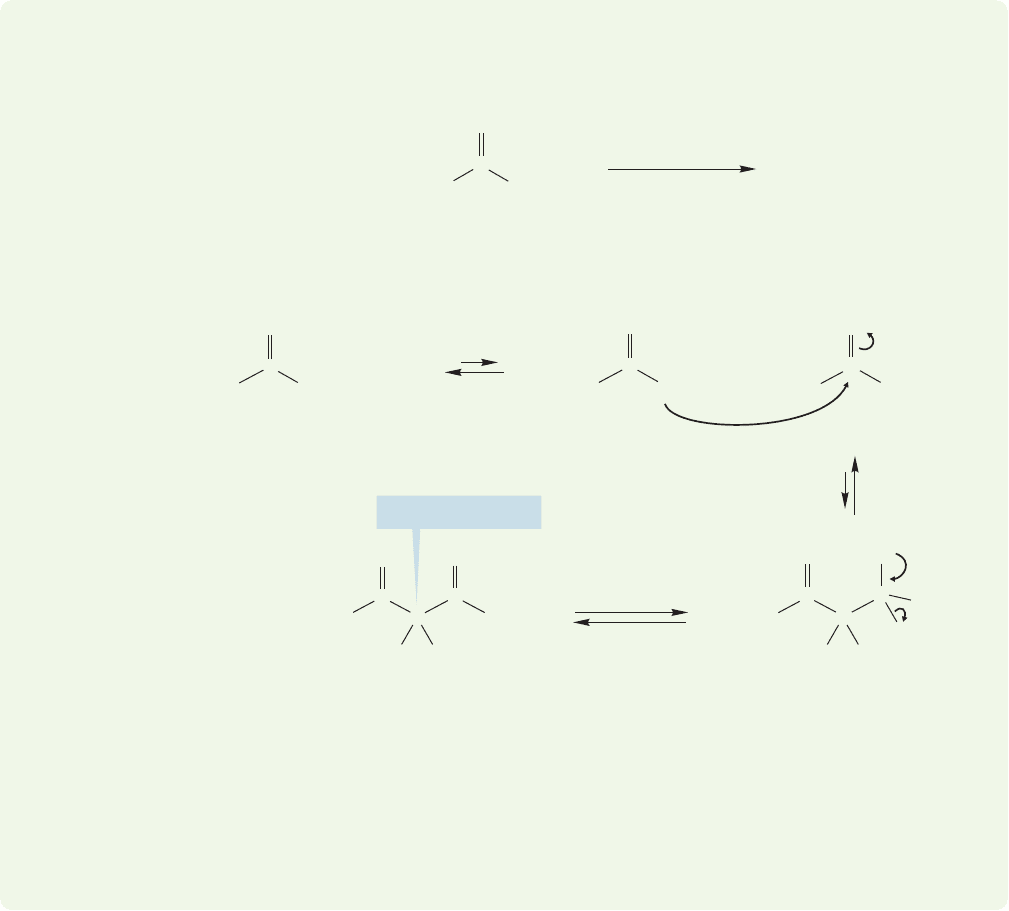
992 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
+
–
..
..
O
CH
3
CH
2
O
..
..
OCH
2
CH
3
..
..
HOCH
2
CH
3
Na
..
..
..
C
Ethyl 2-methylpropanoate
(full equiv.) No useful amount of
Claisen condensation
product
CH(CH
3
)
2
However, the product A contains no doubly α hydrogens! There can be no for-
mation of the stable, highly resonance-stabilized anion that accounts for the suc-
cess of the Claisen condensation. Thermodynamics dictates that the starting
materials be more stable than the product, and the reaction settles out at a point
at which there is little product. The mechanism of the reverse reaction is written
for you: Just read the scheme backward.
19.9 Variations on the Claisen Condensation
So, that’s the Claisen condensation: Two esters combine with an equivalent of base
to make a β-keto ester. Now it’s time to look at how changes in structure of the start-
ing materials affect the structure of the product and the mechanism of its formation.
19.9a Intramolecular Claisen Condensations The Dieckmann condensa-
tion, the cyclic version of the Claisen condensation, is named after Walter Dieckmann
(1869–1925). The reaction transforms an acyclic diester into a cyclic β-keto ester.
Formation of five- and six-membered rings is favored, and there is little beside the
intramolecular nature of the reaction to distinguish the Dieckmann from the standard
–
–
–
O
..
C
..
..
..
..
+
..
..
..
elimination
addition
CH
3
CH
2
O CH(CH
3
)
2
..
O
..
C
..
....
CH
3
CH
2
OC(CH
3
)
2
..
O
..
C
..
..
CH
3
CH
2
O CH(CH
3
)
2
..
O
..
..
C
C
C
..
..
CH
3
CH
2
O
OCH
2
CH
3
CH
3
H
3
C
CH(CH
3
)
2
..
O
..
..
No hydrogens here
O
..
C
C
C
A
..
..
CH
3
CH
2
O
OCH
2
CH
3
CH
3
H
3
C
CH(CH
3
)
2
..
O
..
..
–
..
..
..
RO
19.8c Reverse Claisen Condensations As mentioned,formation of the ini-
tial condensation product in the Claisen condensation is usually endothermic. If no
doubly α hydrogens exist for anion formation, β-keto esters will revert to their
component ester starting materials when treated with alkoxide base.
ANSWER In this classic problem, Claisen condensation leads to product A through
the usual addition–elimination scheme:
WORKED PROBLEM 19.33 Even when a full equivalent of sodium ethoxide is used,
ethyl 2-methylpropanoate fails to give useful amounts of a condensation product.
Explain with a careful mechanistic analysis.
19.9 Variations on the Claisen Condensation 993
Claisen condensation (Fig. 19.107). Like the Claisen, the Dieckmann condensation
requires a full equivalent of base. Unless there is sufficient base available to remove
the doubly α hydrogen and drive the reaction to a thermodynamically favorable con-
clusion, the reaction is not successful. A final acidification liberates the β-keto ester
in the absence of base.
A MORE COMPLEX EXAMPLE
THE STANDARD DIECKMANN
..
..
O
C
–
–
(–)
(–)
(–)
..
..
..
..
OH
2
..
..
+
..
..
H
3
O
..
+
H
2
O
CH
2
C
CH
3
O
..
..
CH
3
O
..
..
CH
3
O
..
..
CH
3
O
H
..
..
..
CH
3
OH
..
..
HOCH
3
..
..
OCH
3
..
OCH
3
..
..
OCH
3
C
C
O
C
enolate formation
intramolecular
condensation
(addition phase)
acidification
elimination
phase
Removable
doubly
hydrogen
C
O
..
..
O
..
..
..
–
–
..
..
OCH
3
O
..
..
CH
..
..
OCH
3
..
..
OCH
3
C
O
..
..
O
–
..
..
..
..
C
C
-Keto esterResonance-stabilized anion
Final product—
the -keto ester
deprotonation
C
H
..
..
..
..
..
..
..
CH
3
O
O
O
C
C
..
..
..
..
..
..
CH
3
O
O
O
C
C
H
..
..
O
..
..
..
O
..
..
O
..
..
CH
3
O
..
..
..
OCH
3
H
3
C
..
(97%)
..
..
CH
3
O
..
..
OCH
3
..
O
..
..
O
H
3
C
1. NaOCH
3
benzene
2. HCl/H
2
O
CH
C
WEB 3D
FIGURE 19.107 The Dieckmann
condensation—an intramolecular
Claisen condensation.
19.9b Crossed Claisen Condensations A naively designed crossed (or
mixed) Claisen condensation is doomed to the same kind of failure as is the
crossed aldol condensation. There are four possible products (Fig. 19.108), and
therefore no easy source of the selectivity we need for a practical synthetic reaction.
O
..
..
C
CH
3
+
O
..
..
C
CH
2
CH
3
–
..
..
/CH
3
OH
..
..
CH
3
O
..
..
CH
3
O
..
..
CH
3
O
..
..
CH
3
O
..
O
..
..
C
CH
3
CH
3
O
..
..
C
..
..
CH
3
O
O
..
..
C
CH
2
CH
3
CH
3
O
..
..
C
..
..
CH
3
O
O
..
..
C
CH
2
CH
3
O
..
..
C
..
..
CH
3
O
O
..
..
C
CH
3
O
..
..
C
+
Na
CH
2
CH
2
CH
CH
FIGURE 19.108 There are four possible—and likely—products from a crossed Claisen condensation.
994 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
PROBLEM 19.34 Show in a general way how these products are produced and write
a mechanism for the formation of one of the four products shown in Figure 19.108.
CH
A SPECIFIC EXAMPLE
A RELATED EXAMPLE
THE “CROSSED CLAISEN”—THE GENERAL MECHANISM
..
..
..
H
3
O
..
+
–
–
(–)
(–)
H
2
O
..
H
3
O
..
..
H
2
O
acidification
..
..
O
H
2
C
..
..
C
enolate
elimination
remove doubly
hydrogen
addition
OR
..
..
OR
..
..
OR
..
..
..
..
O
CH
3
CH
2
O
..
..
C
OCH
2
CH
3
..
..
O
X
X = Ph Benzoates
= H Formates
= RO Carbonates
..
..
C
OR
+
O
X
..
.. ..
C
CH
2
O
..
..
C
..
..
CH
3
O
..
..
CH
(56%)
(78%)
O
..
..
C
..
..
..
OR
O
X
..
..
C
CH
2
O
..
..
C
..
..
OR
O
X
..
..
C
CH
O
..
..
C
–
–
+
..
..
OR
O
X
..
..
C
CH
2
O
..
..
C
..
..
RO
..
OR
..
..
H
..
..
OCH
3
..
..
O
C
NaH/benzene
78 ⬚C
2.
1.
3. /
H
3
O
..
+
..
..
..
H
2
O
1.
2. /
..
CH
3
CH
2
C
..
..
OCH
3
OCH
3
..
..
..
O
..
..
OCH
2
CH
3
..
O
C
–
..
..
OCH
2
CH
3
..
+
Na
CN
C
N
C
(–)
CH
2
FIGURE 19.109 Successful crossed Claisen condensations.
As in the crossed aldol condensation, there are some simple steps we can take to
improve matters.If one of the starting esters has no α hydrogen,there can be no eno-
late formation from it, and it can act only as an electrophile, never as a nucleophile.
It is helpful to add the ester with an α hydrogen to a mixture of the partner without
an α hydrogen and the base. As the enolate is formed it is more likely to react with
the ester that has no α hydrogen since it is present in excess. Using this technique, a
successful crossed Claisen can be achieved. Benzoates, formates, and carbonates are
examples of esters without α hydrogens and are often used (Fig. 19.109).
19.9 Variations on the Claisen Condensation 995
(a)
H
3
O
+
/H
2
O
H
3
O
+
/H
2
O
..
1. NaOR/HOR
NaOR
HOR
2. H
2
O
O
..
..
O
..
..
COOR
COORROOC
CH
3
CH
3
(b)
(c)
(CH
3
OOC)
2
CH
2
NaOCH
3
/HOCH
3
1. NaOCH
3
/HOCH
3
2.
..
..
O
..
..
O
..
..
O
O
..
..
Ph
O
..
..
Ph
O
..
..
O
..
..
..
COOCH
3
COOCH
3
COO
–
CH
3
OOC
OH
We now have some new reactions to use in problems.Put another way, your lives
are now even more complicated than before,as even more sadistically difficult prob-
lems are within your abilities.There really aren’t too many new things to think about.
You only have to add the Claisen-like processes that involve the loss of a leaving
group from a carbonyl carbon that has been attacked by an enolate. In other words,
you always have to keep the addition–elimination reaction in mind.
But be careful—reactions are driven by thermodynamics,not our concepts of for-
ward and backward.The reverse Claisen is much harder to detect in problems than
the forward version, but no less reasonable mechanistically. As always in matters such
as these, experience counts a lot, and there is no way to get it except to work a lot
of problems.
PROBLEM 19.35 Provide mechanisms for the following reactions:
+
–
..
..
..
..
..
OCH
3
..
..
CH
3
O
Na
+
OCH
3
..
..
OCH
3
HOCH
3
H
CHO
O
..
..
O
..
..
O
..
..
..
..
CH
2
COO
–
Na
+
Mixture of stereoisomers
(35%)
PROBLEM 19.36 Provide a mechanism for the following transformation. Be care-
ful. This problem is much harder than it looks at first. Hint: The mechanism
involves a lactone intermediate.
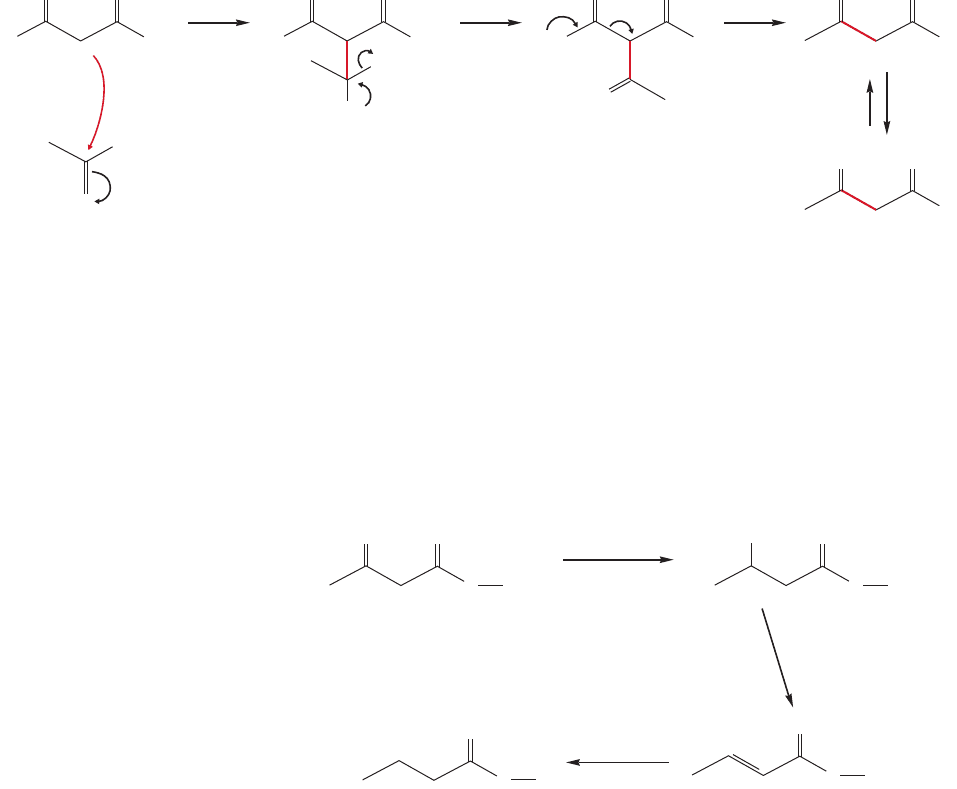
+
(new bond in red)
–
– –
O
OO
–
O
S¬Enzyme
O SR
–
OO
O SR
S¬Enzyme
O
–
OO
O SR
OO
SR
OO
SR
CO
2
FIGURE 19.110 Loss of CO
2
drives this reaction toward product.
19.10 Special Topic: Forward and Reverse Claisen
Condensations in Biology
Both forward and reverse Claisen condensations feature prominently in the biochem-
ical synthesis and degradation of fatty acids (p. 862). A critical step in the construc-
tion of these long-chain carboxylic acids involves enzyme-mediated Claisen
condensations in which activated two-carbon fragments are sewn together. Of
course, Nature has a thermodynamic problem here—how is the endothermicity of
the Claisen condensation to be overcome? The trick is to use an activated malonate
in the condensation. Loss of carbon dioxide is used to drive the equilibrium toward
the product (Fig. 19.110).
996 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
NADPH
NADPH
reduction
reduction
enzymatic
elimination
OO
O
OH
O
O
S R
S R
S R
S R
FIGURE 19.111 A series of enzyme-
mediated reactions leads to an overall
reduction of one carbonyl group to a
methylene.
Nature’s work is not yet done; first,a reduction of the ketone occurs (Fig.19.111).
The reducing agent is NADPH, a molecule closely related to NADH, which we
saw in Chapter 16 (p. 814). Next, there is an enzyme-mediated elimination reac-
tion. Finally, NADPH participates again, reducing the newly formed double bond
to give the final product. Repetition of the steps in Figures 19.110 and 19.111 even-
tually leads to the fatty acid.
In Chapter 17 (p. 863), we discussed briefly the metabolism of fatty acids,
and raised a question: How is a fatty acid cleaved at the bond to give a car-
bon chain that is two carbons shorter? The problem is that the bond needs
to be activated in some way. If the β carbon were oxidized, we would have
α,β
α,β
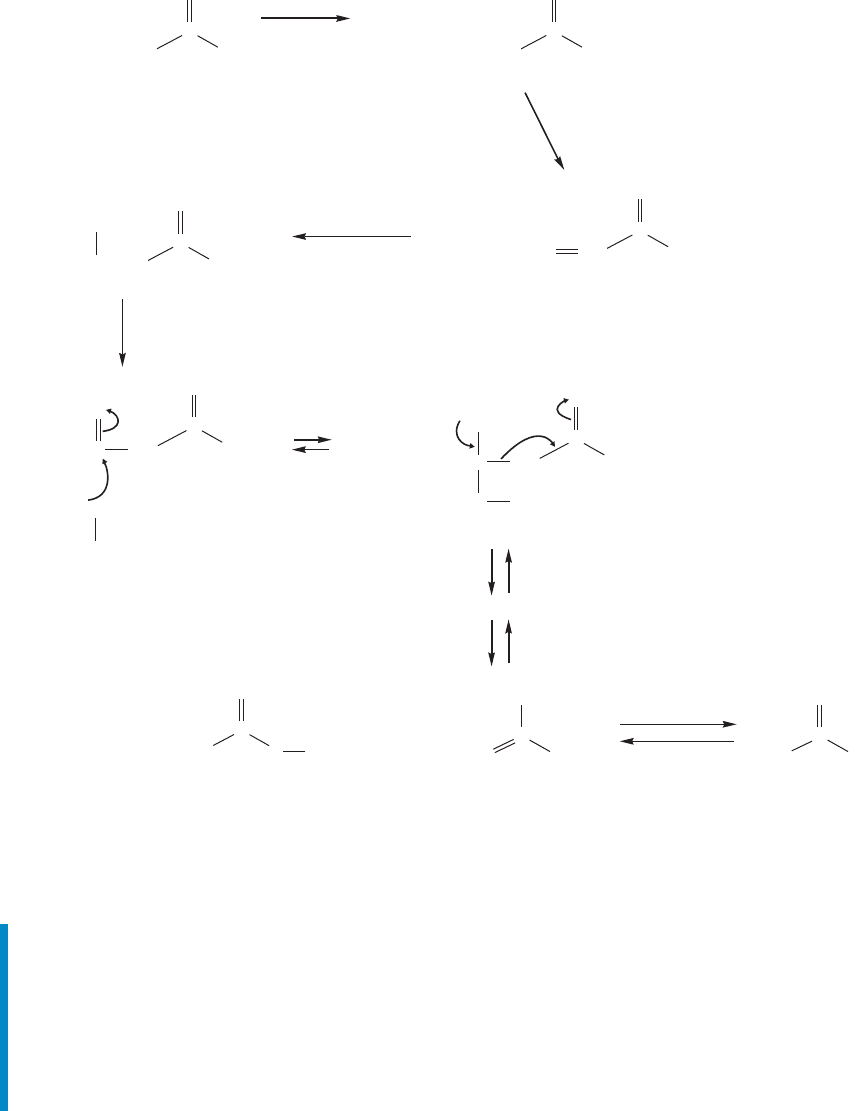
19.10 Special Topic: Forward and Reverse Claisen Condensations in Biology 997
a β-keto ester all set up for a reverse Claisen condensation. That is exactly the
pathway Nature uses. Here, palmitate is first converted into the SCoA deriva-
tive. Then three enzymes act in sequence to dehydrogenate, hydrate, and dehy-
drogenate again to give the β-keto ester (Fig. 19.112). Now the reverse Claisen
does the first two-carbon cleavage reaction, and metabolism of this fatty acid
has begun.
CH
3
(CH
2
)
12
CH
2
CH
2
C
O
O
–
Palmitate
OH
α
β
CH
3
(CH
2
)
12
CH
2
CH
2
C
O
acyl-CoA
dehydrogenase
enoyl-CoA
hydrase
β-OH-acyl-CoA
dehydrogenase
ββ
A β-keto ester
SCoA
CH
3
(CH
2
)
12
CHCH
2
C
O
SCoA
CH
3
(CH
2
)
12
CH CH
C
O
SCoA
C
O
C
O
SCoA
SCoA
+
Myristate
(ready for further degradation)
CH
3
(CH
2
)
12
C
O
–
S
Enzyme
CH
3
(CH
2
)
12
CH
2
C
O
SCoA
CH
2
C
–
O
S Enzyme
H
2
C
OH
S Enzyme
CH
3
(CH
2
)
12
C
SCo
A
Acetyl-CoA
H
3
C
O
C
Summary
There are several variations of the Claisen condensation.The intramolecular vari-
ation is called the Dieckmann condensation. A crossed Claisen condensation is
possible between two different esters, but this reaction can lead to multiple prod-
ucts. Claisen condensations can operate in the “forward” direction or in the
“reverse” direction, and these two processes lead to the construction and decon-
struction of fatty acids.
FIGURE 19.112 Metabolism of a fatty acid containing 16 carbons to one containing 14.
998 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
THE GENERAL CASE
A SPECIFIC EXAMPLE OF THE ROBINSON ANNULATION
..
..
O
+
+
–
–
..
..
..
..
..
..
..
H
2
O
H
2
Oprotonation
..
..
H
2
O
Na
+–
OCH
2
CH
3
HOCH
2
CH
3
–10 ⬚C
O
..
..
O
..
..
..
..
..
O
OH
–
..
..
..
OH
+
+
–
..
..
..
OH
–
–
..
..
..
OH
base
Michael
KOH
H
2
O
100 ⬚C
aldol
elimination
O
H
3
C
–
..
..
....
Note enolate formation
new
enolate
formation
enolate
formation
again
O
H
3
C
–
..
..
O
..
..
..
..
O
..
..
O
..
..
OH
..
..
O
..
HO
(52%) (85%)
..
O
..
..
O
..
..
..
HO
....
CH
3
CH
3
CH
3
..
..
O
CH
3
..
..
..
O
..
..
O
H
3
C
..
..
O
..
..
O
H
2
C
..
..
O
–
..
..
WEB 3D
FIGURE 19.113 The Robinson annulation creates a six-membered ring.
19.11 Condensation Reactions in Combination
Alas, neither Nature nor a typical chemistry teacher shows much mercy, and one is
more likely than not to find condensation reactions in combination. None of these
reactions is particularly difficult to understand when encountered by itself.
Similarly, the synthetic consequences of the reactions are relatively easy to grasp when
they are encountered one at a time, but are by no means simple when combinations
of these reactions are involved. Condensation problems, occasionally called “Fun in
Base” problems (a term that seems to encompass reactions run in acid as well as
base—there are no “Fun in Acid” problems), can be very hard. The best way to
become proficient is to work lots of them, but there are also some practical hints
that may help.
One common sequence is a combination of the Michael addition and aldol
condensations.Indeed, there is a synthetic procedure for constructing six-membered
rings, invented by Sir Robert Robinson (1886–1975) and called the Robinson
annulation, that uses exactly that combination. In this reaction a ketone is treated
with methyl vinyl ketone in base (Fig. 19.113). A Michael addition ensues and
