Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

19.6 Addition of Carbonyl Compounds to the Position: The Aldol Condensation 979
␣
advantage of Michael addition should ultimately win out.This notion suggests that
strong nucleophiles such as hydride (H
) and alkyllithium compounds, which surely
add irreversibly to carbonyls, might be found to give the products of attack at the
carbonyl group, and such is the case (Fig. 19.85).
+
1. LiAlH
4
ether
2. H
2
O
1. CH
3
Li
2. H
2
O
O
H
H
O
H
(94%)
(81%)
No addition to
the alkene portion
(2%)
HO
H
H
H
H
CH
3
H
3
C
O
CH
3
H
3
C
OH
CH
3
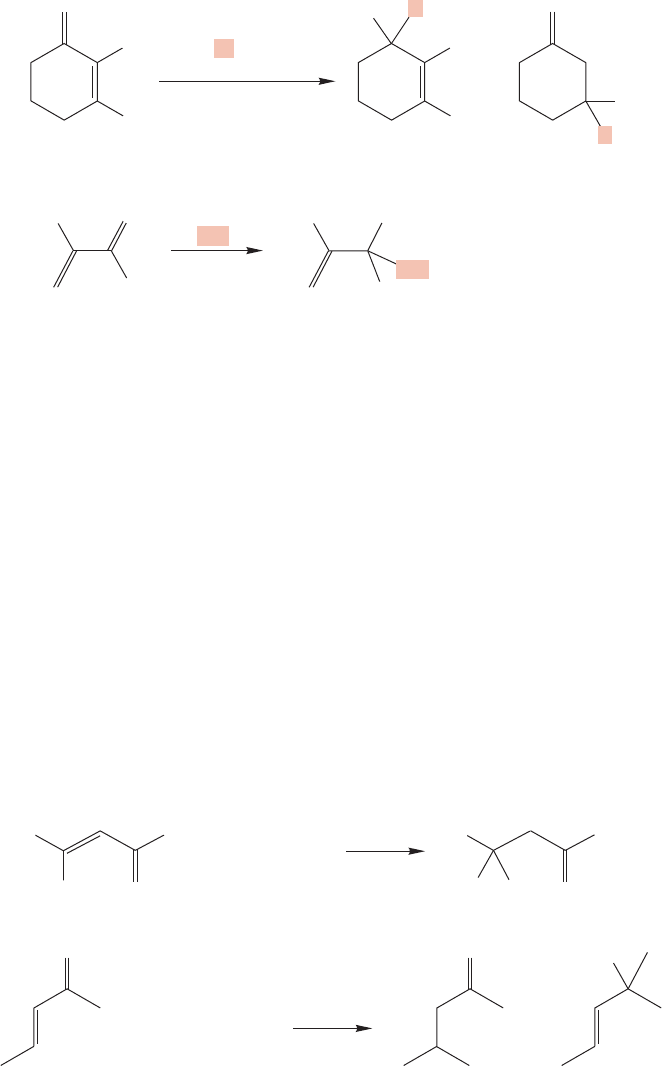
FIGURE 19.85 Two irreversible addition reactions to the carbonyl group of an
α,β-unsaturated carbonyl compound.
1. ether
–70 ⬚C
2. H
2
O
1. catalytic
CuI, ether
2. H
2
O
(Bu)
2
CuLi+
CH
3
MgBr++
O
O
CH
3
H
3
C
H
3
C
O
HO
(53%)
CH
3
CH
3
H
3
C
Bu
O
(87%) (4%)
Cuprate additions
FIGURE 19.86 Cuprates and copper-catalyzed Grignard reagents add in Michael
fashion to α,β-unsaturated carbonyl compounds.
A very common reaction in organic synthesis is the addition of a cuprate (p. 798)
to an enone. Although the organometallic cuprate is a strong base and there-
fore might be expected to add to the carbonyl carbon, this reagent is found to
give Michael addition products (Fig. 19.86). Grignard reagents usually give 1,2-
addition with α,β-unsaturated aldehydes, but the outcome of Grignard addition
to α,β-unsaturated ketones is difficult to predict. Steric factors are thought to con-
trol the selectivity of such Grignard additions. However, Grignard reactions that
are copper catalyzed dependably undergo Michael addition to α,β-unsaturated
compounds.
Summary
In this section, we have seen two variations on the aldol theme, the Knoevenagel
condensation and the Michael reaction. In all of these reactions, a single nucleo-
phile adds to a single electrophile. The reactions are fundamentally the same as
the aldol condensation; the variations arise from the structural differences of the
nucleophiles.
19.7 Reactions Related to the Aldol Condensation
In the next few pages, we will use what we know of the simple aldol condensation
to understand some related reactions. We will start with reactions that are very sim-
ilar to simple aldol reactions,and slowly increase the complexity.The connection to the
simple,prototypal acid- and base-catalyzed aldol reactions will always be maintained.
19.7a Intramolecular Aldol Condensations Like most intermolecular reac-
tions, the aldol reaction has an intramolecular version.If a molecule contains both an
enolizable hydrogen and a receptor carbonyl group, an intramolecular addition will
be theoretically possible. Particularly favorable are intramolecular cyclizations that
980 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
NHCOOCH
3
O
OR
HO
R = complex series of sugar molecules
A B
C
SSSCH
3
NHCOOCH
3
O
OR
Michael
reaction
DNA in
cancer
cells
HO
S
NHCOOCH
3
O
OR
HO
S
NHCOOCH
3
–
O
OR
..
HOHO
S
NHCOOCH
3
–
O
OR
+
Dead
cancer
cell
S
HH
–
–
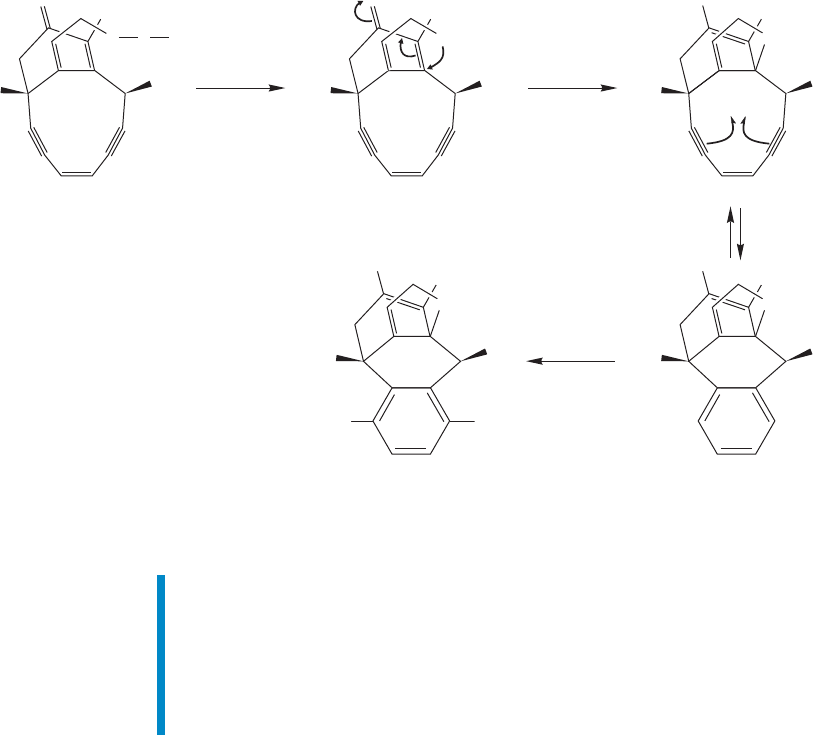
FIGURE 19.87 The operation of the antitumor agent calicheamicin.
The Michael reaction is critical to the operation of the anticancer drug
calicheamicin (Fig. 19.87). In the first step of its operation, the trisulfide bond in
A is broken.The nucleophilic sulfide then adds in Michael fashion to give the eno-
late (B). This addition changes the shape of the molecule, bringing the ends of the
two acetylenes closer together. A cyclization occurs to give a diradical (C), and this
diradical abstracts hydrogen from the cancer cell’s DNA, which ultimately kills the
cell.Calicheamicin depends for its action on the change of shape.Before the Michael
reaction, the ends of the two acetylenes are too far apart to cyclize. They are freed
to do so only when the sulfur adds to the α,β-unsaturated carbonyl.
19.7 Reactions Related to the Aldol Condensation 981
THE GENERAL
BASE-CATALYZED CASE
SPECIFIC BASE- AND
ACID-CATALYZED EXAMPLES
B
BH
BH
+
BH ++
–
–
CO
R
CCH
3
O
..
..
..
..
..
O
CCH
2
O
..
..
..
..
..
O
..
..
H
2
O
KOH
H
2
O
H
2
SO
4
C
R
C
O
C
CH
2
..
....
..
–
HO
..
....
..
C
OH
R
C
CH
2
..
..
O
..
..
C
OH
R
C
CH
R
..
..
..
O
..
..
C
R
C
CH
O
..
..
O
..
..
O
..
..
O
..
..
O
(83%)
(96%)
..
..
O
..
..
CH
3
CH
3
CH
3
H
3
C
–
B
..
–
(–)
–
O
..
..
WEB 3D
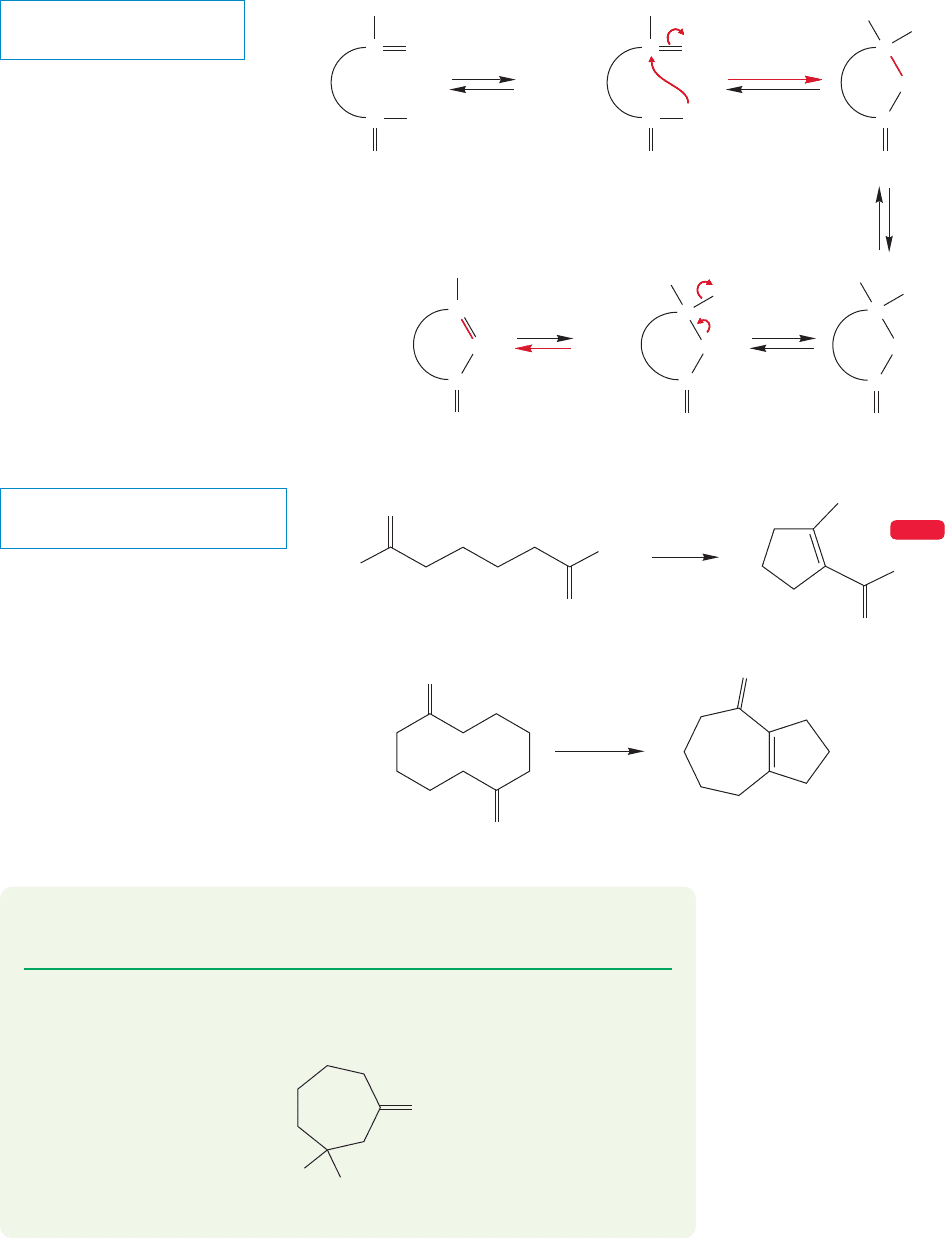
FIGURE 19.88 The mechanism for
intramolecular aldol reactions.
form the relatively strain-free five- and six-membered rings. The products are still
β-hydroxy ketones (or the dehydration products, α,β-unsaturated ketones), and the
mechanism of the reaction remains the same (Fig. 19.88).
PROBLEM 19.28 Write the mechanisms for the acid- and base-catalyzed aldol
condensations of the molecule at the bottom of Figure 19.88.
PROBLEM 19.29 Write the mechanisms for the acid- and base-catalyzed reverse
aldol reaction for 3-hydroxy-3-methylcycloheptanone. Hint: See Problem Solving,
p. 968.
O
H
3
C
OH
3-Hydroxy-3-methylcycloheptanone
982 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
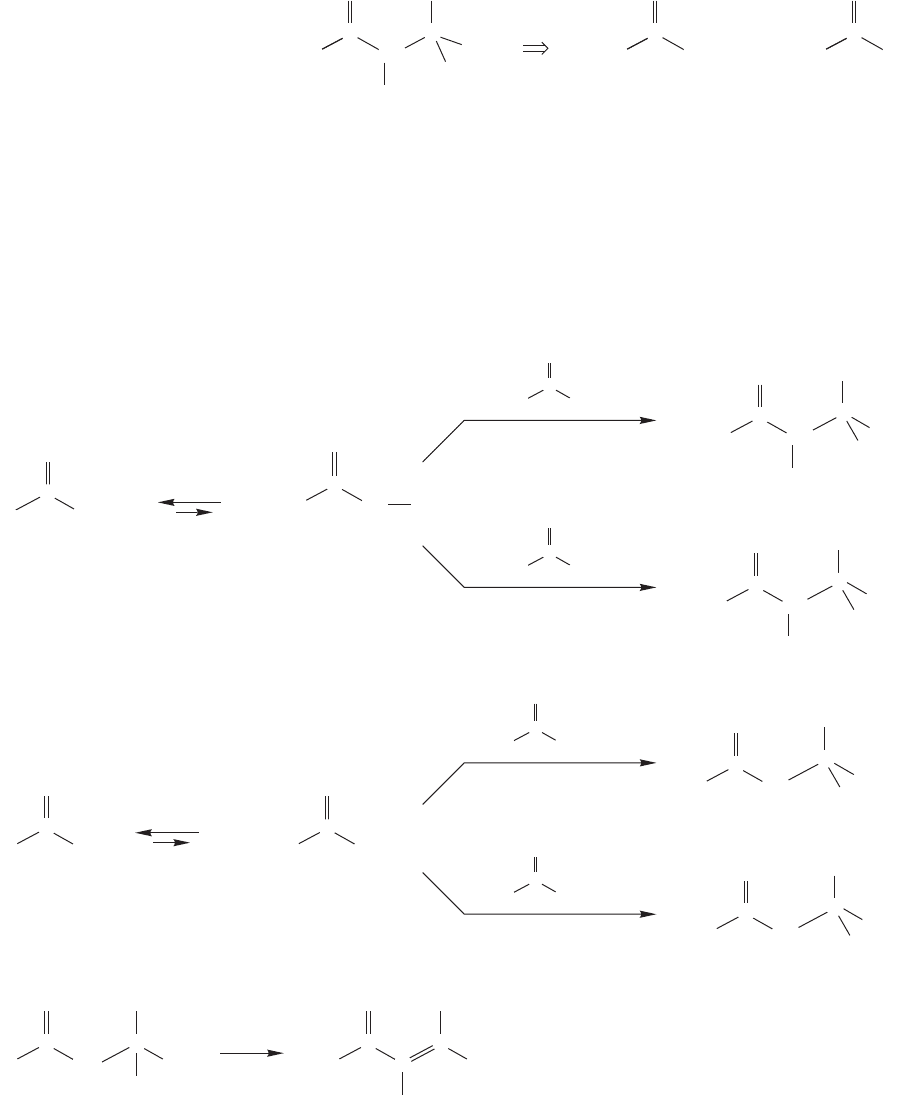
FIGURE 19.89 A retrosynthetic
analysis suggests that a crossed aldol
reaction between diethyl ketone and
acetone should give 5-hydroxy-4,5-
dimethyl-3-hexanone.
One possible dehydration:
H
3
C
C
base
O
CH
3
CH
3
C
OH
D
CH
2
H
3
C
C
O
CH
3
H
C
CH
3
C
CH
2
CH
3
CH
2
CH
3
–
H
3
C CH
3
C
base
O
H
3
C CH
2
C
C
O
H
3
C CH
2
C
C
C
CH
3
CH
3
O
OH
OH
O
H
3
C
O
..
CH
2
C
D
H
3
C
CH
3
C
CH
3
CH
2
CH
2
CH
3
C
O
–
CH
3
CH
2
CH
2
CH
3
C
base
O
CH
3
CH
2
C
C
O
CH
3
CH
2
C
A
B
C
CH
3
CH
3
CH
3
CH
2
CH
3
CH
3
CH
2
CH
3
O
OH
OH
H
3
C
CH
3
C
O
CH
3
CH
2
CH
3
CH
O
..
C
CH
3
CH
2
CH
2
CH
3
C
O
CH
CH
FIGURE 19.90 When this synthetic
route is attempted, four β-hydroxy
ketones, A, B, C, and D, are likely
to be produced.
19.7b Crossed Aldol Condensations In practice, not all β-hydroxy ketones
can be formed efficiently by aldol reactions. Suppose we were set the task of syn-
thesizing 5-hydroxy-4,5-dimethyl-3-hexanone (Fig. 19.89). We recognize it as a
potential aldol product through the dissection shown in the figure, which suggests
that an aldol condensation of diethyl ketone and acetone should be a good route to
the molecule. An aldol condensation between two different carbonyl compounds is
called a crossed (or mixed) aldol condensation.
+
..
..
..
..
CH
C C
OOH
CH
3
CH
2
..
..
C
O
CH
3
CH
2
CH
2
CH
3
..
..
C
O
H
3
CCH
3
CH
3
5-Hydroxy-4,5-dimethyl-3-hexanone
CH
3
CH
3
However, a little thought about the mechanism reveals potential problems.Two
enolates can be formed and each enolate has two carbonyl groups to attack
(Fig. 19.90). Thus, four products, A, B, C, and D, are possible (more if any of the
β-hydroxy ketones are dehydrated to α,β-unsaturated ketones), and all are likely to
be formed in comparable yield. The enolate of diethyl ketone can add to the car-
bonyl of acetone or diethyl ketone to give A and B. Similarly, the enolate of acetone
gives C and D by reaction with the two carbonyl compounds.
19.7 Reactions Related to the Aldol Condensation 983
There are some simple ways to limit the possibilities in a crossed aldol reaction.
If one partner has no α hydrogens, it can function only as an acceptor, and not as the
nucleophilic enolate partner in the reaction (Fig. 19.91). For example, we might hope
..
..
..
..
..
..
..
..
H
2
O
..
..
H
2
O
–
–
CH
3
C
NaOH
H
2
O
O
..
..
H
3
C
C(CH
3
)
3
C(CH
3
)
3
C(CH
3
)
3
C
O
..
..
..
..
..
..
H
C
no
α hydrogens
O
O
..
..
CH
2
C
..
..
(CH
3
)
3
C(CH
3
)
3
C
(CH
3
)
3
C
O
–
..
..
..
O
–
–
..
..
..
O
CH
2
C
Only enolate possible
C
CH
O
C
C
O
..
..
..
..
..
..
O
..
..
O
..
HO
..
..
–
..
OH
H
H
C
CH
O
C
C
O
CH
3
CH
3
CH
2
CH
2
CH
2
CH
2
(CH
3
)
3
C
(CH
3
)
3
C
(CH
3
)
3
C
(CH
3
)
3
C
++
FIGURE 19.91 The crossed aldol
reaction of tert-butyl methyl
ketone and benzaldehyde can give
only two products. Benzaldehyde
has no α hydrogens and cannot
form an enolate.
(CH
3
)
3
C CH
3
C +
O
(CH
3
)
3
C CH
(90%)
CH
C
O
H
C
NaOH
CH
3
CH
2
OH
H
2
O
room temperature
32 h
O
FIGURE 19.92 In fact, there is only one major product in the aldol condensation of tert-butyl methyl ketone
and benzaldehyde.
for better luck in a reaction pairing tert-butyl methyl ketone and benzaldehyde. In
principle,however,there are still two possibilities,because the enolate can add to either
the ketone or the aldehyde to generate two different β-hydroxy ketones. When this
reaction is run, however, only one product is formed in substantial yield (Fig. 19.92).
PROBLEM 19.30 There is a reaction between benzaldehyde and hydroxide ion.
What is it, and why does it not interfere with the aldol condensation?
There are several reasons that this crossed aldol is successful. First, the rate of
addition of the enolate to the carbonyl group of benzaldehyde is much greater than
that of addition to tert-butyl methyl ketone because the carbonyl groups of alde-
hydes are more reactive than those of ketones. Second, the equilibrium constant for
the addition to an aldehyde carbonyl is more favorable than that for addition to a
Mixed aldol condensation
ketone carbonyl (Fig. 19.93).Third, because the addition and dehydration steps are
reversible,if elimination occurs, thermodynamics will favor the most stable product,
which in this case is the phenyl-substituted enone.
984 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
–
(–)
(CH
3
)
3
C CH
2
C
O
..
..
..
(CH
3
)
3
C CH
3
C
O
..
..
Less reactive
K
more
favorable
H
2
O
H
C
More reactive
O
..
..
..
..
..
..
–
..
..
..
O
–
..
..
..
..
..
H
2
O
..
..
O
(CH
3
)
3
C
CC
O
(CH
3
)
3
C
CC
C(CH
3
)
3
CH
3
O
..
..
..
..
..
..
OH
..
..
OH
H
(CH
3
)
3
C
C
C
O
(CH
3
)
3
C
CC
C(CH
3
)
3
CH
3
O
CH
2
H
CH
2
CH
2
CH
2
K
less
favorable
FIGURE 19.93 The carbonyl group of
benzaldehyde is more reactive than
that of tert-butyl methyl ketone, and
equilibrium favors the product for the
reaction with benzaldehyde but not
for the reaction with tert-butyl
methyl ketone.
The crossed aldol reaction is important enough to have been given its own name,
the Claisen–Schmidt condensation (Ludwig Claisen, 1851–1930).
Earlier in this chapter we saw LDA, a base that is effective in producing enolates
without the complications of addition to the carbonyl group. Because LDA is such a
strong base, all the initial carbonyl compound is driven to its enolate (Fig. 19.94).
(–)
–
..
..
..
..
H
2
O
N
–78 ⬚C
Li
+
O
CH
3
..
..
H
O
..
..
O
CH
2
..
..
..
..
–
–
..
..
O
..
..
O
..
..
..
(65%)
O
2-Hexanone
7-Hydroxy-5-decanone
Cyclohexyl ethyl ketone
Butanal
..
OH
1. LDA
THF
–78 ⬚C
2.
3. H
3
O
+
(79%)
OHO
O
OPh
O
H
OPh
FIGURE 19.94 A crossed
aldol reaction with LDA
as base. Because of its size,
LDA selectively
deprotonates the less
substituted side of the
initial ketone making the
kinetic enolate. Because of
its strength, LDA
completely deprotonates
the carbonyl compound. A
second carbonyl
compound can then be
added and undergo the
aldol reaction.The
alkoxide is protonated at
the end of the reaction
when water is added.
19.8 Addition of Acid Derivatives to the Position: The Claisen Condensation 985
␣
No aldol reaction is possible until the LDA is consumed and a second carbonyl compound
is added.This procedure is a convenient way to do crossed aldol reactions in a controlled
fashion. When there is a choice, LDA forms the more accessible, less substituted enolate.
Lithium diisopropylamide is a large,sterically encumbered base and removes a proton from
the sterically less hindered position to give what is called the kinetic enolate. Notice that
this reaction can be used to generate two new stereocenters adjacent to each other. This
procedure is very useful and has received considerable attention in the past 30 years.
19.8 Addition of Acid Derivatives to the ␣ Position:
The Claisen Condensation
The reaction between enolates and esters is an addition–elimination process in which
the enolate is the nucleophile that replaces the alkoxy group of the ester. This reac-
tion is similar to many you saw in Chapter 18.
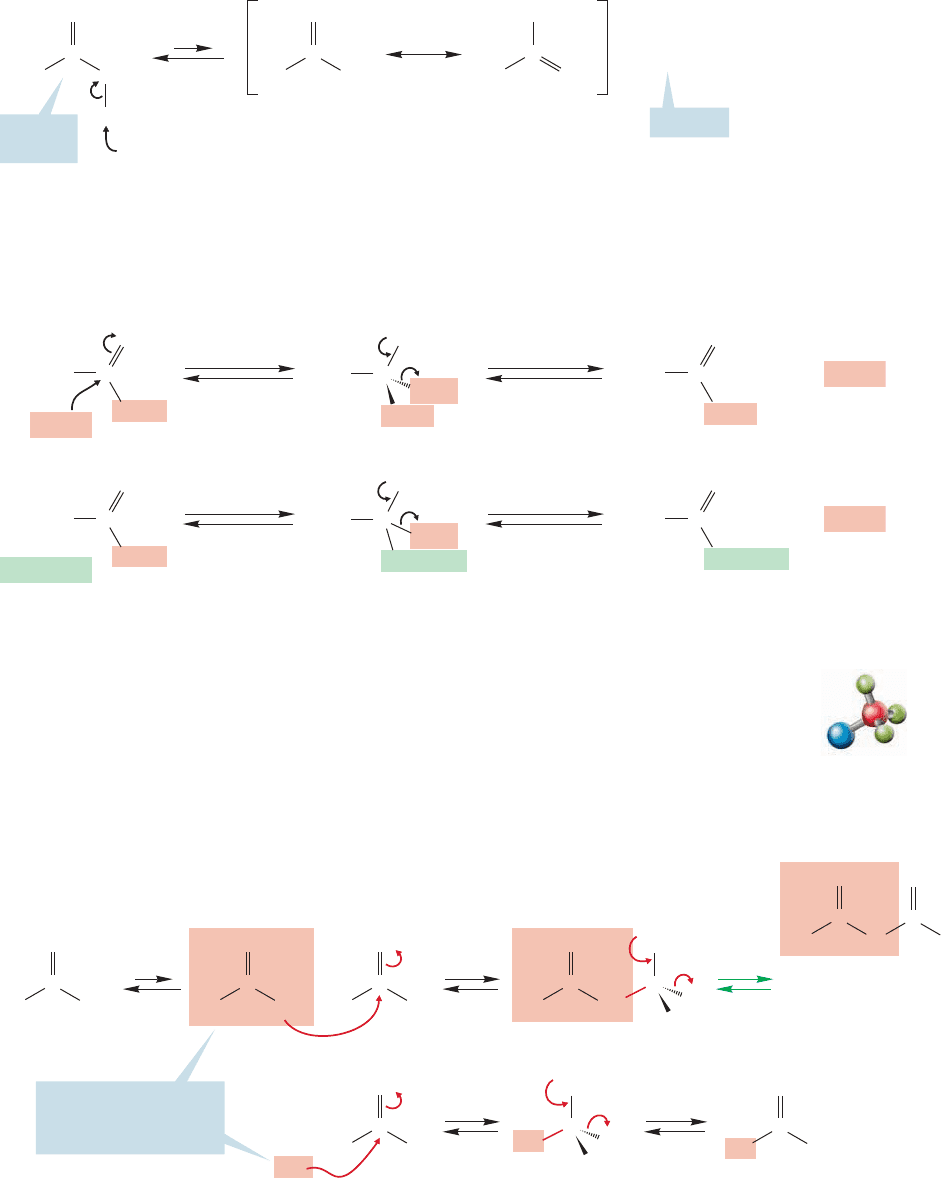
19.8a Condensations of Ketone Enolates with Esters Many reactions can
occur between an ester and a ketone under basic conditions. It is unlikely that con-
densation of the ketone enolate with another molecule of ketone will be a problem,
because aldol reactions of ketones are reversible, and usually endothermic. So even
though the product of the aldol reaction will be formed, starting ketone will be
favored. If ketone is drained away through reaction with the ester, the reversibly
formed aldol product merely serves as a storage point for the ketone. The condensa-
tion of an ester enolate with an ester is usually not a problem because the ketone is a
much stronger acid (pK
a
19) than the ester (pK
a
24, see Table 19.2), and forma-
tion of the ketone enolate will be much preferred to formation of the ester enolate. In
the reaction between the ketone enolate and an ester, the enolate adds to the ester
carbonyl, alkoxide is lost, and a β-dicarbonyl compound is formed (Fig. 19.95). If a
''
C C
O
H
3
C
..
..
C
ether
1. Na
+
–
OEt
2. H
3
O
+
/H
2
O
CH
3
O
H
3
C
..
..
C
CH
2
..
..
O
H
3
C
..
..
C
OCH
2
CH
3
Overall reaction
Mechanism
..
OCH
2
CH
3
..
..
H
3
C
..
C
O
..
CH
3
CH
2
O
O
..
..
CH
3
CH
3
H
3
C
O
..
..
C C
O
O
..
..
CH
3
H
3
C
..
(43%)
+
+
..
–
–
(–) (–)
..
..
..
..
..
–
..
..
–
..
..
C
H HH H
C OEt
++H
2
O
..
..
OEtH
O
..
..
O
..
..
H
3
C
CH
3
C
C
H H
C
O
..
..
O
..
..
H
3
C
CH
3
C
H
C
C
O
..
..
O
..
..
H
3
C
1
2
3
4
5
Enolate formation
Addition
Elimination
Removal of
doubly hydrogen
Acidification
C
H H
C
C
H
3
O
+
/H
2
O
FIGURE 19.95 The mechanism
of a crossed condensation reaction
between a ketone and an ester.
full equivalent of base is used, the doubly α hydrogen of the β-dicarbonyl compound
is removed to give a nicely resonance-stabilized anion.The reaction is completed by an
acidification step that regenerates the β-dicarbonyl compound in the absence of base.
PROBLEM 19.31 Why are esters less reactive in addition reactions than aldehydes
and ketones?
Frequently, the strong base sodamide (NaNH
2
) or LDA is used in condensing
esters with ketones.These conditions favor removal of the kinetically more accessi-
ble hydrogen when there is a choice of possible enolates (Fig. 19.96).
986 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
–
–
..
..
H
3
O
1. NaNH
2
2.
3. H
3
O
+
/H
2
O
..
+
H
2
O
Na
+
–
NH
2
ether
addition
enolate
formation
removal of
doubly
hydrogen
elimination
acidification
C
H
(CH
3
)
2
CHCH
2
(CH
3
)
2
CHCH
2
(CH
3
)
2
CHCH
2
O
..
..
..
C
CH
2
O
..
..
..
–
..
..
C
O
..
..
C
O
..
..
C
EtO
..
..
OEt
..
C
O
..
..
..
OEt
..
..
HOEt
..
..
..
–
O
..
..
O
..
..
O
..
..
O
..
..
O
(74%)
(51%)
O
..
..
O
(–) (–)
..
..
NH
2
H H
CH
2
CH(CH
3
)
2
CH
2
CH(CH
3
)
2
(CH
3
)
2
CHCH
2
CH
2
CH(CH
3
)
2
(CH
3
)
2
CHCH
2
CH
2
CH(CH
3
)
2
(CH
3
)
2
CHCH
2
CH
2
CH(CH
3
)
2
CH
2
THE GENERAL CASE
A SPECIFIC EXAMPLE
O
O
O
OEt
CH
2
C
CH
..
–
CH
2
FIGURE 19.96 Amide bases are often used in crossed aldol condensations of esters and ketones. They lead to formation of
the kinetic enolate through removal of the most accessible hydrogen.
19.8 Addition of Acid Derivatives to the Position: The Claisen Condensation 987
␣
19.8b Ester Enolates React with Esters Esters are approximately seven
pK
a
units less acidic than alcohols. Nevertheless, it is possible for some ester eno-
late to be formed in the presence of a base such as an alkoxide ion, even though the
reaction must be substantially endothermic (Fig. 19.97).
HO
pK
a
~ 17
Enolate
Ester
pK
a
~ 24
–
–
–
..
..
..
..
..
..
..
..
..
..
O
RO
..
..
RO RO
..
..
..
C +
CH
2
O
..
..
C
CH
2
O
..
..
C
CH
2
H
OC(CH
3
)
3
OC(CH
3
)
3
FIGURE 19.97 Formation of
an enolate anion from an
ester. Because tert-butyl
alcohol has a pK
a
of about 17,
and the ester a pK
a
of about
24, this reaction must be
highly endothermic.
Why doesn’t the alkoxide in Figure 19.97 add to the ester carbonyl group and start
a base-catalyzed transesterification reaction? It does! If one is not careful to use the
same OR group in the alkoxide and the ester, then mixtures of products are found
(Fig. 19.98). As long as the OR groups match, however, the base-catalyzed transes-
terification doesn’t change anything—the product is the same as the starting material.
..
..
..
OCH
3
..
OCH
2
CH
3
..
..
OCH
2
CH
3
–
C
..
..
..
..
H
3
CH
3
C
CH
2
CH
3
O
C
addition
elimination
O
..
..
..
OCH
3
–
..
..
..
H
3
C OCH
3
+ C
New ester
O
..
..
–
..
O
..
..
..
..
..
OCH
3
..
OCH
3
..
..
OCH
3
–
C
..
..
..
..
H
3
CH
3
C
CH
3
O
C
addition
elimination
of CH
3
O
O
..
..
..
–
..
..
..
OCH
3
–
..
..
..
H
3
C OCH
3
+ C
No net change!
O
..
..
–
..
O
..
..
FIGURE 19.98 A base-catalyzed transesterification reaction will generate a new ester unless
the OR group of the alkoxide is identical to the OR group of the ester.
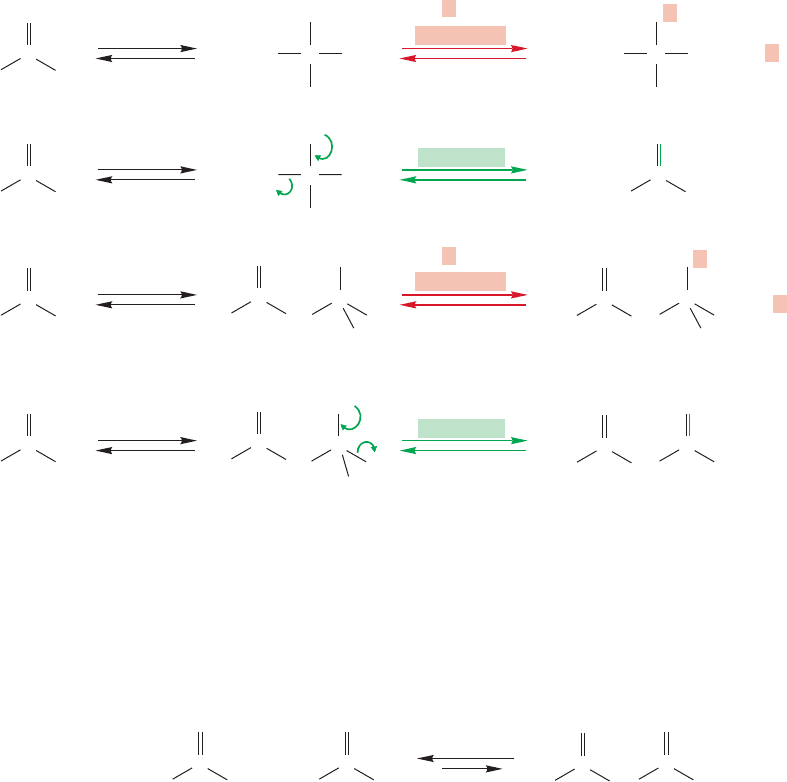
What can the ester enolate do? It can react with the acidic alcohol to reproton-
ate,regenerating the starting ester.However, it can also react with any other electrophile
in the system. The carbonyl group of the ester is just such a species, and it can react
with the ester enolate in an addition reaction. Although the product is more com-
plex, the addition reaction is no different from any other addition of a nucleophile
to an ester carbonyl (Fig. 19.99).The second step of the reaction is also analogous to
previous reactions. The tetrahedral intermediate can expel the enolate in a simple
reverse of the original addition reaction, or it can lose alkoxide to give a molecule of
β-keto ester.This reaction is called the Claisen condensation after Ludwig Claisen,
Both nucleophiles add
to the Lewis acid—the
ester carbonyl
C
–
–
–
–
Nu
Nu
..
–
..
..
O
RO
..
..
RO
..
..
ROH
..
..
..
..
..
C
CH
3
..
..
O
OR
..
..
..
OR
..
..
C
H
3
C
..
..
..
O
RO
..
..
C
CH
2
..
..
O
RO
..
..
O
..
..
C
CH
2
CH
3
C
..
..
O
OR
..
OR
..
..
O
..
..
C
H
3
C
O
..
..
CH
3
..
..
RO
C
CH
2
CH
3
C
+
–
OR
Acetoacetic ester
O
..
..
O
..
..
CH
3
+C
Nu
..
..
..
–
OR
..
..
..
FIGURE 19.99 In the first step of the reaction between an ester enolate and an ester, the nucleophilic ester enolate adds to the
electrophilic carbonyl carbon of the ester. In the second step the alkoxide ion is lost from the tetrahedral intermediate.
Claisen condensation
988 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
who has many reactions named for him. Recall the Claisen–Schmidt condensation,
for example (p. 984). There are more to come.
The Claisen condensation bears the same relationship to the aldol condensation as
the addition of a nucleophile to an ester bears to the addition of a nucleophile to an
aldehyde or ketone. Whereas the tetrahedral alkoxide formed in both aldehyde (or
ketone) reactions can only revert to starting material or protonate, both ester reactions
have the added option of losing the ester alkoxide. What is added in ester chemistry is
the possibility of the elimination phase of the addition–elimination process (Fig.19.100).
Nu
–
..
..
..
O
RO
..
..
RO
..
..
C
CH
3
O
Nu
..
..
C
CH
3
O
H
..
..
..
..
CCH
O
CH
3
CH
3
Nu
..
..
CH
OH
CH
3
Nu
–
..
..
..
C
O
CH
3
O
H
2
..
..
C
CH
3
–
..
O
H
..
..
C
CH
3
CH
2
CH
2
O
H
..
..
CH
3
C
..
O
HH
..
..
C
..
C
-Hydroxy aldehyde
–
..
..
RO
..
+
–
..
..
HO
..
+
–
..
..
HO
..
+
Nu
..
–
addition
Nu
..
–
addition
aldol
elimination
protonation
protonation
OH
..
..
H
2
O
..
..
H
2
O
..
..
O
RO
..
..
C
CH
3
2
–
CH
2
CH
2
–
..
..
..
O
RO
..
..
C
H
3
C
..
..
O
OR
..
..
C
..
..
O
RO
..
..
C
CH
3
O
..
..
C
-Keto ester
..
..
RO
..
+
Claisen elimination
FIGURE 19.100 The aldol condensation is just one example of the addition of a nucleophile to a carbonyl group
followed by protonation. The Claisen condensation is just one example of the addition–elimination sequence
available to esters.
FIGURE 19.101 Thermodynamics
favors the pair of esters (starting
material) in the Claisen condensation,
not the β-keto ester product.The
reaction to form the β-keto ester is
endothermic.
There is an easy way out of this thermodynamic bind, however. In the last step
of the Claisen condensation a molecule of alkoxide catalyst is regenerated.The cat-
alyst is regenerated in the aldol condensation as well, and in the aldol reaction the
catalyst goes on to form another enolate.In the Claisen condensation, however, there
is another, faster reaction possible. The β-keto ester product is by far the strongest
acid in the system as it contains hydrogens that are α to two carbonyl groups. The
anion formed by deprotonation of the β-keto ester is resonance stabilized by both
carbonyl groups (recall Problem 19.25). The pK
a
values of β-keto esters are about
11 (Table 19.3), and therefore these molecules are about 10
13
more acidic than the
starting esters! By far the best reaction for the alkoxide regenerated at the end of
..
..
O
RO
..
..
C
CH
3
..
..
O
RO
..
..
C
CH
3
–
..
..
O
RO
..
..
RO
..
..
C
CH
2
CH
3
O
..
..
C
Two esters stabilized
by ester resonance
Only one ester stabilized
by ester resonance
..
–
..
..
OR
..
..
..
HOR
++
As in aldol condensations of ketones, in the Claisen condensation it is the start-
ing materials that are favored thermodynamically (Fig. 19.101). Esters are stabilized
by resonance and there are two ester groups in the starting materials but only one
in the product.The result is a thermodynamic favoring of the two separated esters.
