Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


19.11 Condensation Reactions in Combination 999
PROBLEM SOLVING
Complex mechanistic problems absolutely require analysis. A good problem
solver always begins by analyzing the problem before writing any arrows. We
suggest first building a map. Ask yourself what atoms in the starting material
become what atoms in the product. You won’t always be able to identify the
fate of every atom, but you should usually be able to see what happens to some
of them. Use attached groups that are not changed in the reaction as markers.
Next, develop a set of goals. Your map should allow you to spot which bonds
in the product must be made and which bonds in the starting material must
be broken.
Finally, after building your map and establishing your goals, you can begin
to think about how to accomplish those goals. We’ll start you off on part (b) of
Problem 19.38, but please try the other problems this way. We guarantee that
you will get more answers right if you do. And a side benefit is that even if you
don’t get the problem completely right, the graders will “see” you thinking and
partial credit definitely flows to such answers.
4
Magid’s first rule is archaic, and we will shortly encounter Magid’s third rule.
PROBLEM 19.37 Although the mechanism shown in Figure 19.113 is the way the
Robinson annulation is always described, there is another (harder) way to write
the reaction. Write a mechanism for the general reaction in Figure 19.113 that
involves doing the aldol condensation first.
Problem 19.37 brings up a serious practical question. You may well be able
to analyze a problem by figuring out the different possible reactions, but choos-
ing among them is more difficult. How do you find the easiest route (there
are very often several) to the product you are after? Clearly, experience is invalu-
able and you are by definition short on that commodity. In such a situation,
it is wise to be receptive to good advice, and here is a practical hint from
one of the best problem solvers known, Ronald M. Magid (b. 1938), in
the form of Magid’s second rule:
4
When there is a choice, always try the
Michael first.
In fact, there are sound reasons for doing the Michael reaction first. Aldol con-
densation products of ketones are not generally favored at equilibrium, for exam-
ple, and doing the Michael reaction makes the subsequent aldol condensation
intramolecular, and thus more favorable.
There is no substitute for practice,though, and Problem 19.38 offers some good
opportunities to practice solving Fun in Base problems. Be sure to look at the
Problem Solving box first.
is followed by an intramolecular aldol condensation to give the new six-membered
ring (Fig. 19.113).
1000 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
C +
+
+
(a)
*(b)
(c)
(d)
(e)
O
Ph
Ph
COOEt
H
KOH
NaOEt
HOEt
NaOH
H
2
O
KOH
H
2
O
KOH
H
2
O
H
2
O
Ph Ph
O
BrCH
2
COEt
O
O
O
O
O
H
H
H
H
O
O
O
O
O
O
OH
O
O
OH
OH
O
–
O
Ph
(f)
KOH
H
2
O
O
O
Hint: There is a 10-membered ring involved.
O
O
H
3
C
CH
3
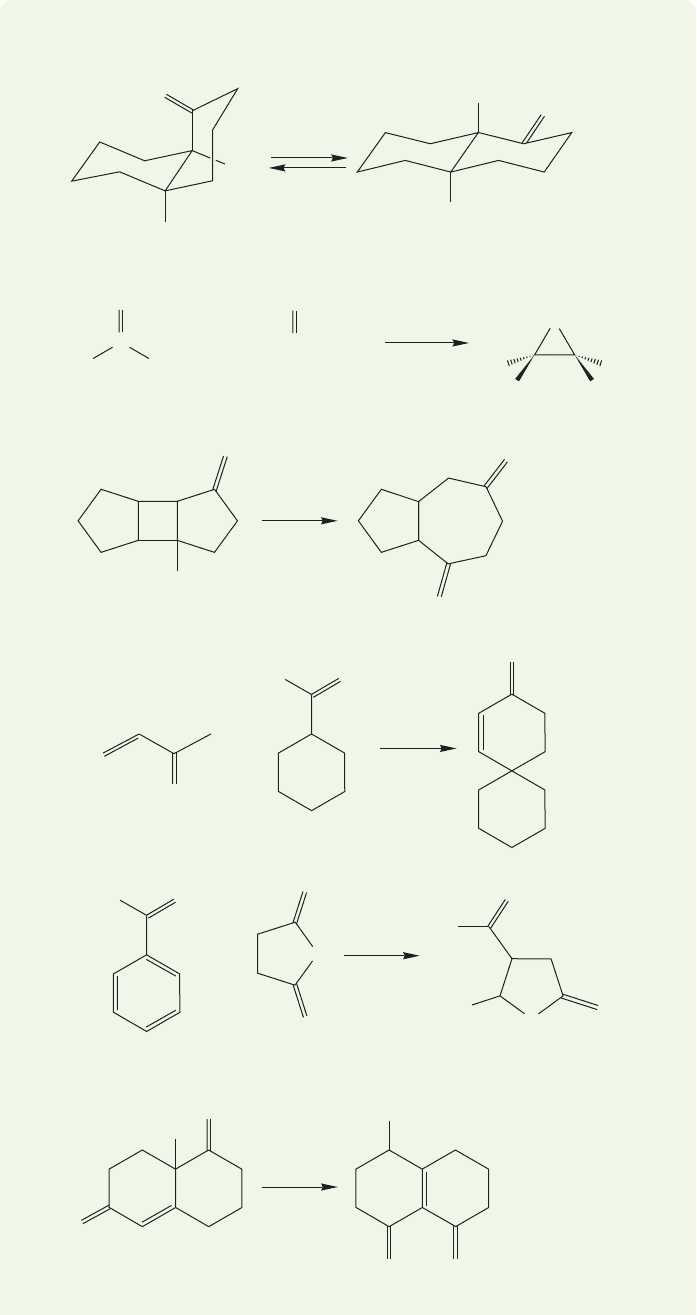
WORKED PROBLEM 19.38 Provide mechanisms for the reactions shown below.
(continued)
19.12 Special Topic: Alkylation of Dithianes 1001
O
Ph
Ph
Ph
Ph
COOEt
O
OEt
Br
O
make
break
??
ANSWER (b) Those two Ph groups are just along for the ride—nothing happens
to them in this reaction. Use them as markers to identify where the carbon
attached to them in the starting material winds up in the product (red dot).
Similarly, that ester group is preserved. Use it as a marker to find the carbon
attached to it in the starting materials (green dot).
Goals:
1. Our map allows us to see that we must make the red dot–green dot bond
(arrow, above), so that’s goal number one.
2. We also must make a bond from oxygen to either the red or green dot—but
we can’t tell which (yet).
3. The bond to bromine must be broken—we know that because there is no Br
in the product.
Okay, now you are on your own. Before looking at the Study Guide, find a way to
make that red–green bond.Once you do that, it will be easy to see how to lose the
Br and how to make that oxygen–dot bond.
C
RH
O
RH
HSCH
2
CH
2
CH
2
SH
H
3
O
+
..
..
S
..
..
S
..
..
FIGURE 19.114 Formation of a
dithiane (a thioacetal).
1,3-Dithiane
(1,3-dithiacyclohexane)
pK
a
= 31.1
–
..
S
..
..
S
HH
H
BuLi
Bu
= CH
2
CH
2
CH
2
CH
3
BuH
Li
+
THF
..
..
S
..
..
S
..
..
+
FIGURE 19.115 Deprotonation
of 1,3-dithiacyclohexane with
butyllithium.
PROBLEM 19.39 Explain how the sulfur atoms operate to increase the acidity of
the adjacent hydrogens in 1,3-dithiane (1,3-dithiacyclohexane).
19.12 Special Topic: Alkylation of Dithianes
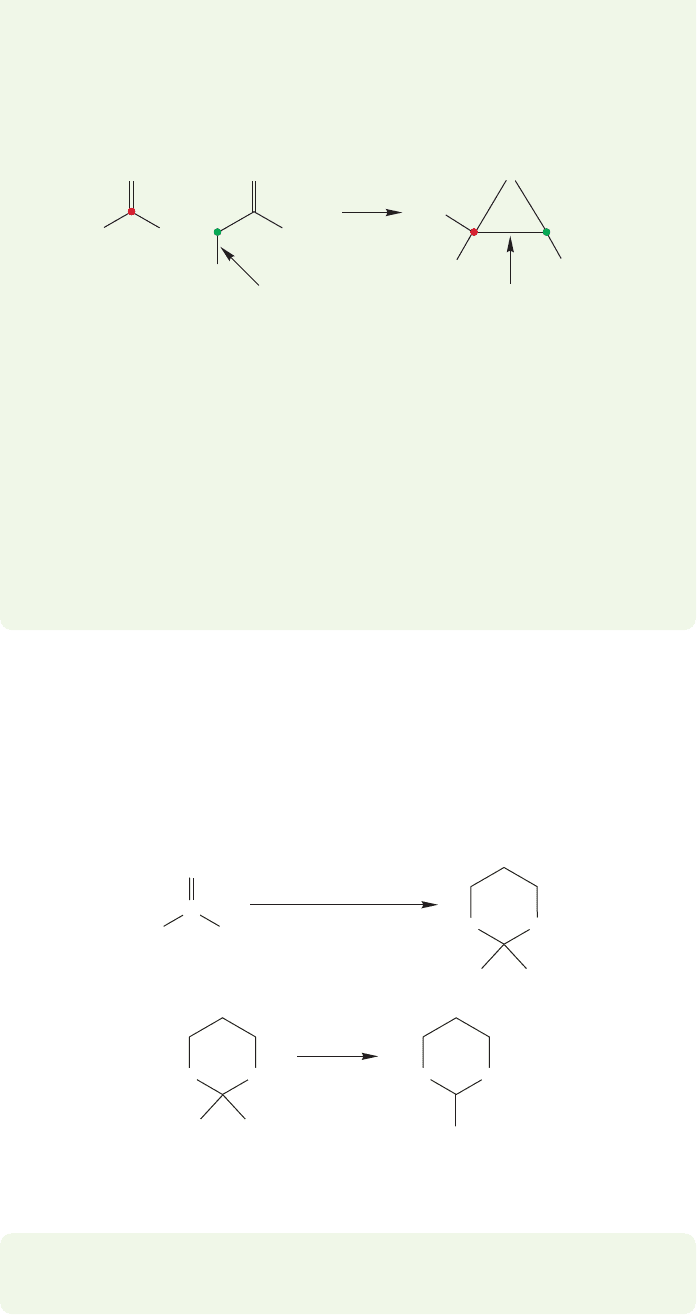
Here is a clever way to alkylate carbonyl compounds at the carbonyl carbon atom.
Thioacetals, also called dithianes, can be made from carbonyl compounds and the
sulfur counterparts of diols (Fig. 19.114). Dithianes are more acidic than typical
alkanes and can be deprotonated with alkyllithium reagents (Fig. 19.115).
1002 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
THE GENERAL CASE
SPECIFIC EXAMPLES
S
N
2
(Hg
2+
)
(Hg
2+
)
H
R
+
–
..
S
..
..
S
H
R
H
..
..
S
..
..
S
R
(84%)
..
..
S
..
..
S
H
I, THF
60 ⬚C
1. BuLi/THF
2.
..
..
R
¬
X
..
..
H
3
O
..
+
/H
2
O
O
..
..
R
O
..
..
SH
..
..
SH
..
..
+
..
..
H
3
O
..
+
+
/H
2
O
SH
..
..
SH
..
..
H
3
C
S
..
..
..
..
S
..
H
3
C
(70%)
S
..
..
S
HH
I, THF
1. BuLi
3. repeat steps
(1) and (2)
2.
..
..
..
H
3
O
Hg
2+
O
..
..
+
..
H
3
O
Hg
2+
S
..
..
S
..
1. BuLi
2. RX
R
R
O
..
..
S
..
..
S
..
..
FIGURE 19.116 A synthesis
of carbonyl compounds using
alkylation of 1,3-dithiane
(1,3-dithiacyclohexane).
–
BuLi repeat
Raney Ni
SS
H
H
H
R I
..
..
..
..
SS
H
R
..
..
..
..
SS
R
R
..
..
..
..
S
N
2
RCH
2
R
SS
..
..
..
..
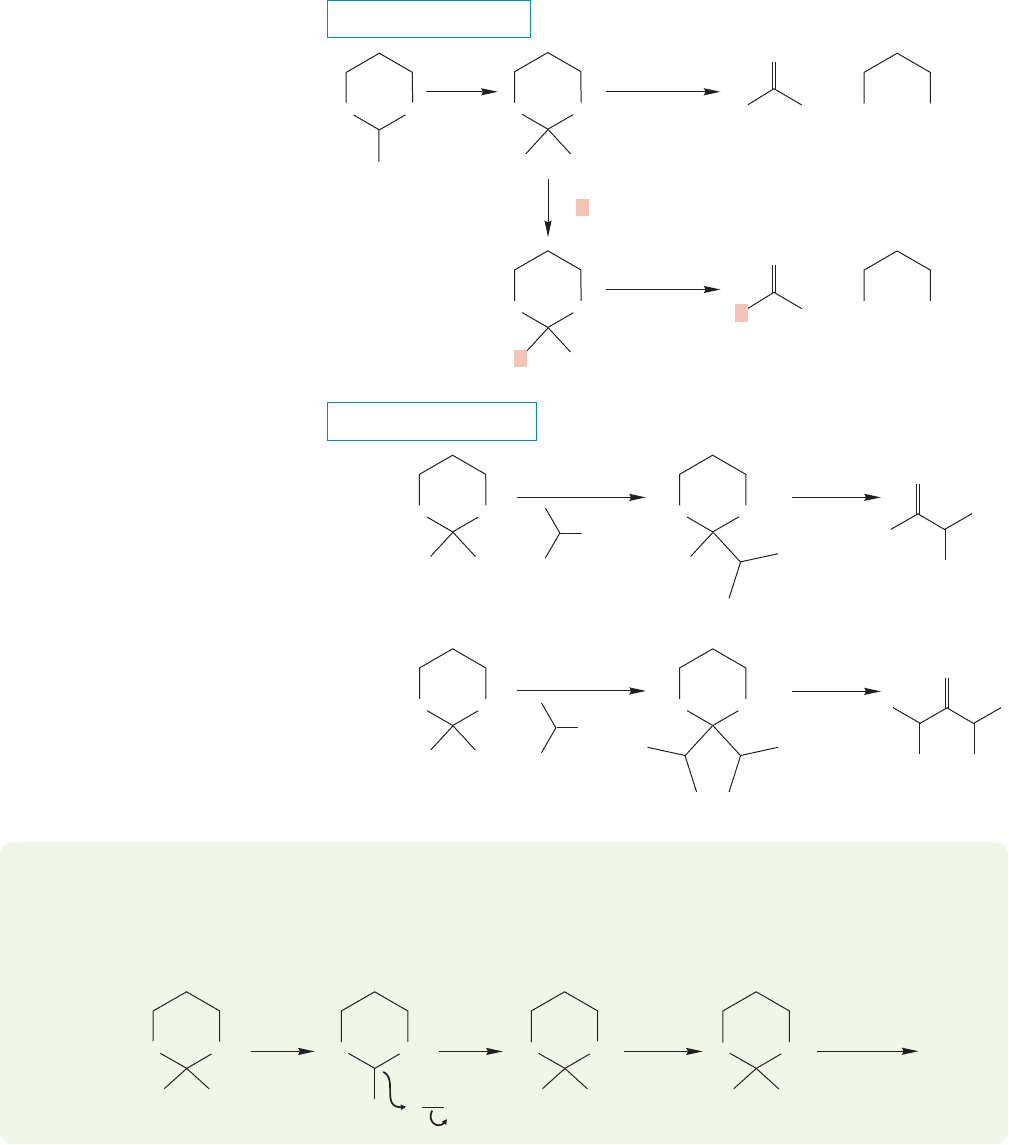
WORKED PROBLEM 19.40 Suggest a synthetic route to alkanes starting from
1,3-dithiane.
ANSWER Easy (and effective in practice). First alkylate the dithiane, then desul-
furize with Raney nickel (p. 253).
The resulting anion is a powerful nucleophile and can undergo both displace-
ment reactions (subject, of course, to the usual restrictions of the S
N
2 reaction) and
additions to carbonyl compounds (Fig. 19.116).The products of these reactions are
dithianes and can be hydrolyzed, usually by mercury salts, to aldehydes or ketones.
The dithiane functions as a masked carbonyl group! Here is another synthesis of
aldehydes and ketones.
19.13 Special Topic: Amines in Condensation Reactions, the Mannich Reaction 1003
19.13 Special Topic: Amines in Condensation
Reactions, the Mannich Reaction
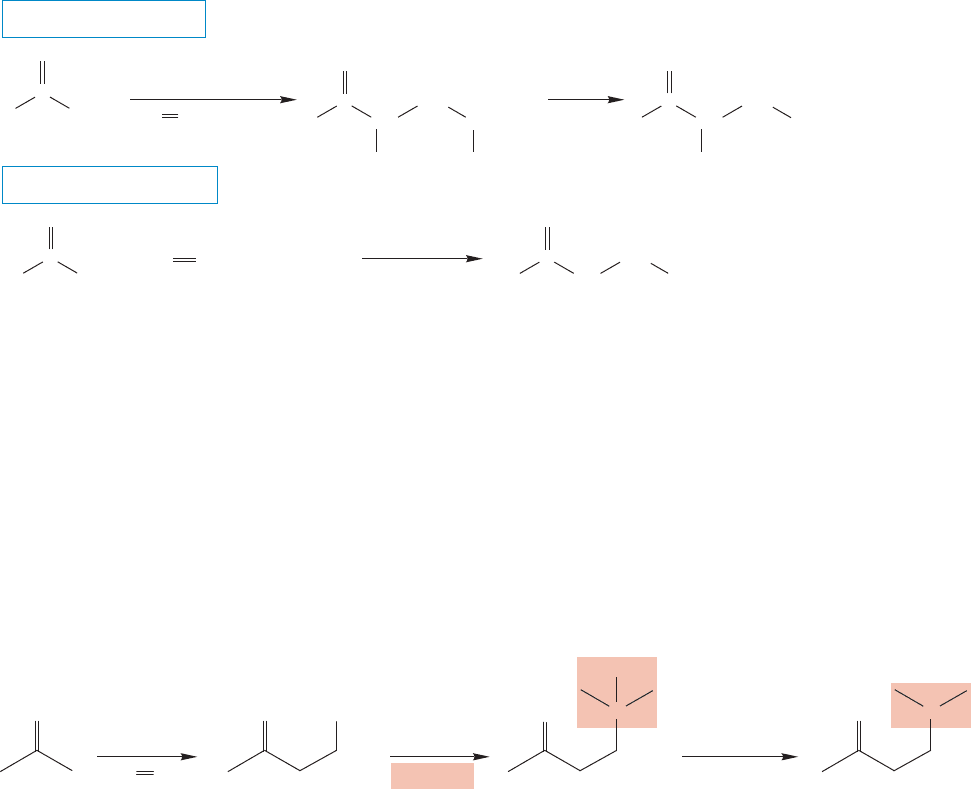
In the Mannich reaction, named for Carl Mannich (1877–1947), an aldehyde or
ketone is heated with an acid catalyst in the presence of formaldehyde and an amine.
The initially formed ammonium ion is then treated with base to liberate the final
condensation product, the free β-amino ketone (Fig. 19.117).
THE GENERAL CASE
A SPECIFIC EXAMPLE
CH
CH
2
N(CH
3
)
2
(70%)
H
2
C
/(CH
3
)
2
NH
HCl/EtOH
NaOH
H
2
O
C
H
R
CH
2
R
O
O
CH
2
(CH
3
)
2
NH
1. HCl/EtOH
80 ⬚C, 2 h
2. base
++
+
–
..
..
C
Cl
R
R
O
..
..
N(CH
3
)
2
H
2
O NaCl
..
..
..
..
..
..
CH
2
CH
2
N(CH
3
)
2
C
Ph
O
..
..
CH
3
C
Ph
O
..
..
CH
CH
2
C
R
R
O
..
..
++
O
..
..
FIGURE 19.117 The Mannich reaction.
The Mannich reaction presents a nice mechanistic problem because two routes
to product seem possible, and it is the simpler (and incorrect) process that is the
easier to find. The difficulty is that we are now experienced in working out aldol
condensations and a reasonable person would probably start that way. An acid-cat-
alyzed crossed aldol condensation (p. 982) leads to a β-hydroxy ketone (Fig. 19.118).
Displacement of the hydroxyl group (after protonation, of course) would lead to
the initial product, the ammonium ion. Treatment with base would surely liberate
the free amine.
N
H
+
A β-hydroxy ketone
(from a crossed aldol
condensation)
HCl/EtOH HCl/ EtOH
(CH
3
)
2
NH
H
2
C
S
N
2
O
O
O
NaOH/H
2
O
deprotonation
OH
H
2
O
O
+
N
O
..
..
..
..
..
..
..
O
..
..
..
..
FIGURE 19.118 An attractive, but incorrect, mechanism for the Mannich reaction.
Sadly, this simple mechanism cannot be correct because, when the β-hydroxy
ketone is made in other ways, it is not converted into Mannich product under the
reaction conditions. What else can happen? Amines are nucleophiles and react rap-
idly with carbonyl compounds in acid to give iminium ions (p. 795). It is this imini-
um ion that is the real participant in the Mannich condensation reaction.
Formaldehyde is more reactive than the ketone, so the iminium ion is preferentially
1004 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
Iminium ion
Enol
(CH
3
)
2
NH/HCl
CH
3
H
3
C
+
+
NaOH
H
2
O
H
2
O
NaCl
++
–
Cl
..
..
..
..
C
CH
3
H
2
C
OH
..
..
HCl
CH
2
(CH
3
)
2
N
OH
..
..
C
N(CH
3
)
2
+
C
H
H
O
..
..
C
O
..
..
C
H
H
CH
3
H
2
C
CH
2
(CH
3
)
2
NH
C
CH
3
H
2
C
O
..
..
CH
2
(CH
3
)
2
N
C
CH
3
H
2
C
O
..
..
..
FIGURE 19.119 The correct
mechanism of the Mannich reaction.
Benzaldehyde
..
..
C
O
H
..
..
C
O
O
..
..
H
2
O
KOH
Potassium benzoate
(oxidized)
Benzyl alcohol
(reduced)
..
..
..
..
..
–
CH
2
OH
K
+
+
FIGURE 19.120 Benzaldehyde gives
benzoate and benzyl alcohol on
treatment with hydroxide ion.
C
O
–
+
+
H
C
O
ONa
Na OD2
D
2
O
OD
CH
2
–
+
FIGURE 19.121 A labeling
experiment shows that no solvent
deuterium becomes attached to carbon
in the Cannizzaro reaction.The
hydrogen that reduces the aldehyde to
the alcohol must come from another
aldehyde, not the solvent.
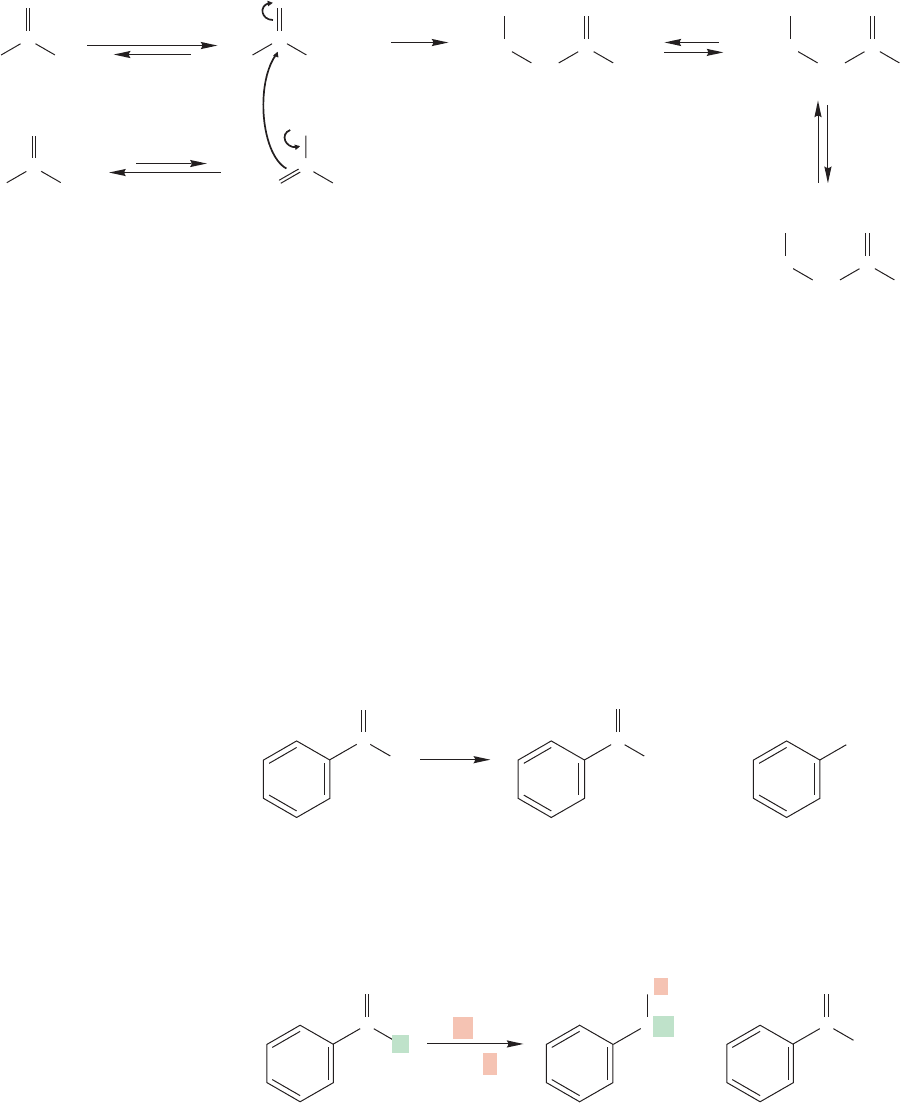
formed from this carbonyl compound (Fig. 19.119). In an aldol-like process,the enol
of the ketone reacts with the iminium ion to give the penultimate product, the ammo-
nium ion. In the second step, the free amine is formed by deprotonation in base.
This process is known as the Cannizzaro reaction, for Stanislao Cannizzaro
(1826–1910). If the reaction is carried out in deuterated solvent, no carbon–deuterium
bonds appear in the alcohol (Fig. 19.121).
19.14 Special Topic: Carbonyl Compounds
without ␣ Hydrogens
Throughout this chapter we have concentrated on molecules capable of forming either
enolates or enols, and the subsequent chemistry has been dependent on those inter-
mediates. It is a legitimate question to ask if there is a base-induced chemistry of
carbonyl compounds that do not have α hydrogens, and cannot form enolates.
19.14a The Cannizzaro Reaction Hydroxide ion will give products from
nonenolizable carbonyl compounds. If we examine a sample of benzaldehyde that
has been allowed to stand in the presence of strong base, we find that a redox reac-
tion has occurred. Some of the aldehyde has been oxidized to benzoate and some
reduced to benzyl alcohol (Fig. 19.120).
Benzaldehyde is especially prone to hydrate formation, and the first step of the
Cannizzaro reaction is addition of hydroxide to benzaldehyde. Much of the time this
reaction simply reverses to re-form hydroxide and benzaldehyde, but some of the
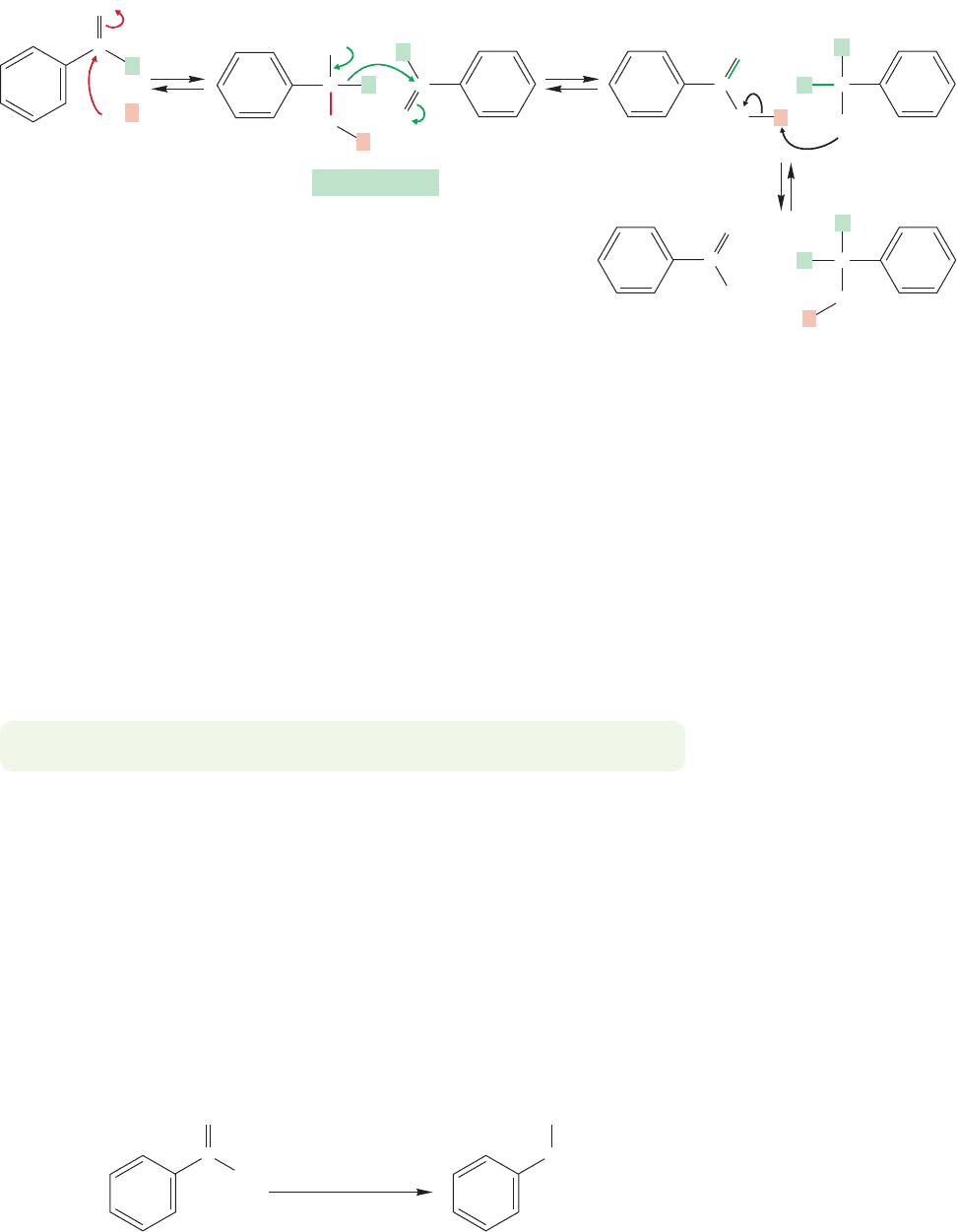
19.14 Special Topic: Carbonyl Compounds without ␣ Hydrogens 1005
time the intermediate finds a molecule of benzaldehyde in a position to accept a
transferred hydride ion (Fig. 19.122).
C
–
+
H
H
OD
O
..
..
C
O
..
..
C
O
..
..
..
..
..
..
..
..
O
D
Hydride transfer
CH
O
..
..
..
..
H
C
O
..
..
..
O
D
C
H
O
..
..
O
..
..
H
C
..
..
..
..
O
–
–
–
D
H
FIGURE 19.122 A hydride shift is at the heart of the
mechanism of the Cannizzaro reaction.
Do not confuse this hydride transfer step with the expulsion of hydride as a leaving
group. Hydride is not a decent leaving group and cannot be lost in this way. However,
if there is an electrophile positioned in exactly the right place to accept the hydride,
it can be transferred in a step that simultaneously reduces the receptor aldehyde and
oxidizes the donor aldehyde. It is very important to be aware of the requirement for
the Lewis acid acceptor of hydride in the Cannizzaro reaction. The hydride must
be transferred, and in the transition state for the reaction there is a partial bond
between the hydride and the accepting carbonyl group.A subsequent deprotonation
of the carboxylic acid and protonation of the alkoxide formed from benzaldehyde
completes the reaction. Notice how critical the observation about the nonparticipa-
tion of the deuterated solvent is. It identifies the source of the reducing hydrogen
atom. Because it cannot come from solvent (all the “hydrogens” in the solvent are
deuteriums) it must come from the aldehyde.
PROBLEM 19.41 Draw the transition state for the hydride shift reaction.
If there were a way to hold the hydride acceptor in the right position, the Cannizzaro
reaction would be much easier, because the requirements for ordering the reactants in
just the correct way (entropy) would be automatically satisfed.The two partners in the
reaction would not have to find each other. This notion leads to the next reaction in
this section, which is largely devoted to hydride shift reactions with wonderful names.
19.14b The Meerwein–Ponndorf–Verley–Oppenauer Equilibration In
the Meerwein–Ponndorf–Verley–Oppenauer (MPVO) equilibration an alu-
minum atom is used to clamp together the two halves (oxidation and reduction) of
the Cannizzaro reaction. An aluminum alkoxide, typically aluminum triisopropox-
ide, is used as a hydride source to reduce the carbonyl compound (Fig. 19.123).
C
O
H
OH
CH
2
..
..
..
..
1. Al[OCH(CH
3
)
2
]
3
HOCH(CH
3
)
2
2. H
2
O
(89%)
FIGURE 19.123 The reduction
of benzaldehyde using the
Meerwein–Ponndorf–Verley–
Oppenauer reaction.
1006 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
..
..
RR
O
..
..
O
+ +
Al
3
..
..
O
A
..
..
O
R
..
..
OO
O
Al
O
H
–
..
..
+
Reduced
Oxidized
R
O
..
..
..
..
O
O
O
..
..
OO
H
..
Al
R
R
FIGURE 19.124 The formation of a bond between
the nucleophilic carbonyl oxygen and the Lewis
acid aluminum alkoxide to give complex A,
which undergoes an intramolecular hydride
transfer facilitated by the aluminum atom
chemical clamp.
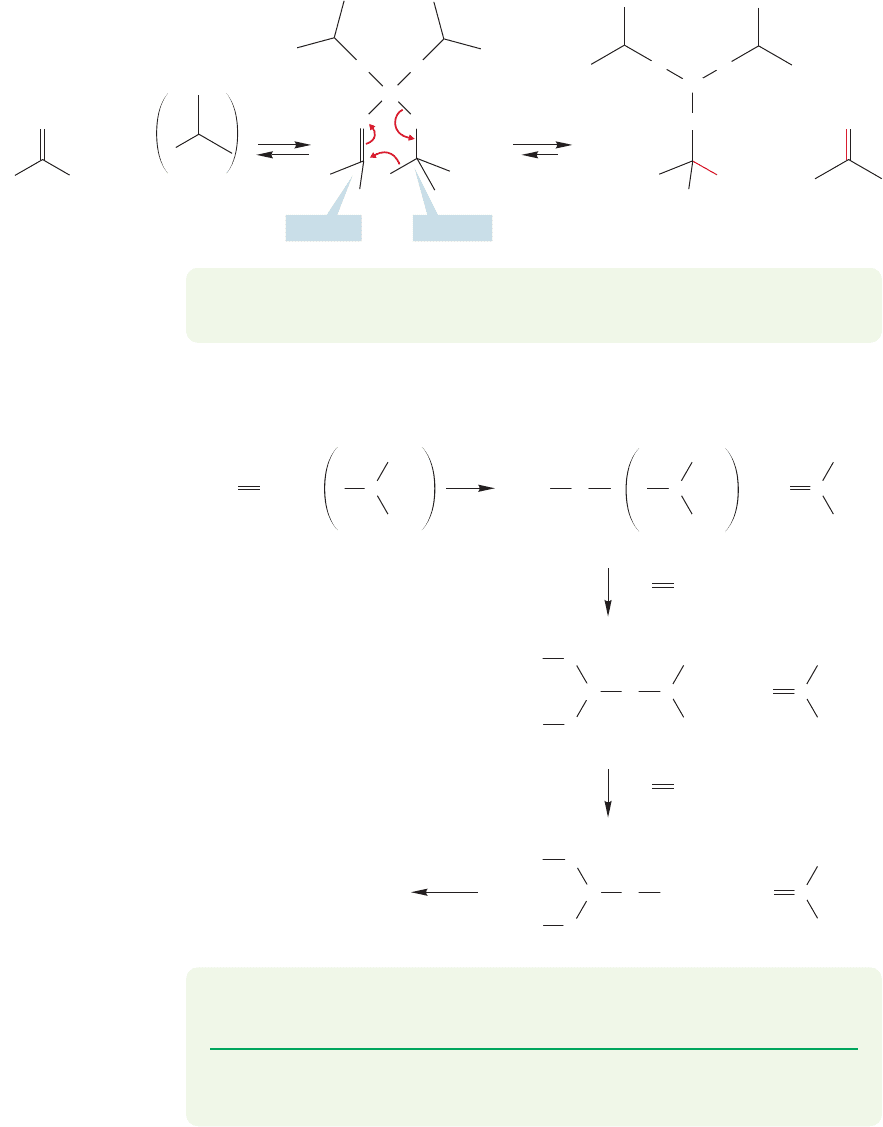
The first step in this reaction is the formation of a bond between the carbonyl
oxygen and aluminum. Aluminum is a very strong Lewis acid and the bond to the
carbonyl oxygen forms easily (Fig. 19.124).This new molecule (A) has the two react-
ing groups clamped together by the aluminum and is nicely set up for an intramol-
ecular transfer of hydride, which simultaneously reduces a molecule of the carbonyl
compound and oxidizes an isopropoxide group to acetone.
PROBLEM 19.43 Write a mechanism for the second stage in the reaction, the
formation of .
PROBLEM 19.44 Write a mechanism for the final hydrolysis reaction of Al(OR)
3
to Al(OH)
3
.
(R
2
CH
O
O)
2
Al
O
O
O
CH(CH
3
)
2
R
2
C
3 R
2
CHOHAl(OH)
3
O
++
R
2
C
O
Al
3
CH
3
CH
CH
3
Al
2
CH
3
CH
CH
3
O
CH
3
C
CH
3
R
2
CH
O
+
O
CH
3
CH
CH
3
O
CH
3
C
CH
3
R
2
CH
O
R
2
CH
O
R
2
C
O
++
O
CHR
2
O
CH
3
C
CH
3
R
2
CH
H
2
O
O
R
2
CH
O
OO
Al
Al
FIGURE 19.125 Repetition and
hydrolysis lead to more acetone
and the new alcohol.
PROBLEM 19.42 Aluminum alkoxides may look very strange to you. Compare the
structure of Al(OR)
3
to that of a trivalent boron compound such as B(OH)
3
.
Three repetitions of this process leads to a new aluminum oxide that is hydrolyzed
at the end of the reaction to give the corresponding alcohol (Fig. 19.125).
19.15 The Aldol Condensation in the Real World, an Introduction to Modern Synthesis 1007
R
HO
R
R
O
R
33+
O
O
R
R
Al
3
O
Al
3
AlCl
3
3 equiv.
FIGURE 19.126 The Meerwein–
Ponndorf–Verley–Oppenauer
equilibration can be used to
oxidize an alcohol.
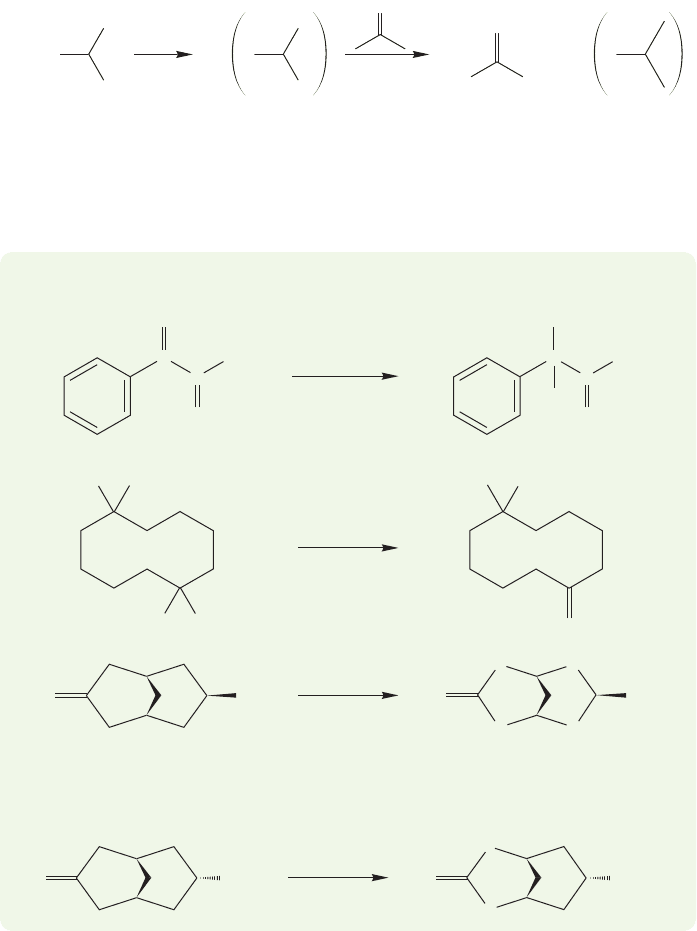
The effect of the aluminum clamp on the rate of the reaction is profound. This
reaction,here shown as a reduction, takes place under very mild conditions and with
few side reactions.
The reaction can also be used to oxidize alcohols to carbonyl compounds.In this
case, acetone is used as the acceptor, and the alcohol to be oxidized is used to form
the aluminium oxide (Fig. 19.126).
–
(a)
C
O
C
KOH/H
2
O
O
H
C
OH
C
O
O
H
+
(b)
HO
H
2
O/H
3
O
D
O
HO
D
CH
3
CH
3
(c)
OH
D
2
O/KOD
O
OD
O
D
2
C
D
2
C
CD
2
CD
2
Hints: Draw a good three dimensional picture of this molecule first! The other
stereoisomer exchanges only five hydrogens for deuteriums.
D
2
O/KOD
O
OD
OH
O
D
2
C
D
2
C
Problems involving hydride shifts tend to be hard enough to thwart even excel-
lent problem solvers. That observation leads directly to Magid’s third rule, which
states, When all else fails and desperation is setting in, look for the hydride shift.
Problem 19.45 involves some practice hydride shifts.
19.15 Special Topic: The Aldol Condensation in the
Real World, an Introduction to Modern Synthesis
In this section,we introduce some of the difficulties of real-world chemistry.The detail
isn’t so important here, although it will be if you go on to become a synthetic chemist!
It is important to get an idea of the magnitude of the problems, and of the ways in
which chemists try to solve them.The general principles and relatively simple reactions
PROBLEM 19.45 Write mechanisms for the following reactions.
1008 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
B
..
base
Two enolates… …lead to two products
–
+
H
..
..
O
CH
3
..
..
..
O
–
(–)
..
..
..
O
–
(–)
BH
1.
..
..
2. H
2
O
B
..
base
–
+
..
..
HO
..
..
O
B
H
1.
..
..
2. H
2
O
CH
2
..
..
O
CH
3
CH
2
H
..
..
O
..
..
O
..
..
O
..
..
OH
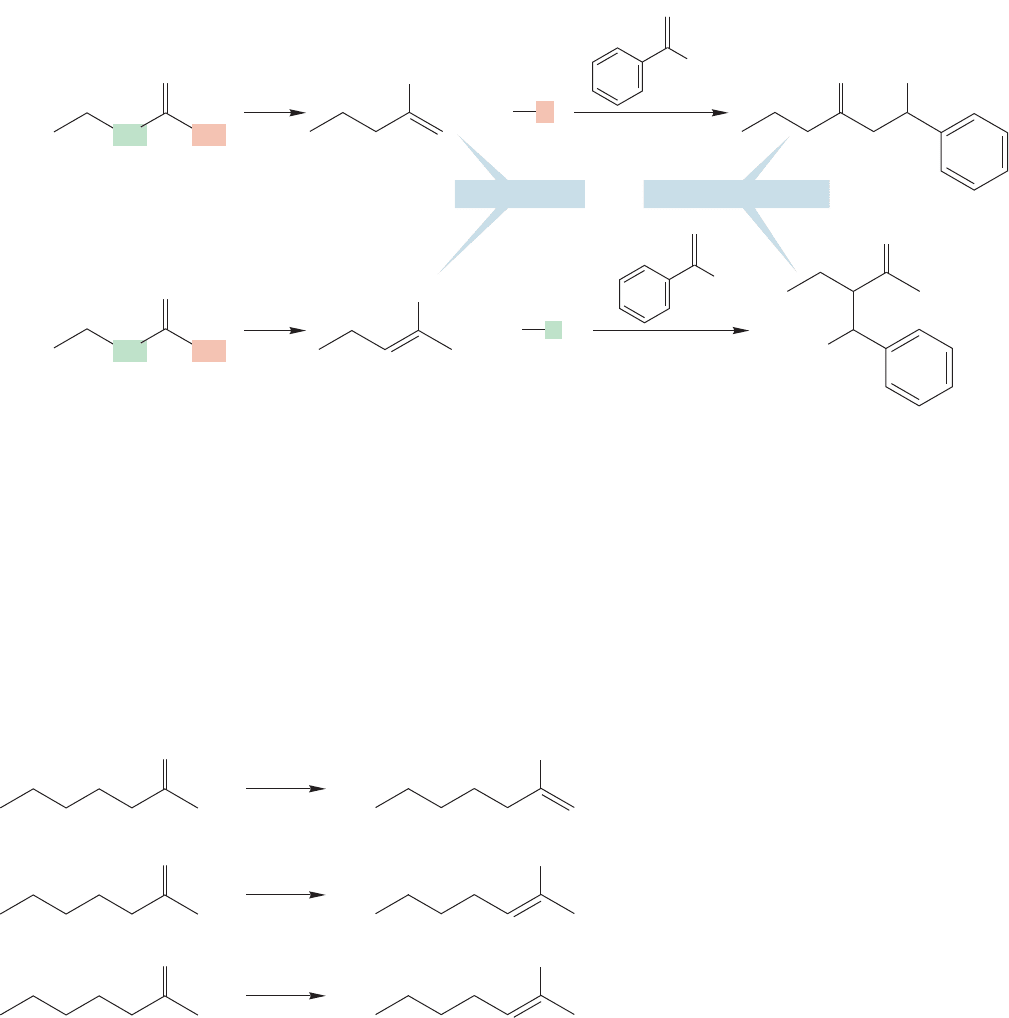
FIGURE 19.127 In principle, an unsymmetrical ketone leads to two enolates, and therefore two products.
The two enolates differ in stability, depending on the number of alkyl groups attached to the carbon-
carbon double bond.
The two enolates are quite different in stability. Consider the enolate resonance
structure that contributes most to the overall structure for the two enolates.One con-
tains a disubstituted carbon–carbon double bond and will be more stable than the
other, which has only a monosubstituted carbon–carbon double bond.
Effective methods have evolved to form the less stable enolate by taking advan-
tage of the relative ease of access to the less hindered α hydrogen (Fig. 19.128a). As
we have learned,LDA is especially effective at forming these less stable, kinetic eno-
lates.The key point is that this strong but large base has difficulty in gaining access
to the more hindered parts of the carbonyl compound. Removal of the less hindered
proton leads to the less stable, or kinetic enolate.
Other methods allow formation of the more
stable, thermodynamic enolate (Fig. 19.128b).
In one of these, boron enolates are formed.
Another option is use of a slightly weaker base
and higher temperatures (thermodynamic condi-
tions) in making the enolate.
We have described only one of the difficul-
ties encountered in just one important syn-
thetic reaction,the aldol condensation.One goal
of all synthetic chemists is selectivity, ideally,
specificity. How do we do only one reaction?
For example, how do we form one, and only
one, stereoisomer of the many often possible?
FIGURE 19.128 Use of the boron
enolate or thermodynamic conditions
to make the more stable enolate.
we have studied (e.g., the straightforward aldol condensation) set the stage for a glance
at the difficulties encountered when chemists try to do something with these reactions.
In the real world of practical organic synthesis, one rarely needs to do a simple aldol
condensation between two identical aldehydes or two identical ketones. Far more com-
mon is the necessity to do a crossed aldol between two different aldehydes, two differ-
ent ketones, or an aldehyde and a ketone. As noted earlier, there are difficulties in doing
crossed aldol reactions.Suppose,for example,that we want to condense 2-pentanone with
benzaldehyde. Benzaldehyde has no α hydrogen, so no enolate can be formed from it.
Some version of the Claisen–Schmidt reaction (p. 984) seems feasible. But 2-pentanone
can form two enolates, and the first problem to solve is the specific formation of one or
the other enolate (Fig. 19.127).
DME
(dimethoxyethane)
25 ⬚C
Ph
3
CLi
O
–
O
BEt
3
25 ⬚C
KH
OBEt
3
O
–
(b)
THF
–78 ⬚C
LDA
O
–
O
(a)
