Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

19.5 Alkylation in the Position 959
␣
19.5d Hydrolysis and Decarboxylation of -Dicarbonyl Compounds
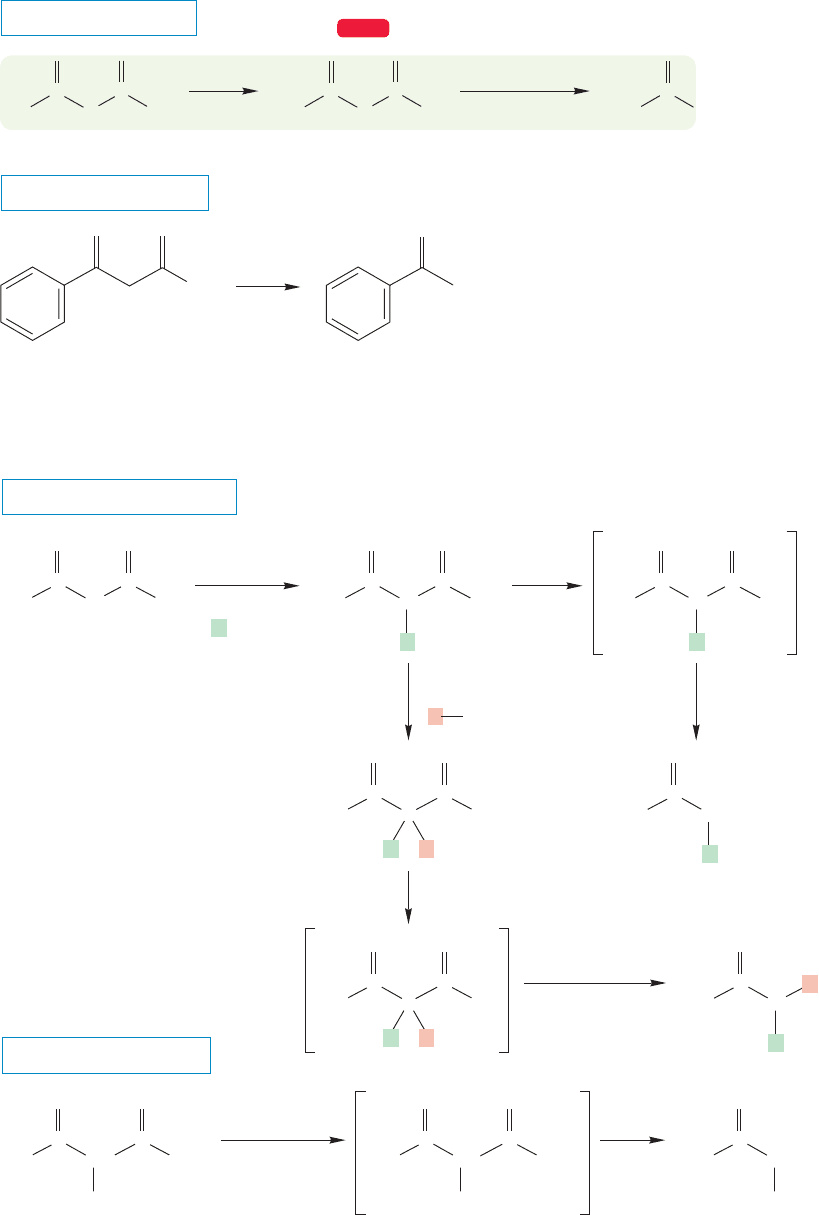
When a β-keto ester such as ethyl acetoacetate is hydrolyzed, the resulting β-keto
acid is most unstable and decarboxylates easily (Fig. 19.56). The end result is one
THE GENERAL CASE
A SPECIFIC EXAMPLE
..
..
H
3
O
..
+
H
2
O
gentle heating
50 ⬚C
O
..
..
C + CO
2
CH
3
O
..
..
+ CO
2
H
3
C
..
..
H
3
CCH
2
O
..
..
C
OR
CH
2
O
..
..
C
An acetoacetic ester
..
..
H
3
C
O
..
..
C
OH
O
..
..
C
..
..
O
..
..
OH
O
..
..
-Keto acid Acetone
WEB 3D
FIGURE 19.56 The acid-catalyzed
hydrolysis and decarboxylation of
acetoacetic esters.
molecule of a ketone and one of carbon dioxide. This reaction is a roundabout
method of making simple ketones. However, the combination of the alkylation
reactions of Figure 19.55 followed by acid or base hydrolysis and decarboxylation is
a useful source of more complex ketones (Fig. 19.57).
1. NaOH/H
2
O
25 ⬚C
25 ⬚C
2.
..
..
..
..
..
..
H
3
O
..
+
/H
2
O
..
..
+ CO
2
C
O
O
C
CH
CH
2
CH
2
CH
2
CH
3
OEt
H
3
C
..
..
..
..
..
..
C
O
O
C
CH
Not isolated (57%)
CH
2
CH
2
CH
2
CH
3
OH
H
3
C
..
..
C
O
CH
2
CH
2
CH
2
CH
2
CH
3
H
3
C
..
..
A SPECIFIC EXAMPLE
THE GENERAL PROCESS
C
O
..
..
O
..
..
C
OR
..
..
H
3
C
CH
2
C
O
..
..
O
..
..
C
OR
..
..
H
3
C
OR
..
..
C
O
..
..
O
..
..
C
R
—
R
X
RX
NaOR/HOR
NaOR/HOR
alkylation
alkylation
decarboxylation
H
3
C OH
..
..
C
O
..
..
O
..
..
C
R
H
3
C
C
O
..
..
R
R R
C
O
..
..
O
..
..
C
OH
..
..
H
3
C
R R
H
3
C
..
..
H
3
O
..
+
H
2
O
..
..
H
3
O
..
+
H
2
O
+ CO
2
C
O
..
..
R
H
3
C
+ CO
2
R
CH CH
CH
2
CH
C
C
decarboxylation
FIGURE 19.57 The decarboxylation
route to substituted ketones.
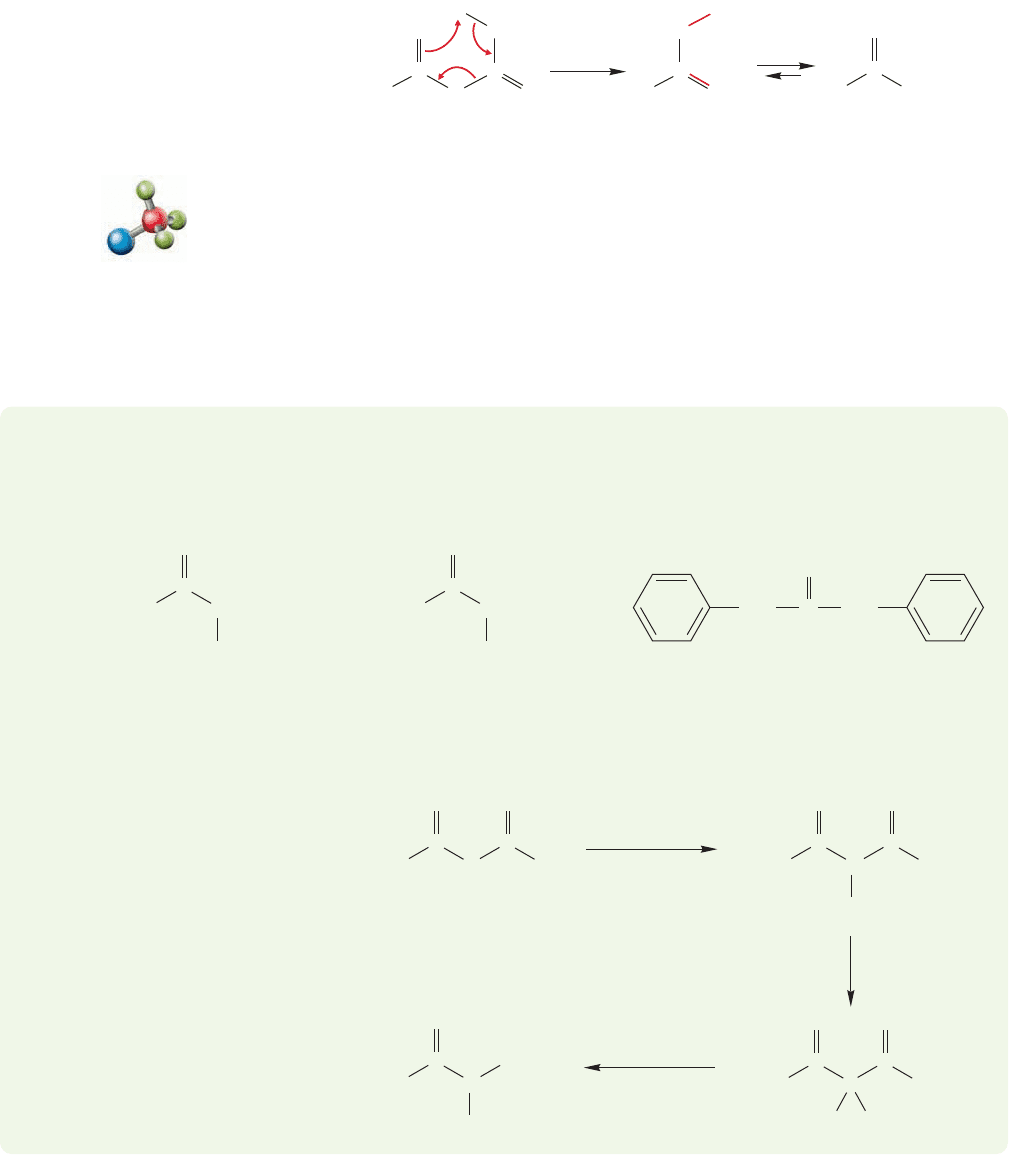
960 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
H
..
..
..
..
C
O
O
C
CH
2
–CO
2
-Keto acids
O
R
H
C
O
CH
2
Enol
R
..
.. ..
..
O
..
..
C
CH
3
R
Ketone
FIGURE 19.58 The mechanism for
the decarboxylation of β-keto acids.
(a)
O
C
CHCH
2
CH
3
CH
3
H
3
C
*(b) (c)
O
C
O
C
CHCH
2
CH
3
CH
3
CH
3
CH
2
CH
2
CH
2
In the decarboxylation reaction, the carbonyl oxygen of the keto group acts as a
base and removes the acidic hydrogen of the carboxylic acid group. Notice the nicely
organized six-membered ring transition state for this reaction (Fig. 19.58). Carbon
dioxide is lost and an enol is initially formed. Normal tautomerization of the enol
to the more stable ketone gives the final product.
Be sure to update your file cards for ketone syntheses with this sequence of reac-
tions, which is called the -keto ester or acetoacetate synthesis.This reaction is not
easy to see, as it requires a number of steps. In practice, you must dissect a potential
target molecule into its parts to determine if it is available through this route. You
must identify the R groups to be attached through alkylation, then piece together
the original β-keto ester (we will learn how to make β-keto esters in Section 19.8b).
Some examples are useful here, and as usual they will appear as problems. For
instance, see Problem 19.16.
ANSWER (b) Ethyl 3-oxopentanoate can be alkylated twice, first with methyl
iodide and then with ethyl iodide. Hydrolysis and decarboxylation give the
desired product.
OEt
O
C
C
O
C
CH
3
CH
2
H
3
CCH
2
CH
3
OEt
O
C
O
C
CH
3
CH
2
CH
2
OEt
O
C
O
C
CH
3
CH
2
CH
CH
3
O
C CH
2
CH
3
CH
3
CH
2
CH
CH
3
1. NaOEt/HOEt
1. NaOEt/HOEt
2. CH
3
CH
2
I
Ethyl 3-oxopentanoate
2. CH
3
I
H
2
O/H
3
O
+
25 ⬚C
WORKED PROBLEM 19.16 Show how the following ketones can be made from
suitable β-keto esters through the acetoacetate synthesis. Mechanisms are not
required.
Decarboxylation
19.5 Alkylation in the Position 961
␣
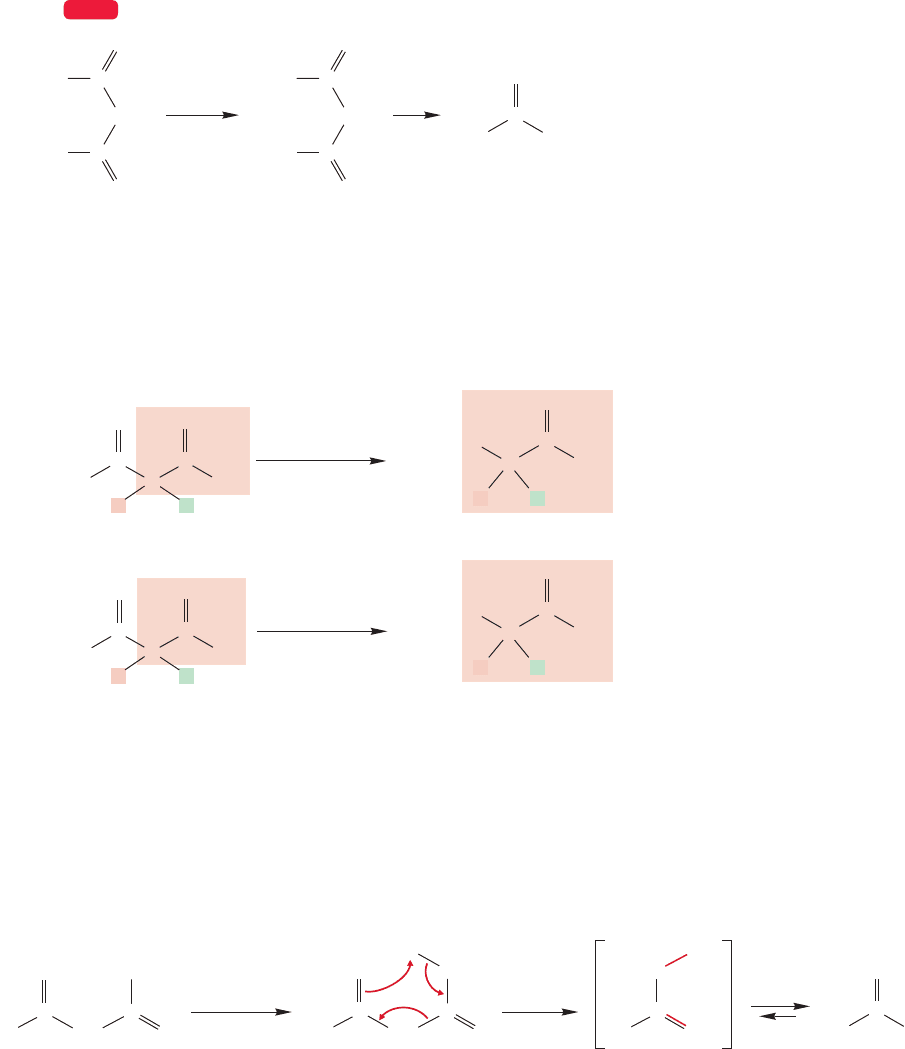
Diesters such as malonic ester can also be used in this kind of synthetic sequence.
The diester is first hydrolyzed in acid or base to the diacid,and then heated to induce
loss of carbon dioxide (Fig. 19.59). This process is known as the malonic ester
synthesis. The end products are carboxylic acids, not the ketones formed in the
related reactions of β-keto esters.
CH
2
CRO
C
Malonic ester
RO
O
..
..
O
..
..
CH
2
CHO
C
Malonic acid
HO
O
..
..
O
..
..
..
HO
..
..
..
..
..
..
..
..
..
..
..
H
3
O
..
+
H
2
O
O
..
..
C + CO
2
CH
3
Acetic acid
WEB 3D
FIGURE 19.59 The formation
of acetic acid from malonic acid.
Note an intuitive point here. The β-keto esters, which contain a ketone, are the
sources of ketones after decarboxylation, and the diesters, which contain only ester
carbonyls, are the source of carboxylic acids after decarboxylation (Fig. 19.60).
1. H
3
O /H
2
O
2.
+
CH
3
O
O
CH
3
C
H
RO
O
C
C
R
R
R
R
O
C
Ketone
O
C
OH
C
H
R
R
Acid
Ketone
+
1. H
3
O /H
2
O
2.
+
CO
2
CO
2
OR
RO
O
C
O
C
Ester
+
C
R
R
FIGURE 19.60 Hydrolysis and
decarboxylation of acetoacetic esters
gives substituted ketones, whereas the
same sequence applied to malonic
esters gives substituted acids.
..
..
..
C
O
OR
C
CH
2
O
RO
..
..
..
..
..
H
..
..
..
C
O
O
C
CH
2
Malonic acids
–CO
2
hydrolysis
O
..
..
HO
..
..
..
..
..
H
C
O
CH
2
Enol
O
..
..
C
CH
3
Acid
HO
..
..
HO
..
..
FIGURE 19.61 Hydrolysis and decarboxylation of a diester is shown.The mechanism of decarboxylation has a
six-membered ring transition state.The initially formed enol is not isolable. It undergoes keto–enol
tautomerization to give the carboxylic acid.
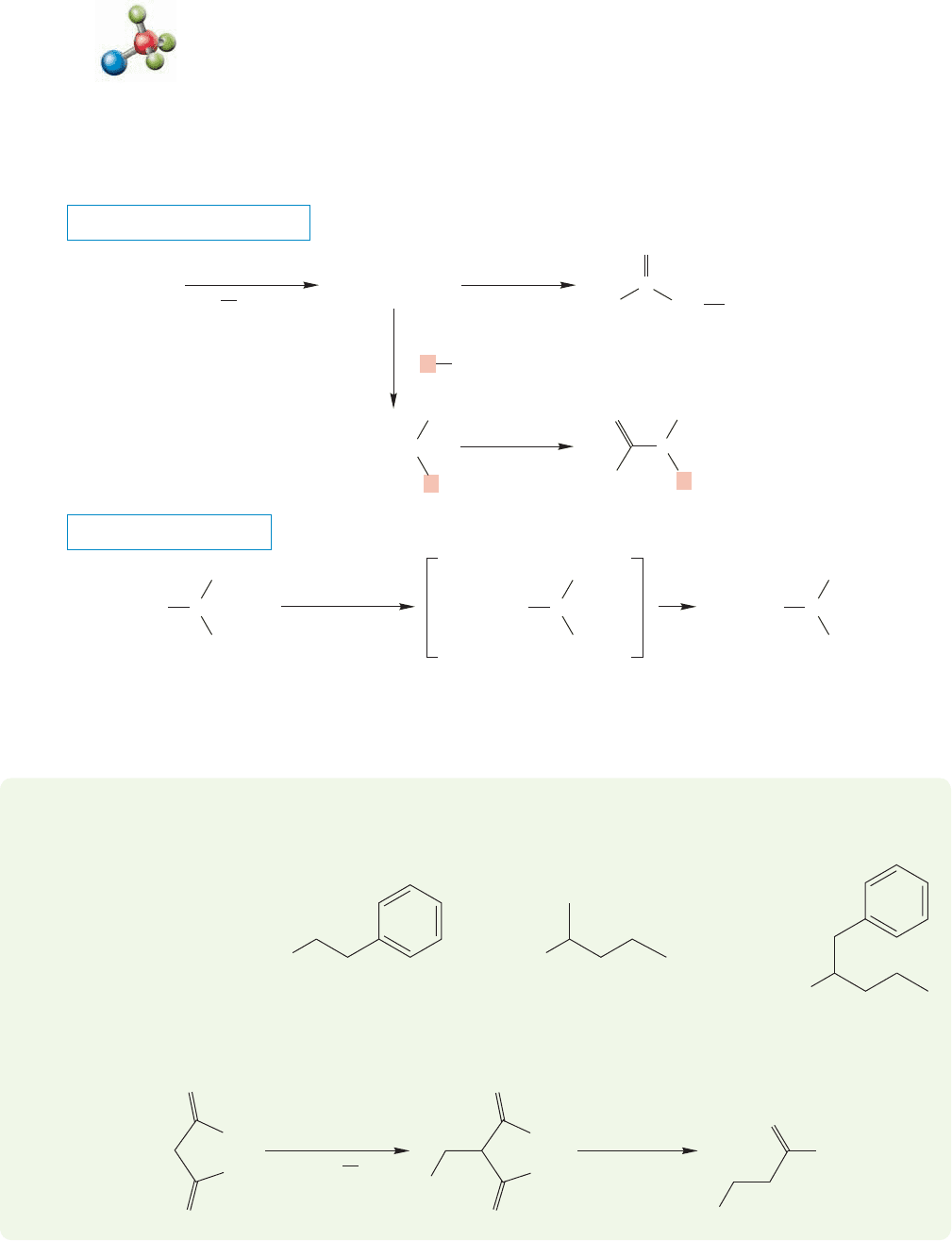
Here is how the reaction works.The hydrolysis converts the diester into a diacid
(Fig. 19.61). These compounds are similar to β-keto acids and will decarboxylate
on heating to give the enols of acetic acids. Notice again the six-membered ring
in the transition state for this decarboxylation. The enols are in equilibrium
with the more stable carbonyl compounds, which are the isolated carboxylic acid
products.
962 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
THE GENERAL SEQUENCE
A SPECIFIC EXAMPLE
–
..
..
..
(EtOOC)
2
CH
2
(EtOOC)
2
CH CH
CH
3
CH
3
CH
2
CH
3
Et = CH
2
CH
3
1. EtO Na
+
2. R X (S
N
2)
(EtOOC)
2
CHR
(EtOOC)
2
C
1. hydrolysis
2.
O
..
..
100 ⬚C, 5 h
1. KOH/H
2
O
2. HCl, 15 ⬚C
–
..
..
..
1. EtO Na
+
2. R X (S
N
2)
CHHOOCCH
2
CH
3
CH
2
CH
3
(HOOC)
2
CH CH
Not isolated (64%)
CH
2
CH
3
C
CH
2
R
R
R
R
R
HO
..
..
1. hydrolysis
2.
O
..
..
CH
HO
..
..
+ CO
2
FIGURE 19.62 The alkylation–decarboxylation sequence for the synthesis of substituted acetic acids from diethyl
malonate.
When this reaction is carried out on the parent diethyl malonate, acetic acid
results, and the process is of no practical synthetic utility. As with the synthesis of
ketones starting from β-keto esters, this process is far more useful when combined
with the alkylation reaction. One or two alkylations of malonic ester give substituted
diesters that can be hydrolyzed and decarboxylated to give carboxylic acids (Fig.19.62).
Substituted acetic acids are potential sources of esters, acid halides, and any other
compound that can be made from a carboxylic acid.
*(a)
HOOC
(c)
(b)
HOOC
CH
3
CH
2
OOC
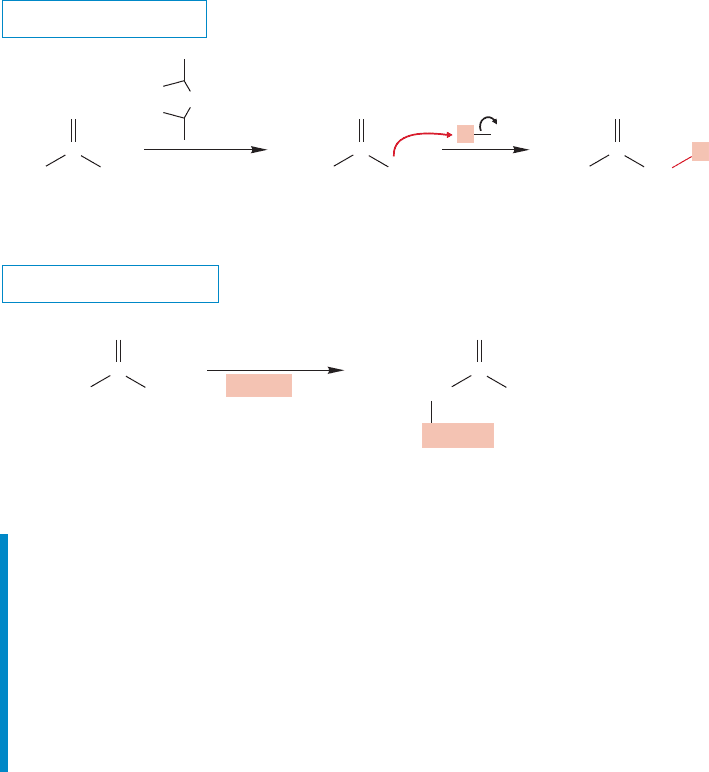
WORKED PROBLEM 19.17 Show how the following molecules can be made through
a malonic ester synthesis.
ANSWER (a) Diethyl malonate is alkylated, hydrolyzed, and decarboxylated.
O
O
OEt
OEt
+
CO
2
O
O
OEt
OEt
1. NaOEt
(one equiv.)
HOEt
2. PhCH
2
I
Ph
OH
1. H
2
O/H
3
O
+
2. Δ
O
Ph
Malonate alkylation
19.5 Alkylation in the Position 963
␣
19.5e Direct Alkylation of Esters It is possible to alkylate esters directly and
thereby avoid the extra steps of the malonic ester or acetoacetate synthesis. Very
strong bases such as LDA can be used to remove the α hydrogens of esters
(Fig. 19.63). When esters are added to these bases at low temperature, an α hydro-
gen is removed to give the enolate anion. This anion can then be alkylated by any
alkyl halide able to participate in the S
N
2 reaction. The end result is an alkylated
ester. This method is usually preferred for small-scale, research laboratory synthe-
ses.The large-scale, industrial syntheses are more likely to use a β-keto ester or mal-
onic ester synthesis, because these methods employ less expensive reagents and can
be performed using milder conditions.
THE GENERAL CASE
A SPECIFIC EXAMPLE
–78 ⬚C
–
–
–
O
..
..
..
..
C
NLi
+
CH
3
CH
3
O
..
..
O
..
..
C
OCH
3
1. LDA, –78 ⬚C
THF, 30 min
2. CH
3
CH
2
Br
–78 ⬚C
CH
3
CH
2
CH
2
..
..
O
..
..
C
(92%)
OCH
3
CH
3
CH
2
CH
CH
2
CH
3
..
..
O
..
..
C
R
LDA
R
X
(S
N
2)
CH
2
CH
3
O
..
..
..
O
..
..
..
C
+ X
CH
2
CH
3
O
..
..
FIGURE 19.63 The low-temperature
alkylation of an ester using LDA as
base.
Summary
This section gives you new ways of making complex molecules by alkylating
ketones,dicarbonyl compounds,and esters.One can efficiently add an alkyl group
to a β-dicarbonyl compound, which can then be converted into a ketone or car-
boxylic acid through the acetoacetate or malonic ester syntheses. Although these
routes are somewhat roundabout—the acetoacetic ester or malonic ester must be
made, alkylated, and finally decarboxylated—in a practical setting they are effi-
cient and easily performed. A more direct method for alkylating esters involves
using LDA at low temperatures.
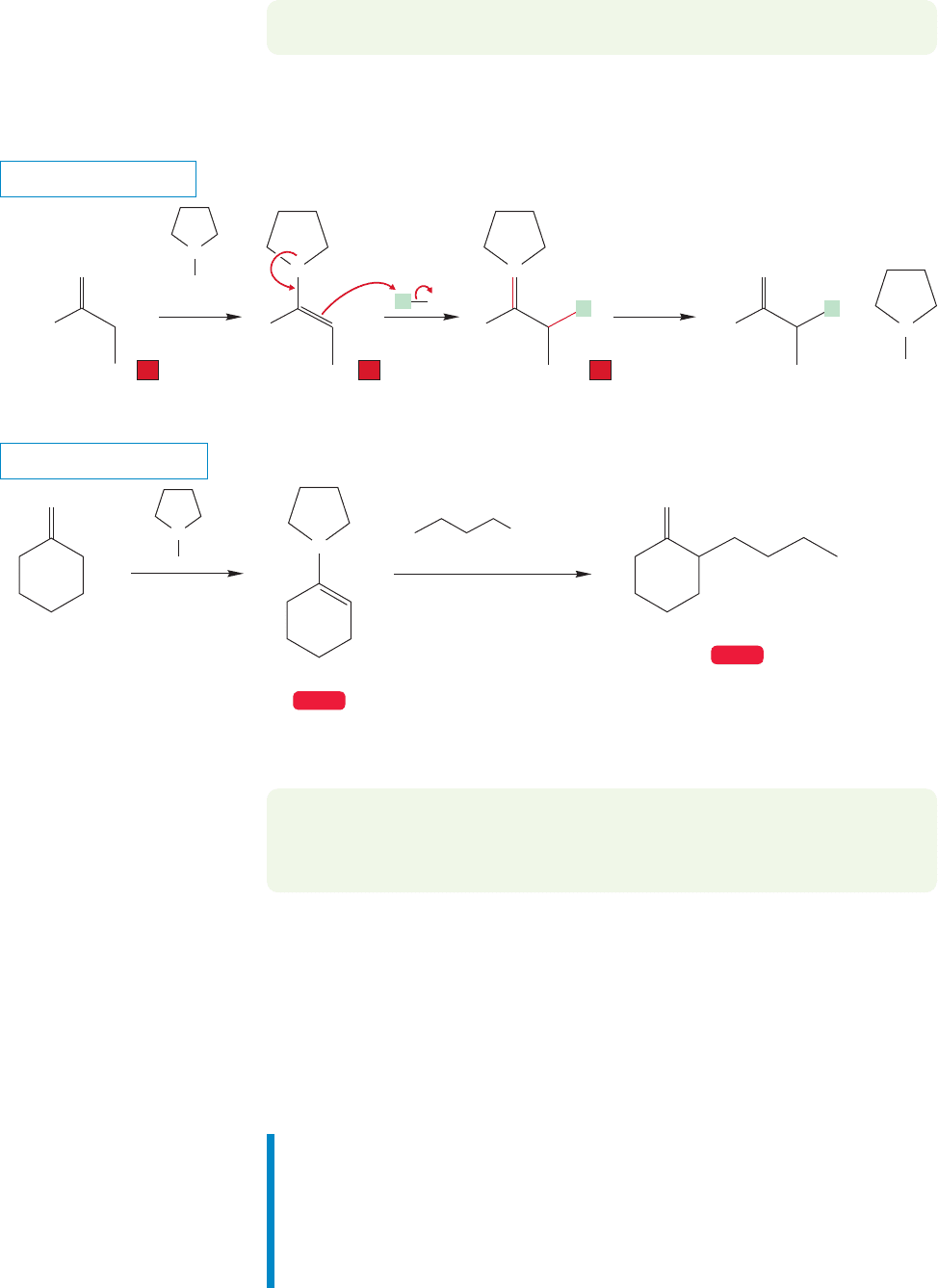
19.5f Alkylation of Enamines Recall that α alkylation of ketones or
aldehydes using hydroxide base resulted in multiple products. Perhaps not
surprisingly, a way around this problem has been found, and it uses enamines
(p. 795) as substitutes for carbonyl compounds. Remember that enamines can be
formed by the addition of secondary amines (not primary or tertiary amines) to
carbonyl compounds. Pyrrolidine is a secondary amine that is often used for
this reaction.
964 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
PROBLEM 19.19 Write the mechanism for the hydrolysis of the iminium ion to
the ketone. Hint: You have seen this reaction before, in reverse, in the mechanism
for acid-catalyzed enamine formation (p. 795).
+
IR
78 ⬚C, 5–8 h
benzene
(~85%)
(57%)
..
..
..
..
..
toluene, 110 ⬚C, 19 h
N
H
..
N
..
N
R
O
..
..
O
R
N
H
S
N
2
H
2
O
+
H
..
..
..
N
..
N
O
..
..
O
I1.
2. 10% H
2
SO
4
/H
2
O
..
..
..
Enamine
formation
1
Hydrolysis
3
Alkylation
2
R R
RR
THE GENERAL CASE
A SPECIFIC EXAMPLE
Pyrrolidine
WEB 3D
WEB 3D
FIGURE 19.64 Alkylation of an enamine initially gives an iminium ion.The iminium ion can be hydrolyzed to give the
alkylated carbonyl compound.
PROBLEM 19.18 Why can’t primary or tertiary amines be used to make enamines?
There are still some problems, however. It is not easy to use alkylations of enamines
made from ketones where more than one possible enamine can be formed. If the two
α positions are similar, then the double bond of the enamine can be formed in either
direction and α alkylation will take place on both sides. There are occasionally difficul-
ties with overalkylation or substitution on nitrogen.If the two α positions of the ketone
are different, then the alkene of the enamine can sometimes be formed selectively on
the more hindered side.
Summary
We have observed several bases employed in the formation of enolates, which can
be alkylated in S
N
2 fashion. A useful alternative involves reactions of enamines.
Figure 19.64 shows the overall procedure: The ketone or aldehyde is first con-
verted into the enamine, the enamine is alkylated, and then the ketone or alde-
hyde is regenerated by hydrolysis.
Enamines are electron-rich and nucleophilic and will attack S
N
2-active halides
(no tertiary halides!) to form iminium ions. The reaction is ended by adding water
to hydrolyze the iminium ions to the corresponding ketone or aldehyde (Fig. 19.64).
19.6 Addition of Carbonyl Compounds to the Position: The Aldol Condensation 965
␣
19.6 Addition of Carbonyl Compounds to the ␣
Position: The Aldol Condensation
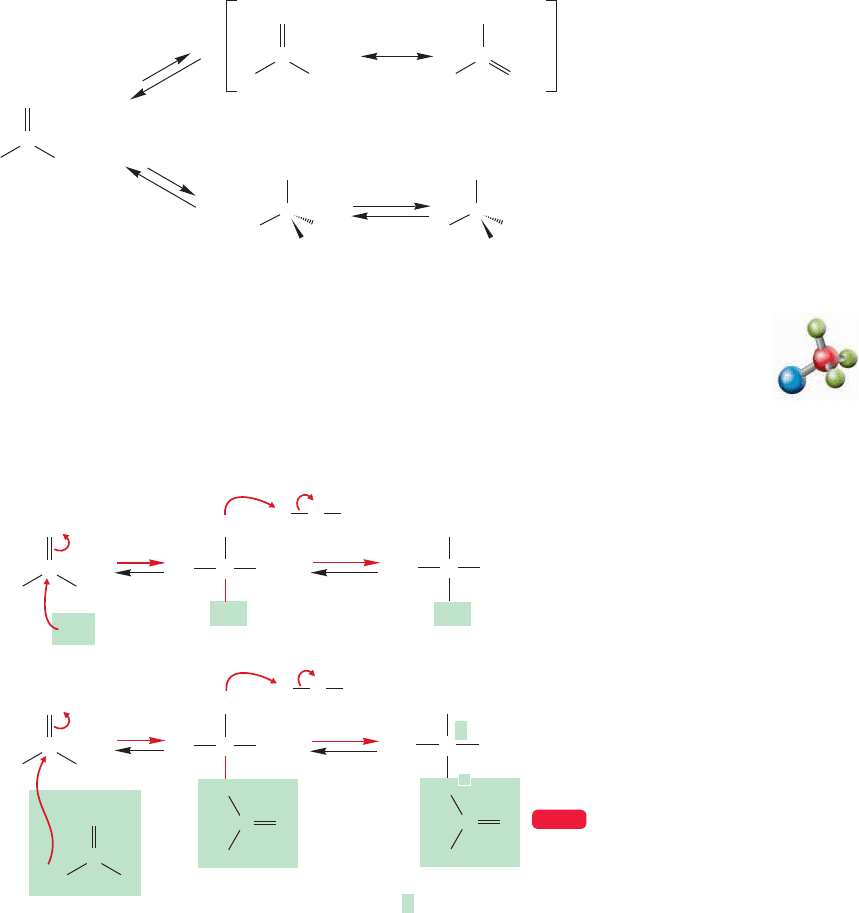
So far, we have seen the reactions of enolates and enols with a variety of electrophiles,
for example, D
2
O, H
2
O, Br
2
,Cl
2
,I
2
, and alkyl halides. What happens if these acids are
absent or, as is the case for water,simply regenerate starting material? Suppose, for exam-
ple, we treat acetaldehyde with KOH/H
2
O (Fig. 19.65). First of all, the answer to the
question of what happens when a base and a carbonyl compound are combined is almost
never, “nothing.” Nucleophiles add to carbonyl groups, and hydroxide will surely initi-
ate hydrate formation.The hydrate is usually not isolable, but it is formed in equilibri-
um with the aldehyde. Moreover, enolate formation is also possible and the enolate, as
well as the hydrate, will be in equilibrium with the starting aldehyde. Although hydrate
formation does not lead directly to an isolable product, formation of the enolate does.
Be very careful about “nothing happens” answers. They are almost always wrong.
O
..
..
..
C
O
HCH
3
..
..
C
O
HHCH
2
..
..
..
C
Enolate formation
CH
2
..
..
C
O
H
CH
3
H
2
O
OH
..
..
..
..
..
H
2
O
..
..
Hydrate formation
–
..
C
OH
H
CH
3
OH
..
..
HO+
..
..
..
..
–
HO
H
2
O
..
..
–
–
–
–
..
..
..
HOO
..
..
..
FIGURE 19.65 Two reactions of
acetaldehyde with hydroxide ion:
addition (hydrate formation) and
enolate formation.
19.6a Ketones and Aldehydes React with Ketone or Aldehyde Enolates
In the solution of acetaldehyde and KOH/H
2
O there is another electrophile in addi-
tion to water, the carbonyl group of acetaldehyde. Nucleophiles add to carbonyl
groups, and the enolate anion is surely a nucleophile. There is no essential difference
between the addition of hydroxide to the electrophilic carbonyl group of acetaldehyde
and the addition of the enolate anion (Fig. 19.66). Nor is there much structural
..
..
CCCH
3
H
O
..
..
O
HCH
3
..
OH
..
..
..
..
OH
..
–
–
..
..
CCCH
3
H
O
..
..
O
HCH
3
..
..
C
O
H
CH
2
..
..
..
..
(–)
CO
H
H
2
C
–
–
β
Aldol
(a
β-hydroxy aldehyde)
CCH
3
H
..
OH
..
..
OH
Hydrate
..
C
+
+
CH
3
H
..
OH
..
..
..
CO
H
H
2
C
OH
..
..
..
–
OH
..
..
..
–
HH
..
O
..
HH
..
O
..
α
WEB 3D
FIGURE 19.66 Addition of hydroxide
and the enolate anion to the carbonyl
group are simply two examples of
the reaction of nucleophiles with
electrophilic carbonyl groups. In
the second step, protonation gives the
hydrate in the top sequence, or aldol
in the lower sequence.
Aldol condensation
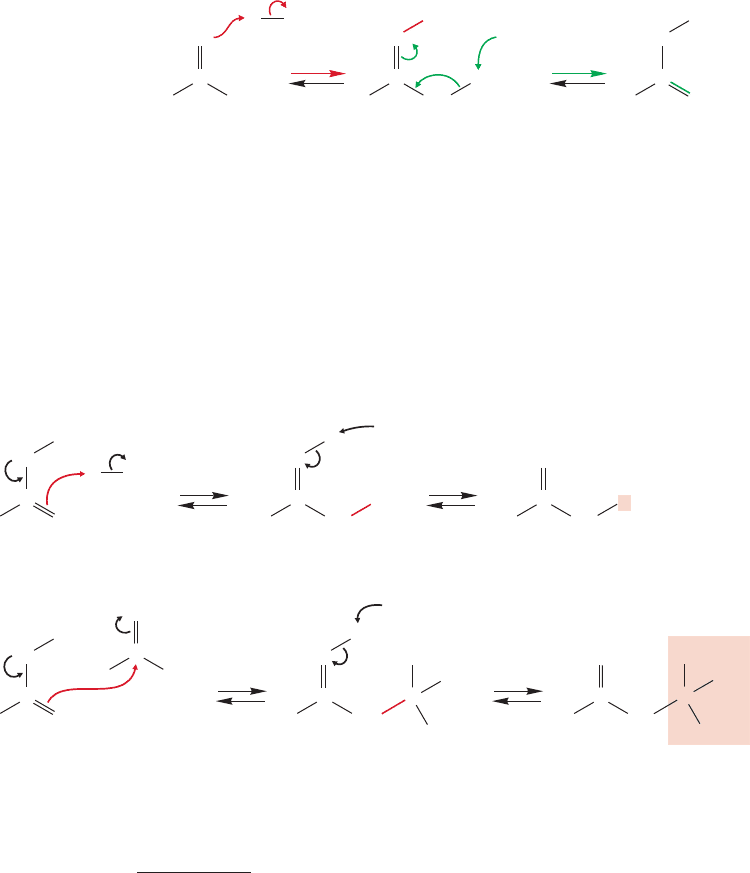
difference between the hydrate formed by protonation of the hydroxide addition prod-
uct, and the molecule known as aldol (ald because of the aldehyde and ol because of
the alcohol) formed by protonation of the enolate addition product.Note that hydrox-
ide ion, the catalyst, is regenerated in the final step of both hydrate formation or aldol
formation. But the aldol is surely more complex than the relatively simple hydrate!
The conversion of an aldehyde or ketone into a β-hydroxy carbonyl compound is
called the aldol condensation or aldol reaction
2
after the product of the reaction of
acetaldehyde, and is quite general for carbonyl compounds with α hydrogens.
As usual,this base-catalyzed reaction has its acid-catalyzed counterpart.For the acid-
catalyzed reaction the catalyst is not hydroxide, but H
3
O
, and the active ingredient is
not the enolate anion, but the enol itself.The first step in the reaction is acid-catalyzed
enol formation, the keto–enol tautomerization we have come to know (Fig. 19.67).
966 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
2
Credit for discovery of the aldol reaction generally goes to Charles Adolphe Wurtz (1817–1884), who coined
the word “aldol” in 1872. However, equal billing at least should also go to Alexander Porfir’yevich Borodin, the
Russian composer–physician–chemist (1833–1887).Borodin was the illegitimate son of Prince Gedianov,and actu-
ally spent his formative years as his father’s serf.Happily, he was given his freedom and became an eminent chemist
who is far better known for the products of his avocation, music, than for his excellent chemistry. Borodin also
discovered the Hunsdiecker reaction (p. 861).
+
HOH
2
..
..
..
..
C
O
HCH
3
..
C
O
H
H
H
CH
2
..
OH
2
..
..
..
+
+
+
CH
3
O
Enol
O
H
H
CH
2
FIGURE 19.67 The acid-catalyzed
aldol condensation begins with
enol formation.
Reaction of the enol with the protonated carbonyl to give aldol
+ H
3
O
..
+
..
..
..
C
O
H
H
CH
2
..
C
O
H
H
CH
2
CH
2
+
+
..
C
OH
HCH
3
..
C
OH
..
..
OH
2
H
CH
3
..
..
C
OH
H
CH
3
..
..
C
O
H
Aldol
Protonation/deprotonation of the enol to regenerate acetaldehyde
+
H
H
3
O
..
+
..
..
OH
2
..
+
+
C
O
H
H
CH
2
..
..
C
O
H
H
H
CH
2
CH
2
..
..
C
O
H
H
OH
2
..
FIGURE 19.68 Two reactions of the weakly nucleophilic enol with Lewis acids. In the first case, it is protonated
to regenerate acetaldehyde; in the second, the enol adds to the strongly Lewis acidic protonated carbonyl group
to give aldol.
The enol, though nucleophilic, is not nearly as strong a nucleophile as hydroxide.
However, the Lewis acid present is the protonated carbonyl, and it is a far stronger
electrophile than the carbonyl group itself.The reaction between the enol and the pro-
tonated carbonyl group is analogous to the reaction of the enol with H
3
O
(Fig.19.68).
Once again, the final step of the reaction regenerates the catalyst, this time H
3
O
.
The acid- and base-catalyzed aldol reactions of acetaldehyde give the same prod-
uct, the β-hydroxy carbonyl compound called aldol. The aldol reaction is a general
synthesis of β-hydroxy ketones and aldehydes.
19.6 Addition of Carbonyl Compounds to the Position: The Aldol Condensation 967
␣
WORKED PROBLEM 19.20 Write the products of the aldol condensations of the fol-
lowing compounds. Write both an acid- and a base-catalyzed mechanism for (b).
O
..
..
CH
3
H
3
C
H
(a)
O
..
..
*(b)
O
..
..
(c)
O
..
..
CH
3
(d)
H
..
..
O
OH
..
..
..
–
–
..
..
O
OH
..
..
..
–
OHH
..
..
..
..
O
..
..
O
..
..
..
O
..
–
..
..
..
–
..
..
O
..
..
H
O
O
+
..
HO
..
..
+
OH
..
..
HO
..
O
+
..
..
..
HO
..
O
..
..
HO
..
..
O
H
+
..
..
H
2
O
+
H
3
O
..
+
H
ANSWER (b) As usual, in base the enolate is formed first and then adds to the car-
bonyl group of another molecule. A final protonation generates the condensation
product and regenerates the catalyst, here hydroxide.
Watch out! In these and many other figures, arrows emanate from one of a pair
of resonance forms, which is done for simplicity’s sake only. You should be able to
write the arrow formalism using the other resonance form.
In acid, the protonated carbonyl group reacts with the enol form of the ketone
to give a resonance-stabilized intermediate. Deprotonation gives the product and
regenerates the acid catalyst, H
3
O
.
968 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
In acid, a second reaction, the dehydration of the β-hydroxy carbonyl compound to
the α,β-unsaturated aldehyde or ketone is very common, and it is generally the dehy-
drated products that are isolated (Fig. 19.69). In the overall reaction,two carbonyl com-
pounds condense to produce a larger carbonyl compound and give off a small molecule—
water in this case. Thus, the aldol reaction is often called the aldol condensation.
..
H
3
O
..
+
H
3
O +
..
+
+
O
..
OH
..
..
CH
2
CH
3
H
C
A
ldol, a β-hydroxy aldehyde An α,β-unsaturated aldehyde
CH
O
..
..
OH
2
..
CH
H
CH
3
H
C
CH
O
..
H
..
C
H
CH
3
H
C
C
α
β
α
β
..
..
H
2
O
..
..
H
2
O
FIGURE 19.69 One possible
mechanism for the acid-catalyzed
dehydration of an aldol.
Let’s consider the mechanism for the loss of water. Hydroxide is a poor leaving
group.Therefore,dehydration is more difficult in base and the β-hydroxy compounds
can often be isolated. However, even under basic conditions dehydration can be
achieved at relatively high temperature,forming the conjugated carbonyl compound.
PROBLEM SOLVING
Success in solving what are often called “fun in base” problems has a lot to do
with recognition, of seeing the clues that are present in almost every problem.
We will have more to say on this subject later, but for now remember that “all”
β-hydroxy aldehydes and ketones and “all” α,β-unsaturated aldehydes and
ketones come from aldol condensations. The quotes around “all” are there just
to remind you that there will inevitably be exceptions.
-Hydroxy aldehydes and ketones
β
α
RR
O
HR
O
RH
3
C
OOH
,-Unsaturated
aldehydes and ketones
β
α
RR
O
HR
O
RH
3
C
O
RR
OOH
,-Unsaturated aldehydes and ketones
β
α
RR
O
-Hydroxy aldehydes and ketones
β
α
RR
OOH
However, when you are dealing with a condensation problem, one of the
first things you should do is scan for β-hydroxy aldehydes and ketones, and
α,β-unsaturated aldehydes and ketones. If you find one, and you will very
often, you can be almost certain that an aldol condensation is involved.
A second point is to remember that in the aldol condensation that forms
these compounds, it is the α,β bond that is made.
