Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

19.6 Addition of Carbonyl Compounds to the Position: The Aldol Condensation 969
␣
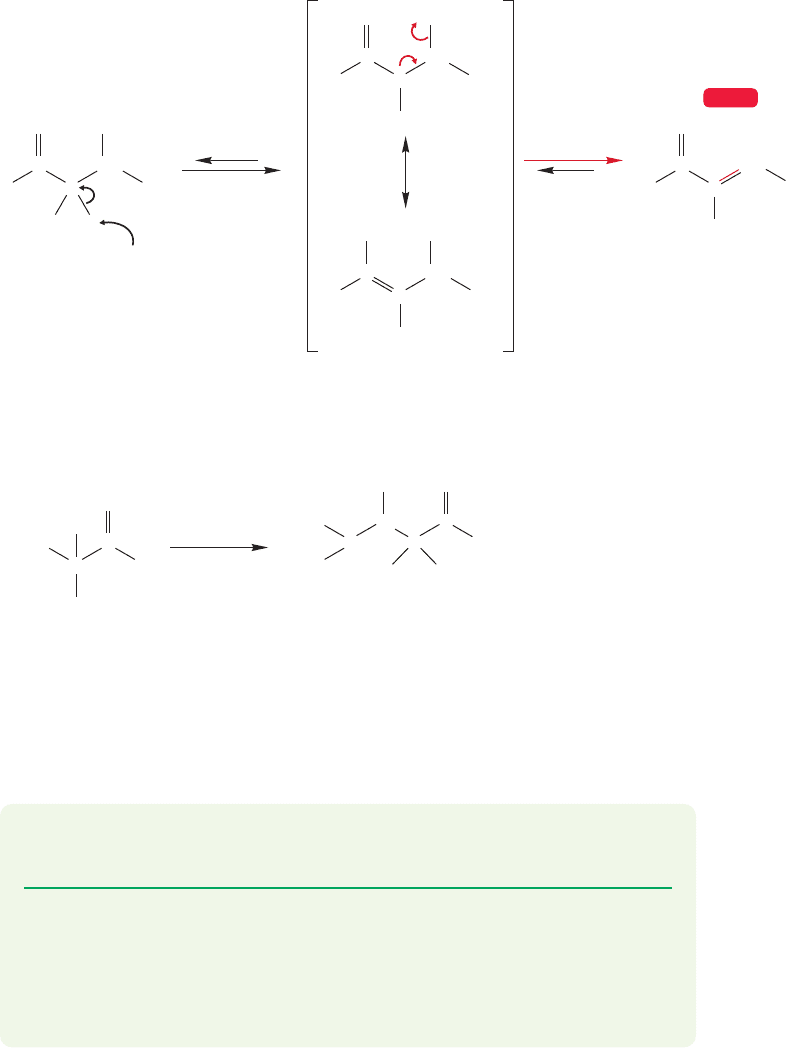
But the mechanism is not a simple E2 reaction (Fig.19.70). Removal of the α hydro-
gen is relatively easy, as this process gives a resonance-stabilized enolate anion. In a
second step, hydroxide is lost and the α,β-unsaturated compound is formed. This
kind of elimination mechanism, called the E1cB reaction, was the subject of discus-
sion in Chapter 7 (p. 309).
PROBLEM 19.21 What are the products of dehydration for the reactions of
Problem 19.20?
PROBLEM 19.22 Figure 19.69 shows protonation of the hydroxyl oxygen, not the
carbonyl oxygen. That picture is correct as the pK
a
of a protonated alcohol is
about 2, and the pK
a
of a protonated aldehyde is about 10. First explain the
relevance of the pK
a
data, and then explain why the hydroxyl oxygen is protonat-
ed in preference to the aldehyde. Hint: Think hybridization.
..
H
2
O
NaOH
O
HC
H
H
3
C
H
3
C
C
(85%)
(no α hydrogens)
OH
..
..
H
3
C
H
3
C
CH
3
H
3
C
CH
HC
C
CH
O
..
..
–
..
H
2
O
O
..
OH
..
..
..
OH
..
–
..
..
HO
..
..
–
–
–
..
..
OH
..
..
C
HH
CH
3
H
CCH
O
..
..
C
H
CH
3
+
H
CCH
..
..
O
..
OH
..
..
C
H
CH
3
H
CCH
.. ..
O
..
OH
..
..
C
H
Resonance-stabilized enolate
CH
3
H
CCH
WEB 3D
FIGURE 19.70 The base-catalyzed elimination of water from aldol.The reaction mechanism is E1cB.
If no α hydrogen is available for elimination, then the initial aldol product can be isolated as shown in
the specific example.
Like many reactions, the aldol condensation is a series of equilibria and it is
not always clear what is the slow step, the rate-determining step. For example,
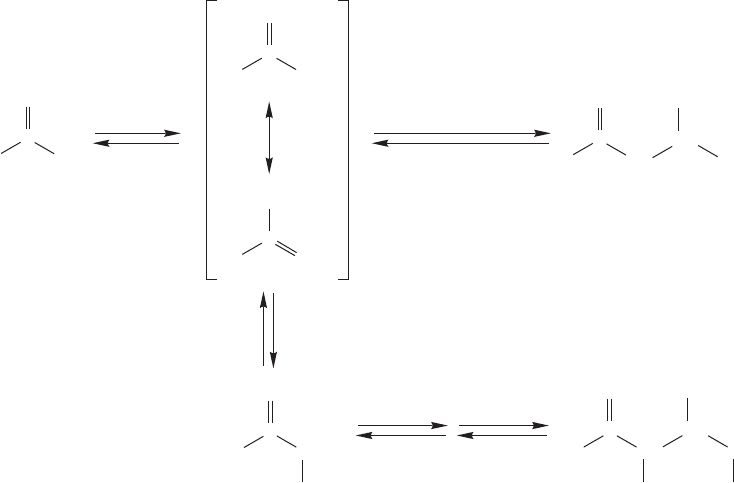
970 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
consider the reaction of acetaldehyde in D
2
O/DO
(Fig. 19.71). In the aldol
formed, deuterium incorporation at carbon is a function of acetaldehyde con-
centration. At high acetaldehyde concentration the aldol product initially formed
is not deuterated on any carbon, but at low acetaldehyde concentration it is deuter-
ated at two positions. The reason for the concentration dependence is that the
rates of the first two steps of the reaction are similar. Once the enolate is formed
it can either revert to acetaldehyde, which will result in exchange in D
2
O, or
go on to the aldol product in a bimolecular reaction with a molecule of acetalde-
hyde. At high acetaldehyde concentrations the enolate is more likely to form
the aldol.
DO
aldol condensation
aldol condensation
..
..
..
–
–
–
D
2
O
..
..
..
..
O
..
..
CH
3
H
C
O
..
..
CH
2
H
C
O
..
..
CH
2
H
C
O
..
..
CH
2
H
C
D
(D) (D)
high aldehyde
concentration
low aldehyde
concentration
..
O
..
OD
..
..
CH
2
Aldol
(not deuterated on carbon)
CH
3
H
C
CH
..
O
..
OD
..
..
CH
Carbon-deuterated
aldol, deuterated at one
or both positions shown
CH
2
H
C
CH
D
2
O
..
..
FIGURE 19.71 A high concentration of acetaldehyde results in a relatively rapid aldol reaction and little
or no exchange at carbon. If, however, the concentration of acetaldehyde is low, the bimolecular reaction
will be slowed and the enolate can revert to acetaldehyde. In D
2
O, this reaction leads to exchange of
hydrogens attached to carbon.The deuterium can appear in either or both of the two positions and is
shown as (D).
If the concentration of acetaldehyde is high, the rate of the bimolecular reaction
is faster (rate k[enolate][acetaldehyde]), and acetaldehyde is not re-formed from
the enolate.Under these conditions, it is the formation of the enolate that is the slow
step in the reaction, the rate-determining step (p. 350). If the concentration of
acetaldehyde is low, the enolate is reconverted to acetaldehyde, and in D
2
O this rever-
sal results in exchange. When exchanged acetaldehyde goes on to form aldol, the
product also contains deuterium attached to carbon. At low acetaldehyde concen-
trations it is the addition step itself that is the slower one, and therefore the rate-
determining step.
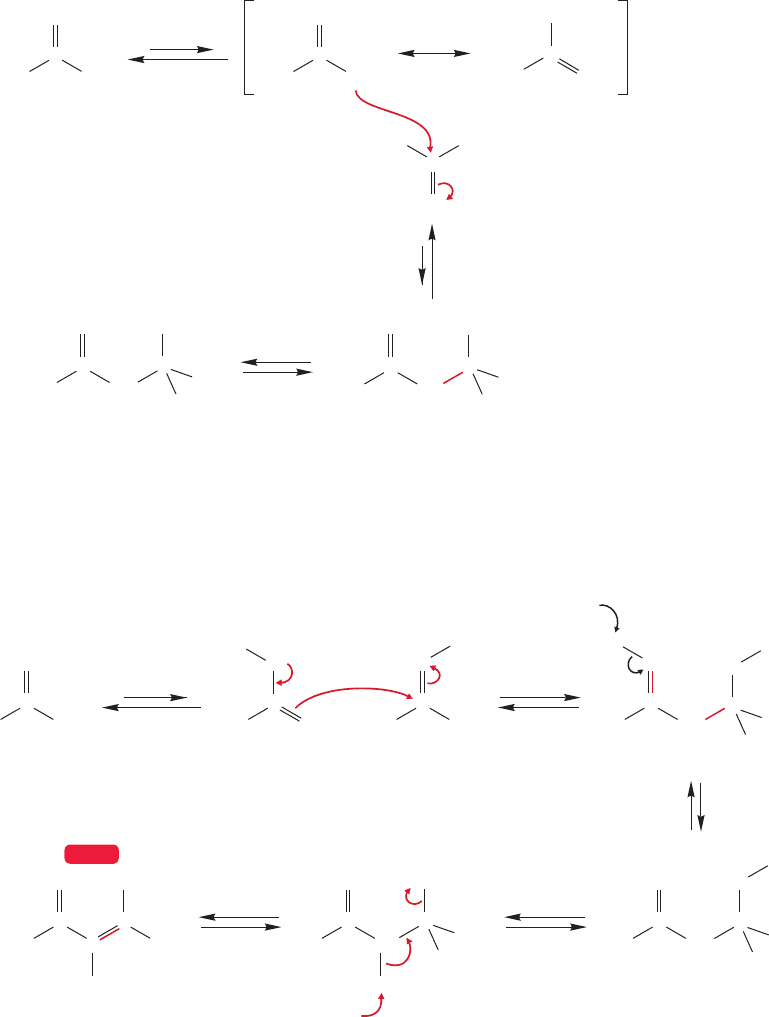
19.6 Addition of Carbonyl Compounds to the Position: The Aldol Condensation 971
␣
Ketones can undergo the aldol reaction too, and the mechanism is similar to that for
the aldol condensation of aldehydes. We will use acetone as an example (Fig. 19.72).
In base, the enolate is formed first, and adds to the electrophilic carbonyl compound.
Protonation by water yields a molecule once known as diacetone alcohol, 4-hydroxy-
4-methyl-2-pentanone.
–
OH
..
..
..
..
..
..
H
2
O
H
2
O
–
–
–
..
..
CH
3
H
3
C
C
O
..
..
O
..
..
O
..
..
O
..
..
O
..
CH
2
H
3
C
H
3
C
C
O
..
..
C
O
..
..
CH
2
CH
3
H
3
C
C
..
..
..
–
HO +
..
CH
2
H
3
C
C
C
..
..
4-Hydroxy-4-methyl-2-pentanone
(diacetone alcohol)
CH
3
CH
3
CH
3
CH
3
H
3
C
C
C
CH
2
OH
FIGURE 19.72 The base-catalyzed
aldol reaction of acetone.
OH
2
..
H
3
O
..
aldol
condensation
+
H
3
O
..
+
H
3
O
..
+
H
3
O
..
+
+
+
+
..
..
H
2
O
..
..
H
2
O
CH
3
H
3
C
C
..
O
CH
3
H
3
C
C
O
H
H
CH
2
H
3
C
C
Enol
dehydration
deprotonation
Protonated
carbonyl
..
O
O
..
..
CH
2
H
3
C
C
C
H
H
..
O
..
O
..
..
CH
2
H
3
C
C
C
H
O
..
..
..
CH
H
H
3
C
C
C
CH
3
O
..
..
C
4-Methyl-3-penten-2-one
CH
3
H
H
3
C+
CC
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
..
..
O
..
(
mesit
y
l oxide
)
WEB 3D
FIGURE 19.73 The acid-catalyzed
aldol condensation of acetone.The
first product, diacetone alcohol, is
generally dehydrated in acid to give
4-methyl-3-penten-2-one.
In the acid-catalyzed condensation of acetone, the enol is the intermediate, and it
adds to the very strong Lewis acid, the protonated carbonyl group, to give initially the
same product as the base-catalyzed reaction,diacetone alcohol (Fig.19.73).Dehydration
generally follows to give the α,β-unsaturated ketone,4-methyl-3-penten-2-one (mesityl
oxide).
We have been at pains to point out the similarities between these aldol reactions
and other addition reactions of carbonyl compounds. Like simple additions, such
as hydration, the aldol condensations are series of equilibria. They are affected by
structural factors in the same way as the simple reactions. Product formation is
unfavorable in base-catalyzed aldol reactions of ketones, just as it is in hydration
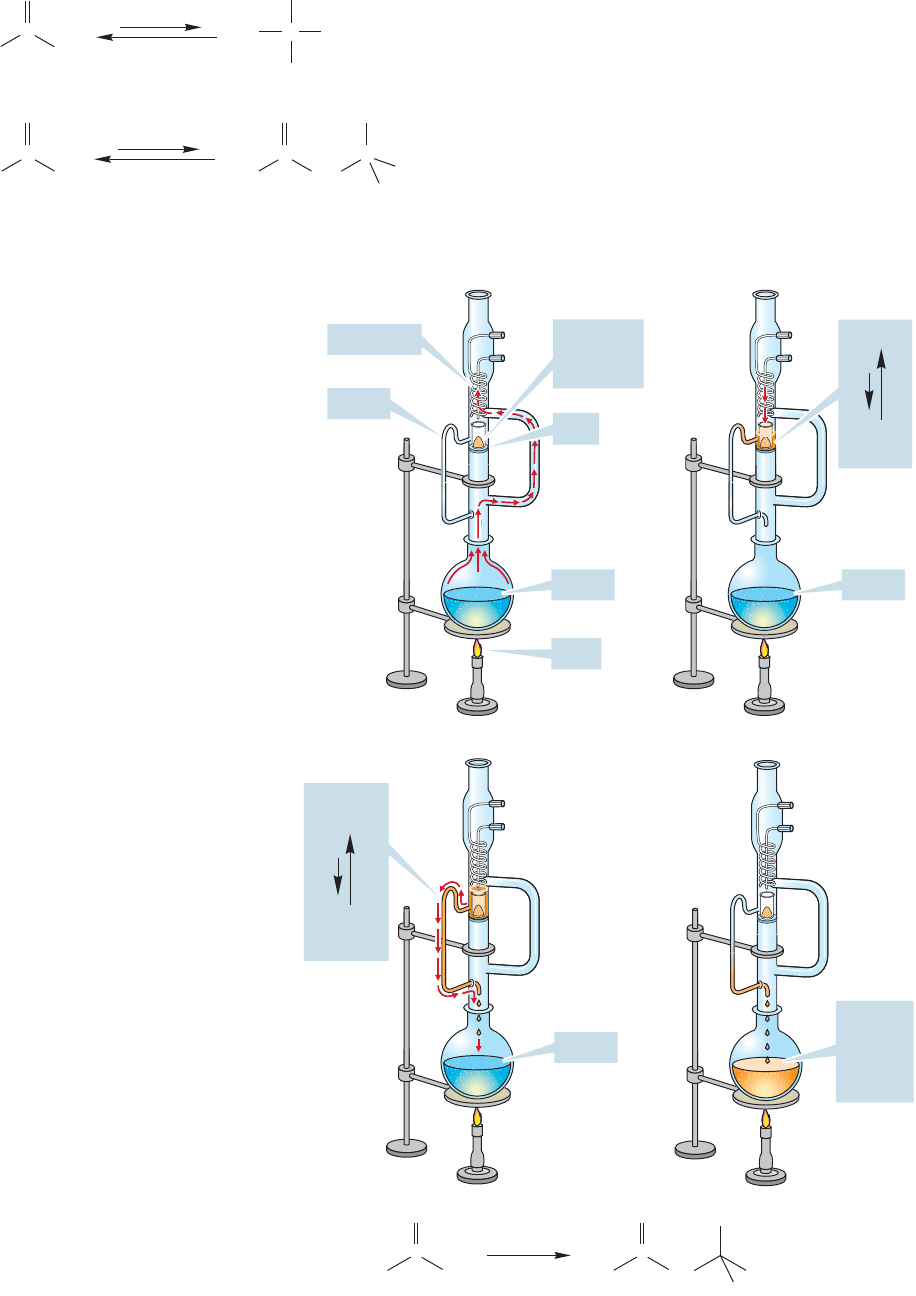
972 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
CH
3
CH
3
C
O
Ba(OH)
2
(71%)
OH
H
3
CCH
2
C
O
H
3
CCH
3
Stage 3
Siphoning
ketone
Aldol
product
Stage 4
Ketone
Ketone
+
aldol
product
Ketone
Aldol
product
Stage 1
Stage 2
Condenser
Siphon
Ketone Ketone
Heat
Ba(OH)
2
(insoluble
catalyst)
Seal
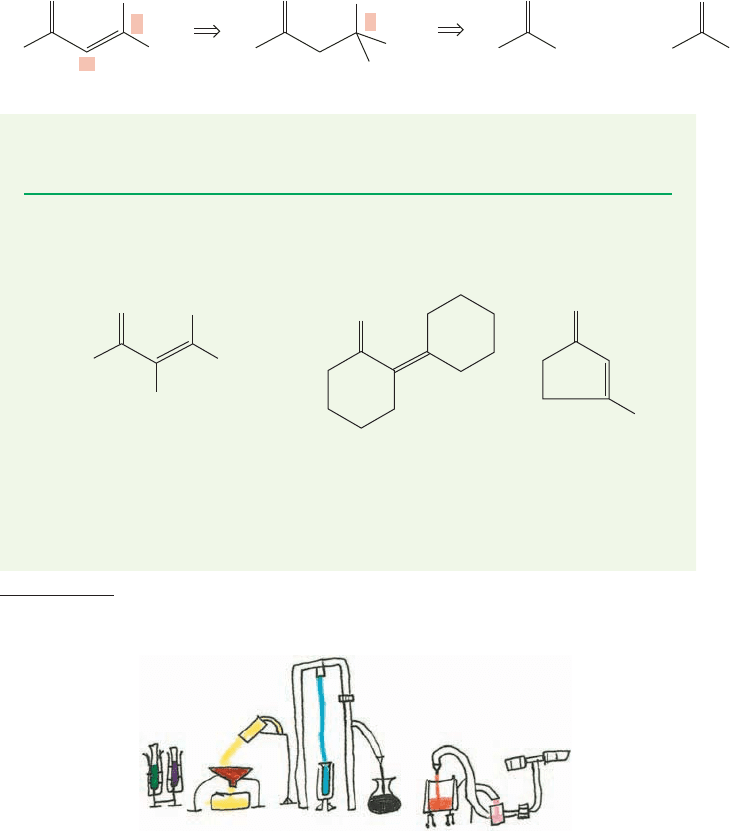
FIGURE 19.75 The operation of a
Soxhlet extractor.
(Fig.19.74).In acid,dehydration generally occurs and formation
of the α,β-unsaturated ketone pulls the equilibrium to the right.
How can the aldol condensation of ketones be useful? If
equilibrium favors starting material, how can the product be
isolated? Ingenious ways have been found to thwart the dic-
tates of thermodynamics. In one, a spectacularly clever piece
of apparatus called a Soxhlet extractor is used. The ketone
is boiled in a flask separated from the catalyst, usually bari-
um hydroxide, Ba(OH)
2
, which is insoluble in organic ma-
terials. The ketone distills to the condenser, and drips from
it to come in contact with the catalyst (stage 1, Fig. 19.75).
–
H
2
O
/H
2
O
CH
3
H
3
C
H
3
CCC
OH
OH
OH
CH
3
HO
CH
3
H
3
C
C
CH
2
H
3
C
C
C
CH
3
CH
3
..
..
..
....
..
O
..
..
O
..
..
..
..
..
..
..
..
..
O
..
..
2
FIGURE 19.74 In the hydration of ketones, the
hydrate is usually not favored at equilibrium.
Similarly, in the aldol reaction of ketones the
initial product molecule is not usually favored,
but the dehydrated product is.
19.6 Addition of Carbonyl Compounds to the Position: The Aldol Condensation 973
␣
Now the aldol condensation takes place to give an equilibrium mixture of start-
ing ketone (favored) and the product of aldol condensation (unfavored) (stage 2).
As the starting ketone continues to boil, and the level of the liquid in the trap
rises, the whole solution eventually is siphoned from the chamber into the flask
that previously contained only ketone (stage 3). There is still no catalyst in the
flask, and so the aldol product cannot revert to starting material. The mixture in
the flask continues to boil, the remaining ketone, which has a much lower boil-
ing point than the product, continues to come in contact with the catalyst in the
isolated chamber, and slowly the concentration of product in the flask increases
to a useful amount (stage 4).
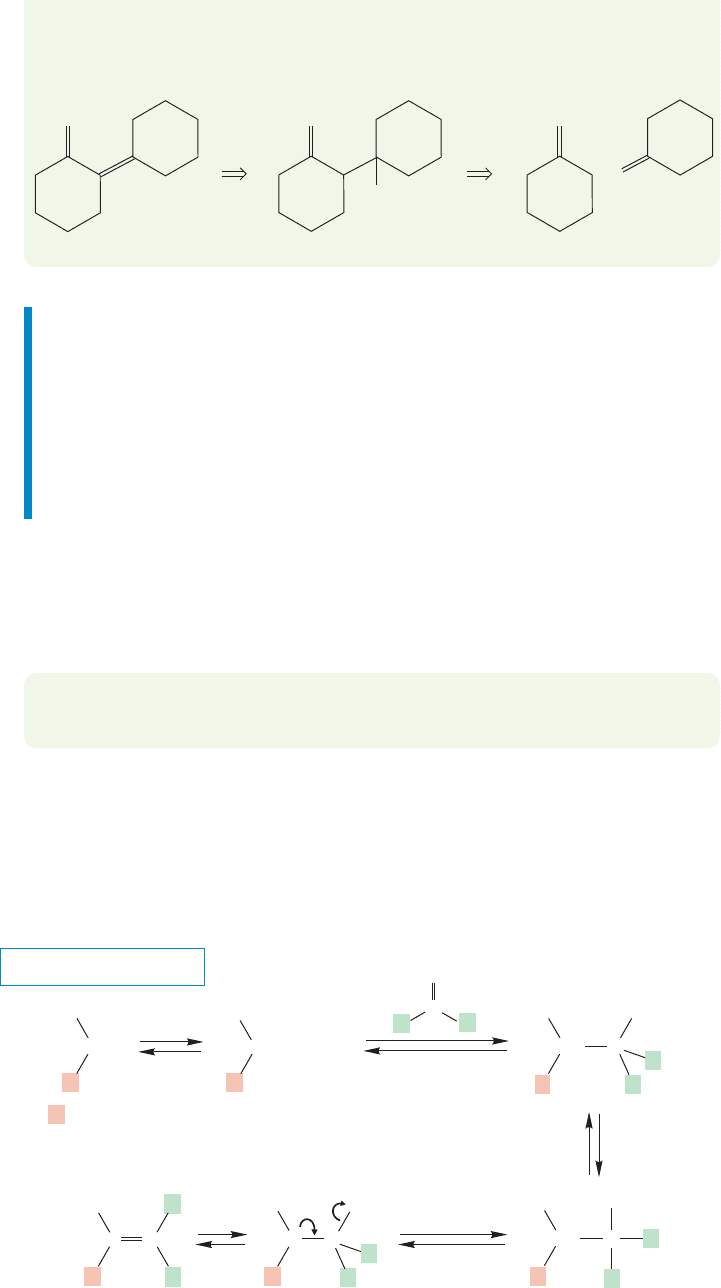
3
All β-hydroxy carbonyl compounds are potential products of an aldol reac-
tion. Whenever you see one, your thoughts about synthesis must turn first to the
aldol reaction.The same is true for the dehydration products, the α,β-unsaturat-
ed carbonyl compounds. It is important to be able to go quickly to the new bond,
to find the one formed during the aldol condensation, and to be able to dissect
the molecule into its two halves. In doing this operation, you are merely
carrying out a retrosynthetic analysis and following the mechanism backward
(Fig. 19.76).
CH
3
R
R
α
β
CH
3
CH
3
H
3
C
R
R
OH
R
+
β
R
..
..
O
..
..
O
..
..
O
..
..
O
..
..
FIGURE 19.76 A retrosynthetic
analysis for the product of an
aldol condensation. Note the
retrosynthesis arrows that mean
“can be formed from.”
PROBLEM 19.23 Write mechanisms for the acid- and base-catalyzed reverse aldol
reaction of diacetone alcohol shown in Figure 19.72.
WORKED PROBLEM 19.24 Perform retrosynthetic analyses on the following three
molecules:
3
One would be wrong to think that the day of clever apparatus has passed. See, for example, Charlie Biggs’
(age 5) creation here. It’s not totally clear exactly what it’s for, but there must be a good use for it.
(a)
*(b)
(c)
CH
2
CH
3
CH
3
CH
2
CH
3
O
O
O
CH
3
CH
2
CH
3
ANSWER (b) In any aldol, or aldol-like condensation, the carbon–carbon π bond
is formed through an elimination reaction (Fig. 19.73). So, the first part of our
retrosynthetic analysis recognizes that the precursor to the final product is
β-hydroxy ketone A.
(continued)
974 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
Now, where does A come from? All β-hydroxy carbonyl compounds are aldol
products, so the σ bond joining the two rings must come from the following aldol
reaction:
Summary
Base- and acid-catalyzed aldol reactions of aldehydes generally lead to useful
amounts of aldol product, the β-keto alcohol, or the product of dehydration, the
α,β-unsaturated carbonyl compound. Aldol reactions of ketones are less useful
because equilibrium does not usually favor product. Special techniques can be
used (Soxhlet extractor) to thwart thermodynamics. If dehydration to the
α,β-unsaturated carbonyl compound occurs, as it often does in acid-catalyzed
aldols, the reaction may be practically successful.
19.6b Ester Enolates React with Ketones or Aldehydes Any com-
pound that can support an anion through resonance stabilization can attack a
ketone or aldehyde. As we have seen, malonate esters and similar compounds read-
ily form resonance-stabilized anions when treated with bases.
PROBLEM 19.25 Draw resonance forms for the anions of each compound in
Table 19.3 (p. 958).
Bases, often amines, can generate significant amounts of enolates from the
β-dicarbonyl compounds in Table 19.3, and similar molecules. In the presence of
an acceptor ketone or aldehyde, condensation occurs to give, ultimately, the α,β-
unsaturated diester shown in Figure 19.77.This reaction is called the Knoevenagel
condensation after Emil Knoevenagel (1865–1921).
THE GENERAL CASE
R
–
–
–
–
B
..
–
–
B
..
–
B
..
BH
CH
2
(R, R are anion-
stabilizing groups)
R
R
CH
C
O
O
+ BH
R
R
R
R
R
CH C
R
R
OH
R
R
CH C +
OH
(dehydration often occurs)
+
R
R
OH
R
R
R
R
CC
R
R
CC
R
R
FIGURE 19.77 The Knoevenagel
condensation.
OH
A
O O O
O
19.6 Addition of Carbonyl Compounds to the Position: The Aldol Condensation 975
␣
SPECIFIC EXAMPLES
100 ⬚C, 1 min
25 ⬚C, 24 h
benzene, pyrrolidine
C
O
O
C
C
H
H
O
CH
3
CH
2
O
CH
3
CH
2
O
(CH
3
)
2
N
C
O
H
(CH
3
)
2
N
C
CHNO
2
CH
2
C
O
O
C
CH
3
CH
2
O
CH
3
CH
2
O
C
(89%)
(83%)
C
H
+ CH
3
NO
2
pentanamine
WEB 3D
FIGURE 19.77 (continued)
Many other reactions, some with names, many without, are conceptually simi-
lar, in that they involve the addition of a complex anion to a ketone or aldehyde.
But, in these other reactions the source of the nucleophilic anionic partner is not
always an enolate. For example, cyclopentadiene (pK
a
15) is a strong enough acid
to give an anion when treated with a base, and the cyclopentadienide ion (p. 589)
can add to ketones.The end result is a synthesis of the beautifully colored compounds
known as fulvenes (Fig. 19.78).
WEB 3D
–
–
–
–
..
..
..
..
..
..
OCH
2
CH
3
..
..
..
HOCH
2
CH
3
H
H
..
..
O
..
..
O
..
..
OH
CH
2
CH
3
..
..
OHCH
2
CH
3
..
..
O
–
..
..
..
OH
CH
2
CH
3
+
H
H
H
H
C
+
+
CH
3
6,6-Dimethylfulvene
(75%)
CH
3
–
..
C
H
3
C CH
3
CH
3
CH
3
C
O
..
..
..
O
H
C
CH
3
CH
3
CH
3
CH
3
FIGURE 19.78 A condensation reaction leading to fulvenes.
With Knoevenagel reactions, your analytical task is more complicated than with
aldols. It is quite easy to learn to dissect β-hydroxy ketones or α,β-unsaturated
ketones into their components, but how is one to deal with the less obvious com-
pounds like fulvenes? There is no easy answer to this question. A carbon–carbon
double bond is always the potential result of a condensation reaction. You must learn
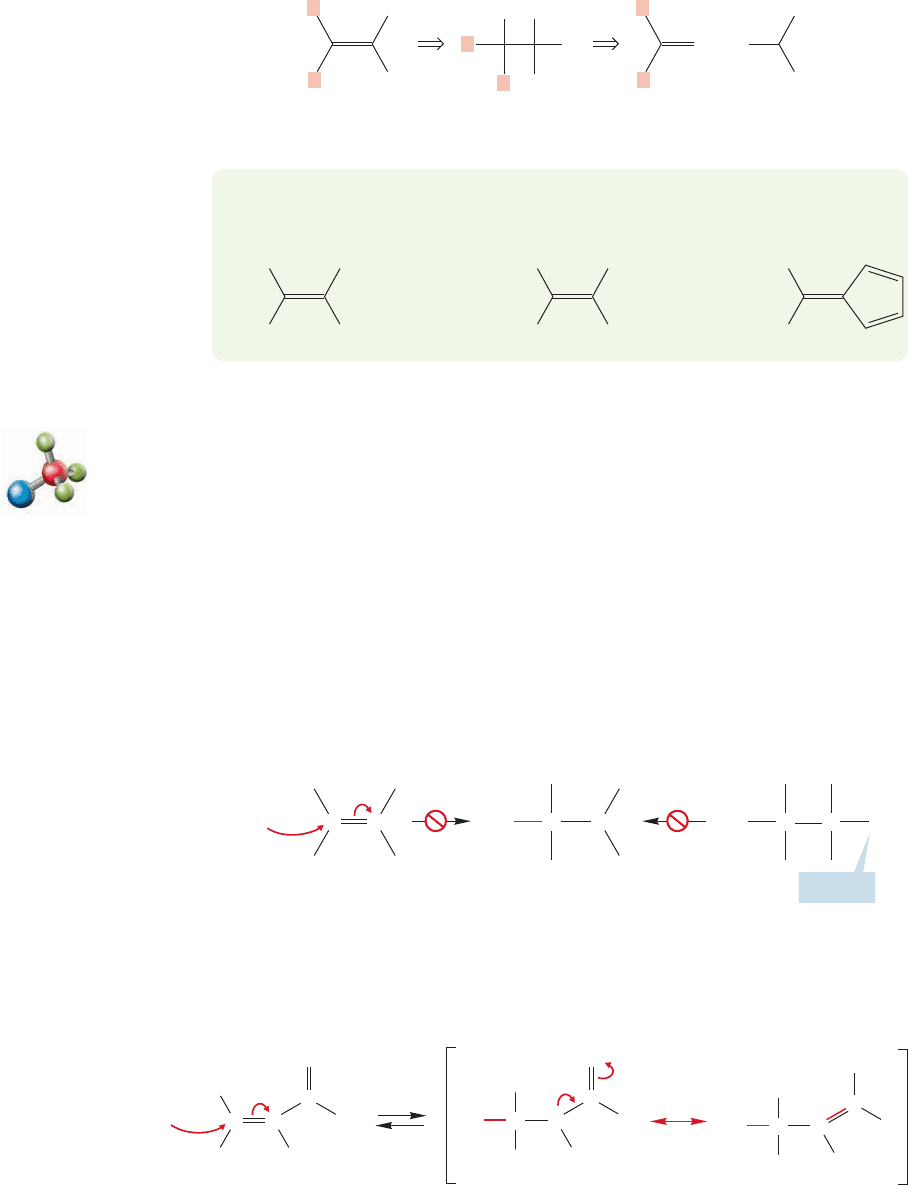
FIGURE 19.79 Any carbon–carbon
double bond is the formal result of a
condensation reaction.
976 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
(a)
Ph
Ph
CN
COOCH
2
CH
3
(b)
(CH
3
)
3
C
H
COOCH
2
CH
3
COOCH
2
CH
3
(c)
Ph
H
to look on them that way, and to evaluate the pieces thus created as parts of a pos-
sible synthesis (Fig. 19.79).
The following problem gives some practice at devising syntheses of molecules
formed by condensation reactions.
pK
a
60
–
–
CC C
An unstabilized anion
Nu C
..
..
C C HNu
Nu
FIGURE 19.80 Addition of a base
such as alkoxide to a simple alkene
would yield an unstabilized anion.
A measure of the difficulty of this
reaction can be gained by examining
the acidity of the related hydrocarbon.
Such species are extraordinarily weak
acids and their pK
a
values are very
difficult to determine.
19.6c Ester Enolates Add to Enones: The Michael Addition We now
have a synthetic route to α,β-unsaturated carbonyl compounds, the aldol (and related)
condensation reactions. There is an especially important process associated with
these compounds, called the Michael reaction, after its discoverer, the American
chemist Arthur Michael (1853–1942).
Ordinary carbon–carbon double bonds are unreactive toward nucleophiles. In
particular, addition is not observed, because it would require formation of an unsta-
bilized anion, the conjugate base of an alkane.Alkanes are extraordinarily weak acids.
Their pK
a
values are difficult to measure, but numbers as high as 70 have been
suggested (Fig.19.80). When working on problems in organic chemistry, an unsta-
bilized carbanion is the functional equivalent of a methyl or primary carbocation.
It is a stop sign, a signal that you are almost certainly wrong!
PROBLEM 19.26 Propose syntheses for the following molecules:
By contrast, many nucleophiles add easily to the double bonds of α,β-unsaturated
carbonyl compounds,also known as enones.This process is called the Michael reac-
tion. Notice the resonance stabilization of the resulting enolate anion (Fig. 19.81).
–
–
CC
..
..
..
Nu
C
R
O
R
R R
CNu C
..
..
..
C
R
O
R
R
R
–
CNu C
An enolate
(resonance stabilized)
..
..
..
C
R
O
R
R
R
An
α,β-unsaturated
carbonyl compound
FIGURE 19.81 Nucleopilic additions
to α,β-unsaturated carbonyl
compounds are common.
R
R
R
R
R
R
R
RHO
H
R
R
–
..
R
O
H
R must be an
anion-stabilizing group
R
Michael addition
19.6 Addition of Carbonyl Compounds to the Position: The Aldol Condensation 977
␣
The reason is the initial formation, not of a bare, unstabilized carbanion as was the
case in Figure 19.80, but of a nicely resonance-stabilized species.The carbonyl group
stabilizes any α anion, no matter how it is formed. The Michael addition is another
way of generating an enolate.
When the species adding to the α,β-unsaturated compound is itself an enolate,
the reaction is properly called the Michael reaction, but related additions are often
also lumped under this heading (Fig. 19.82).
–
–
..
..
..
..
..
..
..
..
..
C
O
O
C
RO
RO
HC
CC
..
..
C
R
O
H
H R
..
..
..
..
..
..
..
..
..
C
O
O
C
RO
RO
CH
CC
..
..
C
R
O
H
H
A real Michael reaction
Enolate
R
–
–
(–)
..
..
..
NC
A
nucleophile
CC
..
..
C
R
O
H
H R
..
NC
CC
..
..
C
R
O
H
H
Still usually called a Michael reaction
R
(–)
FIGURE 19.82 Two Michael
reactions.
THE GENERAL CASE
A SPECIFIC EXAMPLE
–
..
+
Enol
R
X
R
O
HX
X
R R
..
..
O
..
..
O
Br
HBr
..
O
R R
H
+
..
..
O
R R
H
..
..
O
H
Ketone
R
X
R
..
..
O
..
..
FIGURE 19.83 An acid-catalyzed
Michael reaction.
There is also an acid-catalyzed version of the Michael reaction. In this reaction
the carbonyl group is initially protonated, and the carbon–carbon double bond is then
attacked by a nucleophile to give the enol (Fig. 19.83). The enol equilibrates with
the more stable carbonyl compound to produce the final product of addition.
Ph
Ph
Ph
HBF
O
O
Ph
Ph
Ph
O
O
4
+
..
..
..
..
..
..
..
..
α,β-Unsaturated compounds present a general problem in predicting reaction
products.There are just too many reactions possible. How, for example, can we decide
whether a nucleophile will add to the carbonyl group (1,2-addition) or to the dou-
ble bond (Michael)? This question captures in microcosm the difficulties presented
by condensation problems in particular, and by the chemistry of polyfunctional com-
pounds in general. How is it possible to decide which of a variety of reactions is the
best one (either in energy terms or in the more practical sense of finding the route
to the end of an exam problem or making a desired compound)?
At least the specific question above about the Michael reaction has a rational
answer. First, notice that the product of Michael addition is likely to be more sta-
ble than the product of addition to the carbonyl group (Fig. 19.84). The product of
Michael addition retains the strong carbon–oxygen double bond, and the simple
addition product does not.
978 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
PROBLEM 19.27 Write a mechanism for the following reaction:
FIGURE 19.84 The two possible reactions of a nucleophile (Nu
) with an α,β-unsaturated
carbonyl compound. Michael addition preserves the carbonyl group and is usually favored
thermodynamically.
..
Strong C
O
double bond
remains
addition
to C
O
Nu
..
–
Nu
..
–
Nu
..
–
..
..
O
or
R
Nu
NuH
..
..
..
O
R
Nu
..
..
O
H
R
Michael
Nu
..
–
–
–
–
..
..
O
R
NuH
..
..
O
R
..
..
..
O
R
..
..
O
R
Nu
Nu
Nu
+
+
But this is a thermodynamic argument, and as we have often seen, the site of
reactivity is many times determined by kinetics, not the thermodynamics of the
overall reaction. However, most nucleophiles add reversibly to the carbonyl group,
and this preference holds for ,-unsaturated carbonyls as well. So even if addi-
tion to the carbonyl group occurs first, for most nucleophiles the thermodynamic
