Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

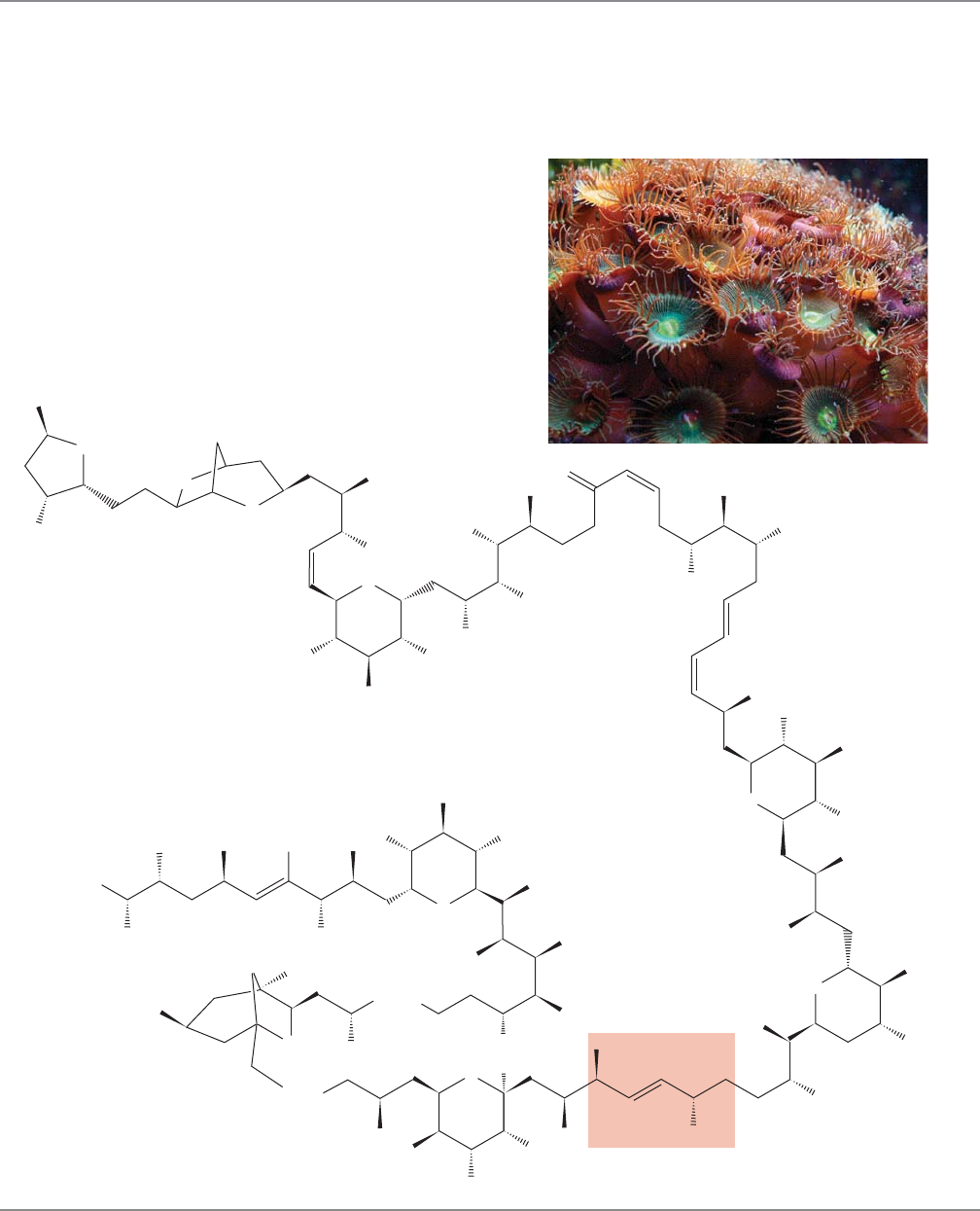
19.15 The Aldol Condensation in the Real World, an Introduction to Modern Synthesis 1009
Molecules found in Nature are sometimes quite spectacularly complicated. Yet we
are now able to modify the simple reactions we have been describing so as to be able
to make breathtakingly complicated molecules, such as the example that follows.
PALYTOXIN
Professor Yoshito Kishi (b. 1937) of Harvard University and
his co-workers set out to make a molecule called palytoxin, a
compound of the formula C
129
H
223
N
3
O
54
, found in a coral
resident in a small tidal pool on the island of Maui in Hawaii.
A derivative of this extraordinarily toxic molecule, palytoxin
carboxylic acid, is shown here. Palytoxin is difficult to make,
not so much because of its size, but because of its stereochem-
ical complexity and delicacy. It contains no fewer than 61
stereogenic atoms, each of which must be generated specifical-
ly in order to produce the real palytoxin.
Several aldol-related reactions were necessary in Kishi’s
spectacular synthesis. For example, one of the last critical
reactions in making palytoxin was a variation of the aldol
CH
3
H
3
C
O
O
H
2
NCH
2
HO
OH
OH
OH
OH
O
O
O
HO
OH
OH
OH
OH
OH
HO
CH
3
HO
OH OH
OH
OH
OH
OH
OH
OH
OH
HO
HO
HO
HO
HO
OH
OH
OH
OH
OH
OH
OH
OH
OH
CH
3
HO
OH
HO
O
(CH
2
)
5
CH
3
OH
OH
CH
3
CH
3
HOOC
O
O
O
O
(CH
2
)
3
condensation, a process called the Horner–Emmons
reaction, which sewed the molecule together at the red
position with an α,β-unsaturated carbonyl group that was
later reduced (stereospecifically) to give palytoxin.
1010 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
19.16 Summary
New Concepts
R
H
C
..
..
HO
R
C
..
..
HO
+
H
R
C
C
..
HO
+
H
H
C
H
R
C
..
..
O
Protonated carbonyl
Enol
acid
base
Enolate
R
H
C
..
..
..
..
O
R
H
C
C
..
..
O
–
–
R
R
R
R
R
R
H
C
H
FIGURE 19.129 Enolate formation in base and enol formation in acid
are typical reactions of carbonyl compounds bearing α hydrogens.
Y
CH
2
R
Y = H (aldehyde)
R (ketone)
OR (ester)
H
3
O
+
Br
2
if Y = H
or R
if Y = OR
D
3
O
+
....
..
..
..
..
Y
OH
R
Y
OH
+
+
Y
D
Br
..
..
..
Y
Br
OD
..
..
Y
D
O
..
..
O
..
..
O
repeat
..
..
Y
DD
O
Y
OH
..
..
OH
+
Y
Y
..
..
O
Y
Y
R
..
..
O
Y
O
..
..
CHR
..
..
OH
Y
CHR
CHR
CHR CHR
+
..
..
O
R
R
R
RR
R
R
FIGURE 19.130 Reactions of enolates and enols with various electrophiles.
This chapter deals almost exclusively with the consequences of
the acidity of a hydrogen α to a carbonyl group. This concept
is really not new, however. You have encountered the idea of
resonance stabilization of an enolate before (for example,
Problem 9.5), and you could surely have dealt with the ques-
tion of why an α hydrogen is removable in base whereas other
carbon–hydrogen bonds are not acidic.
In base, most carbonyl compounds containing α hydrogens
equilibrate with an enolate anion. In acid, it is the enol that
is formed in equilibrium with the carbonyl compound
(Fig 19.129).
Enol or enolate formation leads to exchange, halogenation,
and alkylation at the α position, as well as the more complicated
enolate additions to ketones, aldehydes, or esters. Many
of the condensation reactions are name reactions. Despite
the apparent complexity of these reactions, they all involve
fundamentally simple nucleophile plus electrophile chemistry
(Fig. 19.130).
This chapter also introduces the notion of forming an eno-
late indirectly, through Michael addition to an α,β-unsaturated
carbonyl group (Fig. 19.82).
The idea of transferring a hydride ion (H
) is elaborated in
Section 19.14. Remember: Hydride is not a good leaving group;
it cannot be displaced by a nucleophile, but it can sometimes
be transferred, providing only that a suitable acceptor Lewis
acid is available.
Y = H (aldehyde)
R (ketone)
OR (ester)
Y
CH
2
R
D
2
O
–
OD
O
..
..
O
..
..
O
..
..
..
..
Y
Y
D
Y
O
..
..
Y
DD
O
..
..
O
repeat
..
..
Y
Br
O
..
..
O
..
..
O
..
..
Y
O
repeat
Y
..
..
..
Br
..
..
..
Br
..
..
..
OH
–
OR
–
OH
–
O
..
..
..
–
O
..
..
..
–
RO
..
..
..
..
O
Y
–
..
..
O
Y
RO
Br
2
CHR
CHR
CHR
CHR
O
..
..
O
..
..
–
OR
H
2
O
R
HO
..
..
R
R
R
R
R
R
R
R
R
R
R
R
R
R
FIGURE 19.130 (continued)
19.16 Summary 1011
Key Terms
aldol condensation (p. 966)
Cannizzaro reaction (p. 1004)
Claisen condensation (p. 987)
Claisen–Schmidt condensation (p. 984)
crossed (mixed) aldol condensation
(p. 982)
crossed (mixed) Claisen condensation
(p. 993)
Dieckmann condensation (p. 992)
dithiane (p. 1001)
enolate (p. 934)
enone (p. 976)
haloform (p. 949)
haloform reaction (p. 948)
Hell–Volhard–Zelinsky (HVZ) reaction
(p. 950)
keto–enol tautomerization (p. 939)
β-keto ester or acetoacetate synthesis
(p. 960)
kinetic enolate (p. 985)
Knoevenagel condensation (p. 974)
lithium diisopropylamide (LDA) (p. 944)
Magid’s second rule (p. 999)
Magid’s third rule (p. 1007)
malonic ester synthesis (p. 961)
Mannich reaction (p. 1003)
Meerwein–Ponndorf–Verley–Oppenauer
(MPVO) equilibration (p. 1005)
Michael reaction (p. 976)
position (p. 933)
Robinson annulation (p. 998)
thermodynamic enolate (p. 1008)
Reactions, Mechanisms, and Tools
The beginning point for almost all of the reactions in this chap-
ter, from which almost everything else is derived, is enolate for-
mation in base or enol formation in acid.
The enols and enolates are capable of undergoing many reac-
tions at the α position, among them exchange, racemization,
halogenation, alkylation, addition to ketones or aldehydes, and
addition to esters. The aldol condensation involves reaction of the
enolate, a strong nucleophile, with the electrophilic carbonyl
compound, or of the less strongly nucleophilic enol with the
powerful electrophile, the protonated carbonyl.
Intramolecular aldol, crossed aldol, and the related
Knoevenagel condensations involve similar mechanisms.
Another way of forming an enolate anion is the
Michael reaction, in which a nucleophile adds to the β position
of a carbon–carbon double bond of an α,β-unsaturated carbonyl
group (Fig. 19.82).
Enolates also add to esters. The biologically important
Claisen condensation is the standard reaction of this kind.
Variations of the Claisen condensation include the Dieckmann
condensation and the reverse Claisen condensation.
1012 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
Syntheses
The new synthetic procedures of this chapter are summarized
below.
1. Acids
The Cannizzaro reaction; R may not have
an
α hydrogen
The haloform reaction; the haloform (CHX
3
) is
also formed; works for methyl compounds,
X = Cl, Br, and I
RH
O
C
RO
O
C
RCH
2
OH
KOH
H
2
O
–
+
HO
O
R
R
HO
O
HO
O
R
R
CO
2
heat
+
HO
O
HO
O
1. 2 equiv.
LDA
2. RX
3. H
3
O
+
RCH
3
CH
3
O
C
RO
O
C
HCX
3
X
2
KOH/H
2
O
–
+
C
C
C
C
C
CH
R
C
CH
2
2. Alcohols
The MPVO reaction; the mechanism involves
complex formation followed by hydride shift
R
R
O
C
RR
OH
CH
3 Acetone
Al[O CH(CH
3
)
2
]
3
+
–
See also the Cannizzaro reaction under “Acids,”
and “
β-Hydroxy Aldehydes and Ketones”
3. Alkylated Acids, Aldehydes, Esters, and Ketones
The enamine is an intermediate
R
RCH
2
O
C
R
CH
R
O
C
1. R
2
NH
2. RX
3. H
2
O
R
R
RO CH
2
O
C
RO
CH
R
O
C
1. LDA
2. RX
R
R
RCH
2
O
C
R
CH
R
O
C
1. LDA
2. RX
R
R
HO CH
2
O
C
HO
CH
R
O
CH
R
C
1. 2 equiv.
LDA
2. RX
3. H
3
O
+
R
R
O
OO
1.
–
OR
2. RX
R
O
OO
R cannot be tertiary
C C C C
H
3
C H
3
CCH
2
A variety of reactions involving hydride shifts is described.The
Cannizzaro reaction is the most famous of these and involves, as
do all hydride shift reactions, a simultaneous oxidation of the
hydride donor and reduction of the hydride acceptor (Fig. 19.122).
Enamines, formed through reaction of carbonyl groups with
secondary amines, can be used to alkylate α positions. The
carbonyl group can be regenerated through hydrolysis of the
iminium ion product (Fig. 19.64).
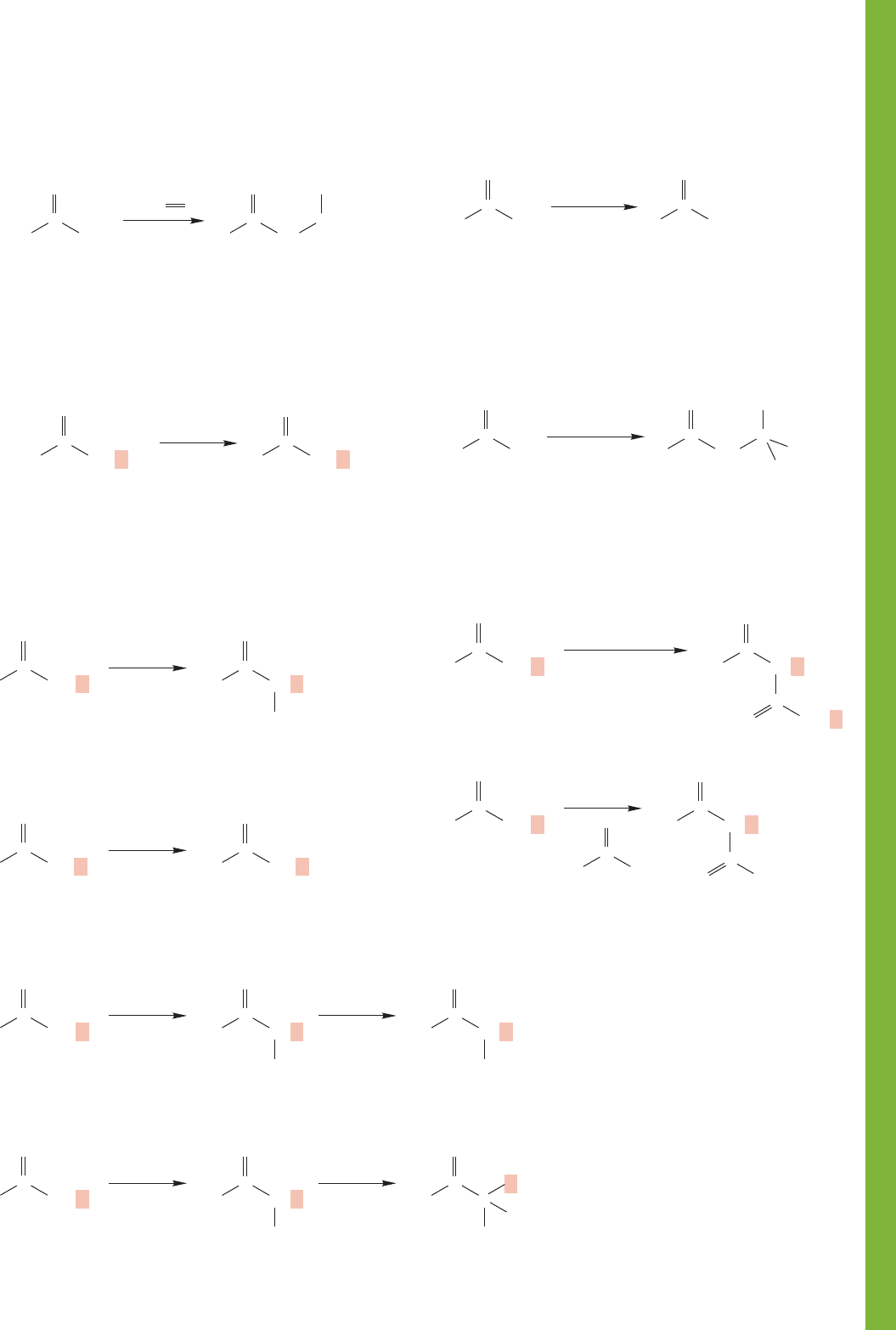
19.16 Summary 1013
4. -Aminoketones (and aldehydes)
6. ␣-Halo Acids, Aldehydes, Ketones, and Esters
In acid, the reaction stops after one halogenation;
X = Cl, Br, and I
In base, up to three
α hydrogens can be
replaced by X; the trihalo carbonyl compounds
react further in the haloform reaction
R R
RCH
2
O
C
RCH
X
O
C
H
3
O
X
2
+
Hell–Volhardt–Zelinsky (HVZ) reaction
R R
HO CH
2
O
C
Br CH
Br
O
C
Br
2
PBr
3
R
HO CH
Br
O
C
H
2
O
R R
RO CH
2
O
C
RO CH
Br
O
C
RO
–
Br
2
RO C
Br
B
r
O
C
RR
RCH
2
O
C
RCX
2
O
C
base
X
2
R
5. Deuterated Aldehydes and Ketones
Mannich reaction; there is an intermediate iminium
ion; of course this is also an amine synthesis
H
3
CCH
3
O
C
H
3
C
CH
2
CH
2
O
C
1. HCl/EtOH/
(CH
3
)
2
NH
CH
2
O
2. H
2
O
NaOH
N(CH
3
)
2
All α hydrogens will be exchanged
in either acid or base
R R
RCH
2
O
C
RCD
2
O
C
KOD/D
2
O
or
D
3
O
+
7. Haloforms
8. -Hydroxy Aldehydes and Ketones
9. -Keto Esters
Starting carbonyl compound must have
a methyl group; X = Cl, Br, or I
RCH
3
O
C
ROH
O
C
HCX
3
1. base, X
2
2. H
3
O
+
+
The aldol condensation; dehydration often occurs
in acid, and on occasion in base
RCH
3
O
C
R
R
C
CH
3
CH
2
OH
O
C
acid or base
Claisen condensation
R R
RO CH
2
R
CH
2
O
C
RO CH
C
O
O
C
1. full equiv. RO
–
2. H
3
O
+
3. H
3
O
+
2
Crossed Claisen condensation
R R
RO CH
2
CH
3
O
C
RO CH
3
O
C
RO CH
C
O
O
C
1. LDA
2.

1014 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
11. ␣,-Unsaturated Acids, Aldehydes, Esters, and
Ketones
Common Errors
10. Ketones
The MPVO reaction; the first step is formation of
a new aluminum alkoxide; the mechanism involves
complex formation and a hydride shift
R
R
OH
CH
RR
O
C
RR
O
C
1. AlCl
3
2.
H
3
C
CH
3
O
C
Decarboxylation of acetoacetic acid
RO
C
RR
C
O
CH
3
C
C
RH
S S
O
RC
R
CH
O
CH
3
1. NaOH
2. H
3
O
+
3. heat
1. LDA
2. RX
3. H
2
O, Hg
2+
The dehydration of the products of
aldol condensations is usually acid-catalyzed
R
OH
C
RCH
2
O
C
H
3
O
+
CH
3
CH
3
R
C
H
RC
O
C
Knoevenagel condensation
OR
C
RO CH
2
O O
OR
C
O
C
RO C
O
C
R¿R¿
C
R¿R¿
O
1. RO
–
2.
Condensation reaction problems can be daunting. At the same
time, many chemists agree that these mental exercises are fun,
and almost everyone has his or her favorites. A common error is
to set out without a plan. Don’t start on a complicated problem
without an analysis of what needs to be accomplished. Make a
map and set some goals. Ask yourself if the reaction is done in
acid or base. What connections (bonds) must be made? Are
rings opened or closed in the reaction? These are the kinds of
questions that should be asked before starting on a problem.
Two conventions, used widely in this chapter, have the poten-
tial for creating misunderstanding. In the less dangerous of the two,
charges or dots in parentheses are used to indicate the atoms shar-
ing the charge, electron, or electrons in a molecule best described
by more than one electronic description (resonance form).
The second involves drawings in which arrows of an arrow
formalism emanate from only one of several resonance forms.
Although extraordinarily convenient, this practice is dangerous,
as it carries the inevitable implication that the resonance form
used has some individual reality. It does not. What can be done
with one resonance form can always be done from all resonance
forms. For simplicity, and bookkeeping purposes, we often draw
arrow formalisms using only one of several resonance forms.
This practice can be dangerous unless we are very clear about
the shortcuts we are taking.
With the appearance of the addition–elimination process,
essentially all the major reaction types are in place.
Problems and errors become more general as complexity
increases. The most common mistake in solving problems
is a failure to analyze the problem before starting to “push
arrows.”The following Problem Solving section makes this
point again and gives an example of the kind of analysis we
are talking about.
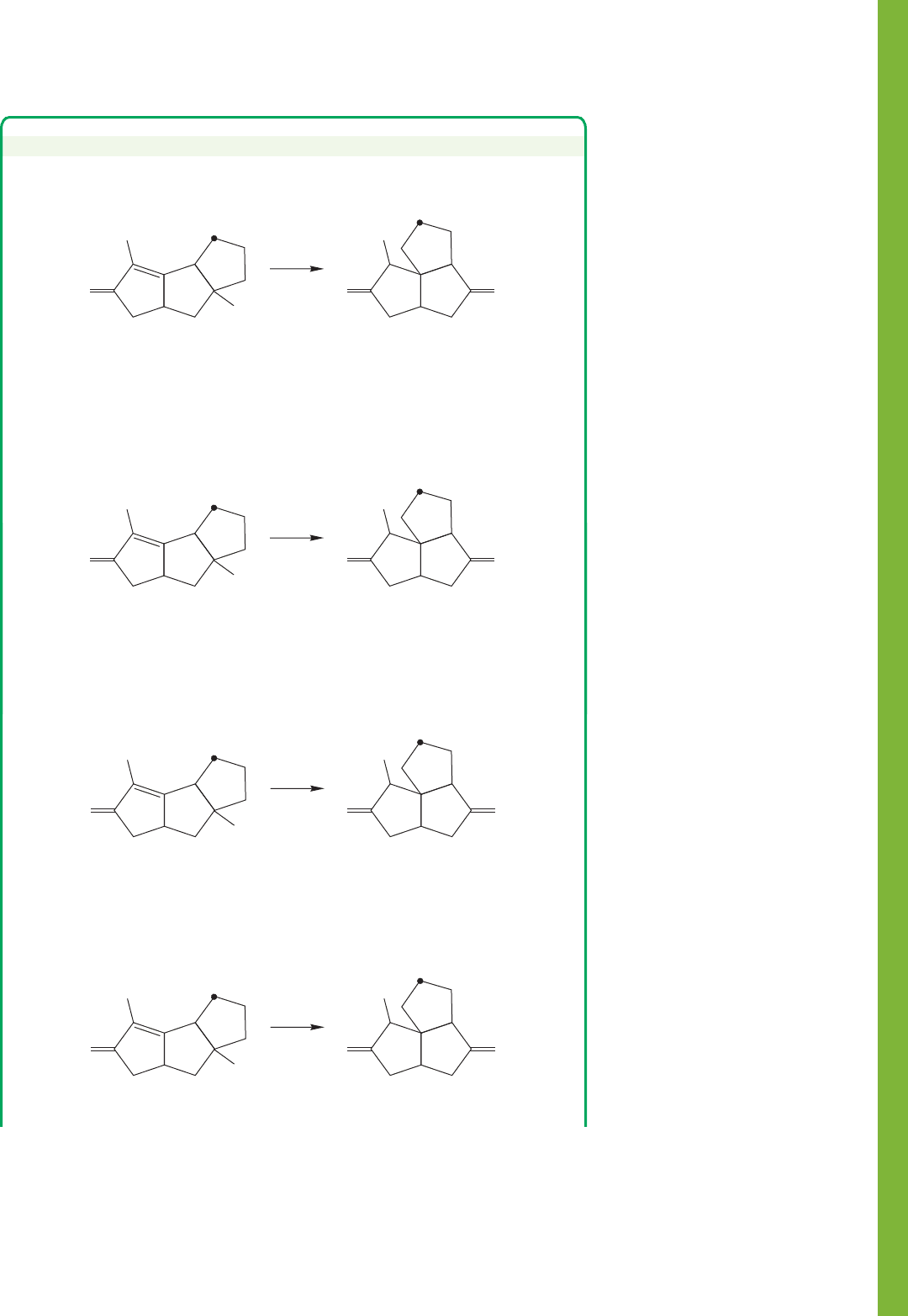
19.16 Summary 1015
RO
–
ROH
OO
H
3
C
OH
O
H
3
C
PROBLEM SOLVING
Provide a mechanism for the following transformation. Note the position of the
label (dot).
Use that methyl group to help create a map of what atoms in the starting
material become what atoms in the product. Methyl groups don’t have much
chemistry, and are usually unchanged in a reaction (unless they are adjacent to a
carbonyl group). If we label that methyl as 1, the adjacent atoms can be called 2
and 3. Note that the carbonyl group “A” on the left remains adjacent to what we
have called carbon 2.
RO
–
ROH
OO
H
3
C
OH
O
H
3
C
2
2
3
3
4
4
11
AA
If we count four atoms counterclockwise from A, we come to carbon “E”
which is attached to an oxygen. In the product, carbon 4 is still attached to
carbon 3 and is adjacent to the label (dot), but it is no longer attached to carbon
E as it is in the starting material. We immediately have a goal—we must break
the bond!
4
O
E
What bond must we make? The map tells you—we must make the
bond. How do we know that? That bond is not present in the starting material,
but it is present in the product. So now we have a second goal—make ! We
also have a third goal—oxidize carbon E from an alcohol to a carbonyl.
F
O
3
F
O
3
RO
–
ROH
OO
H
3
C
OH
O
H
3
C
2
2
3
3
4
4
11
AA
E
E
F
F
RO
–
ROH
OO
H
3
C
OH
O
H
3
C
2
2
3
3
4
4
11
AA
B
C
B
C
D
E
D
E
(continued)
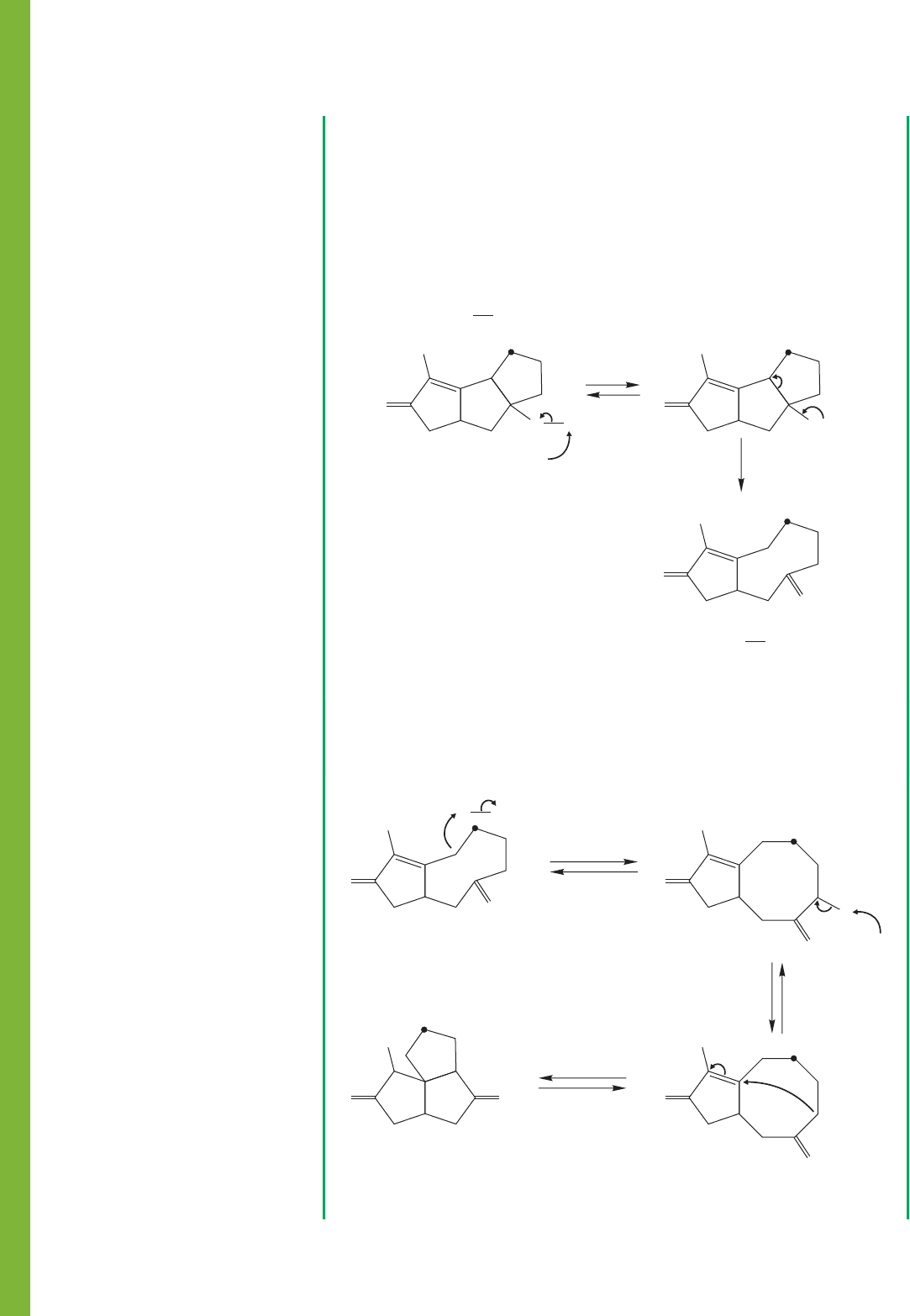
1016 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
Now we are more than halfway there. We only have to make , and a
Michael reaction easily does that.
F
O
3
O
O
O
H
3
C
(–)
(–)
(–)
(–)
–
–
4
4
3
E
A
O
H
H
3
C
F
F
E
–
HOR
protonate
and redraw
Michael
deprotonate
RO
–
O
4
3
O
H
3
C
F
E
OO
H
3
C
3
4
F
E
So now this problem has evolved from an amorphous “How do we get from
starting material to product?” to “How do we break , make , and do
the required oxidation?” That’s a much more specific set of questions. This kind
of analysis can make very difficult problems doable, and moderate problems
almost trivial. As you start to try out reactions, those that accomplish goals feel
good and can be followed up, whereas those that do not can be discarded. Here is
a set of reactions that solves this problem:
F
O
34
O
E
O
RO
–
H
O
H
3
C
3
4
A
F
O
O
H
3
C
Accomplishes two goals at
once! Breaks E 4 and forms
the new carbonyl. Note resonance
stabilization of the new anion
Goals: Break E 4, oxidize carbon E
(–)
(–)
4
E
A
F
O
–
–
O
H
3
C
4
A
EE
F
(continued)
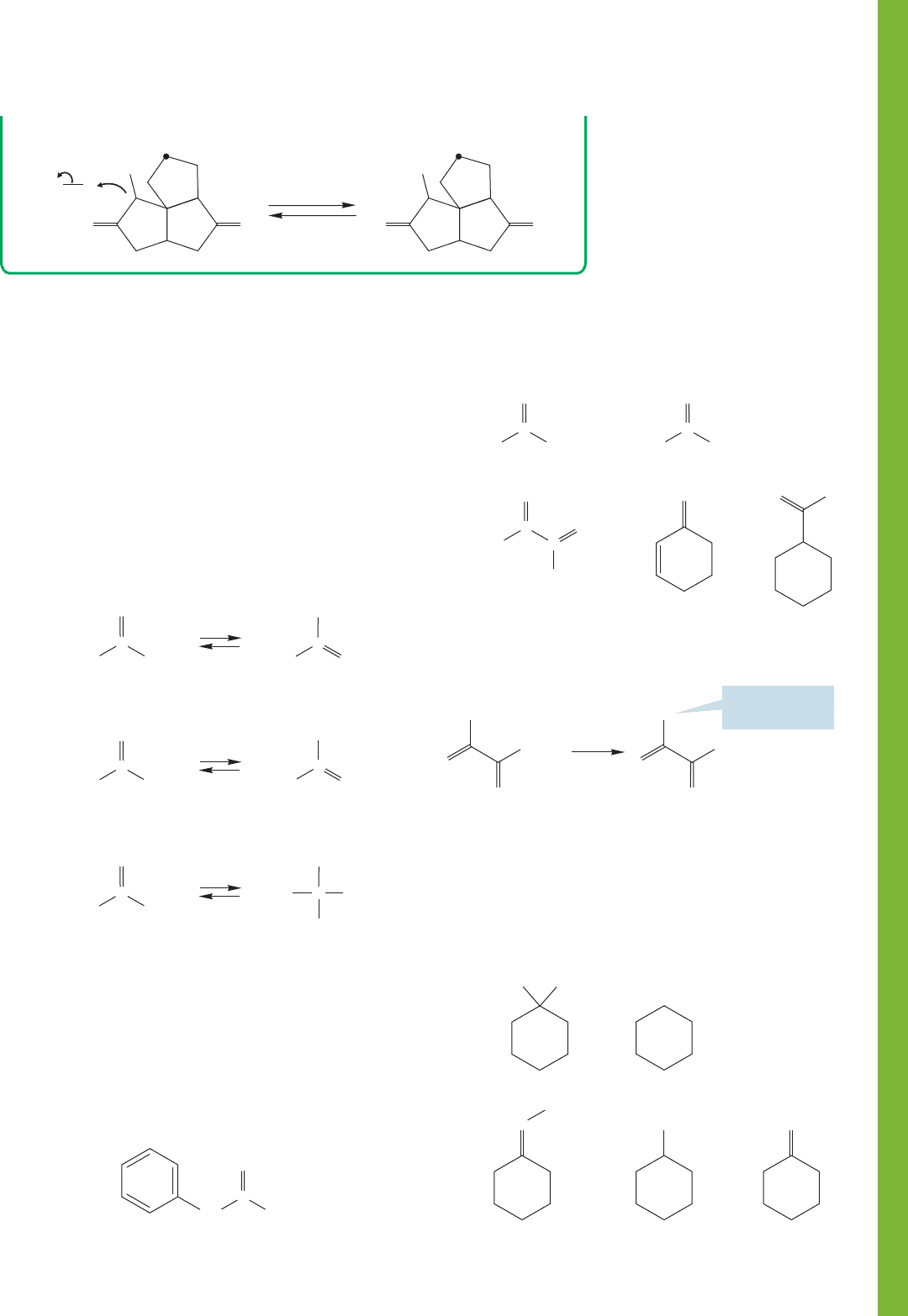
19.17 Additional Problems 1017
A final protonation completes the problem.
19.17 Additional Problems
PROBLEM 19.46 The “normal” bond length for a carbon–
oyygen single bond is 1.43 Å. In hydroxyethene, the enol form
of acetaldehyde, it is only 1.38 Å. Why is this carbon–oxygen
bond shorter than usual?
PROBLEM 19.47 Write mechanisms for the following acid-
and base-catalyzed equilibrations. There is repetition in this
drill problem, but being able to do these prototypal reac-
tions quickly and easily is an essential skill. Be certain you
can solve this problem easily before going on to more chal-
lenging ones.
(–)
–
H
RO
OO
H
3
C
OO
H
3
C
PROBLEM 19.48 The following compound contains two
different types of α hydrogens. Removal of which α hydro-
gen will yield the more stable enolate? Which α hydrogen is
likely to be removed faster? Explain why these questions
need not have the same answer. That is, why might the less
stable enolate be formed faster than the more stable enolate
and vice versa?
H
3
C
CH
3
H
3
C
C
CH
3
OH
OH
(c)
O
C
H
3
C
C
CH
3
NH
H
3
C
C
CH
2
(b)
NH
2
H
3
C
C
CH
3
O
H
3
C
C
CH
2
OH
(a)
CH
2
C
CH
3
O
PROBLEM 19.49 Which positions in the following molecules
will exchange H for D in D
2
O/DO
?
H
3
C
C
CH
2
CH
3
O
(a)
H
C
CH
2
CH
3
O
(b)
H
3
C
C
C
H
CH
2
(c)
O
(d)
O
(e)
O
H
H
3
CO OCH
3
N
(a)
(c)
(b)
NHPh
(c) (d)( (e)
OH
CH
2
PROBLEM 19.50 As we saw in Problem 19.49c, methyl vinyl
ketone exchanges the three methyl hydrogens for deuterium in
D
2
O/DO
. Why doesn’t the other formally “α”hydrogen
exchange as well?
D
2
O
DO
CH
3
O
H
CD
3
O
H
–
This H does not
exchange
PROBLEM 19.51 The proton-decoupled
13
C NMR spectrum of
2,4-pentanedione consists of six lines (δ 24.3, 30.2, 58.2, 100.3,
191.4, and 201.9 ppm), not the “expected” three lines. Explain.
PROBLEM 19.52 Provide simple synthetic routes from cyclo-
hexanone to the following compounds. Hint: These questions
are all review.
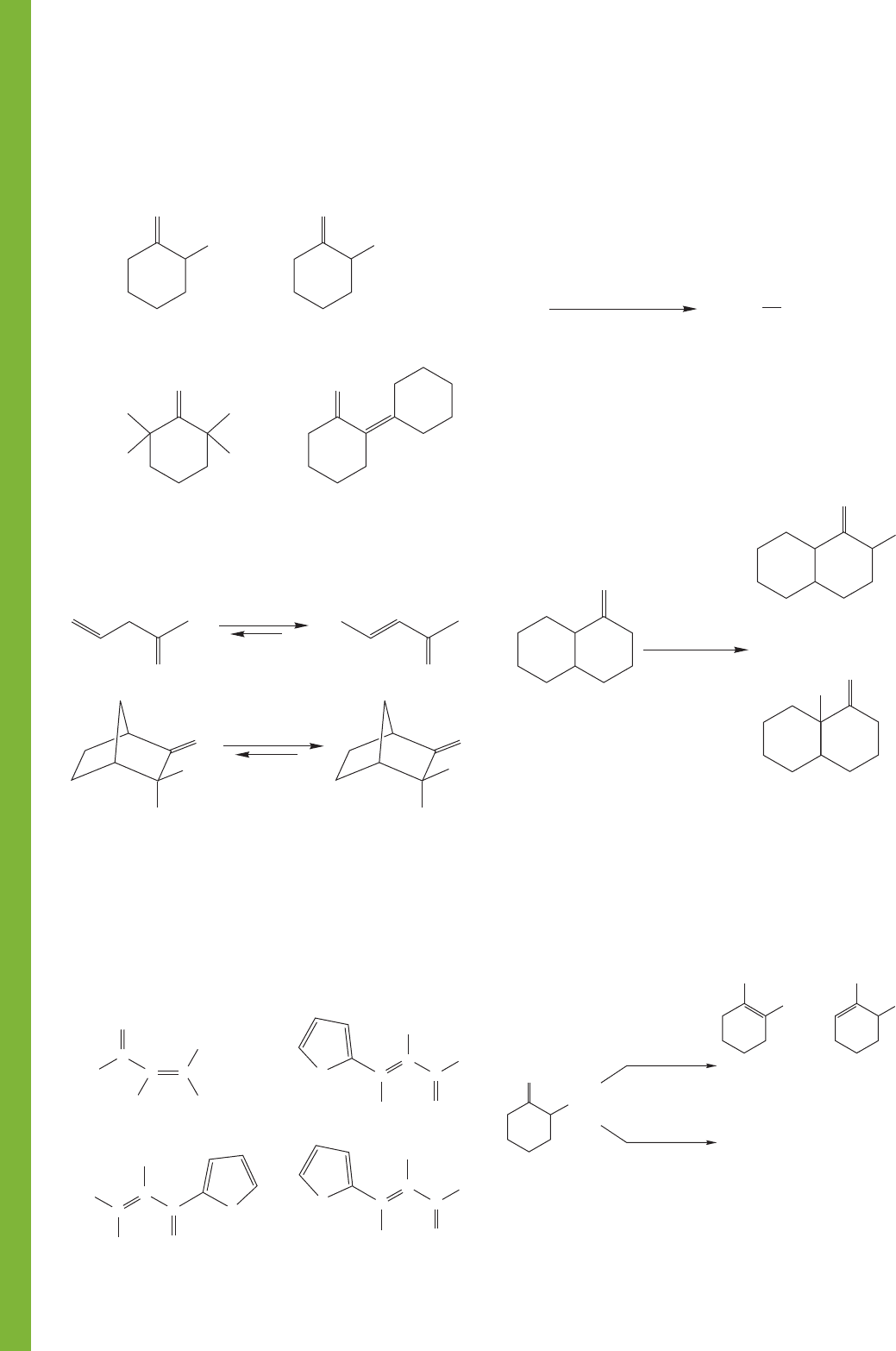
1018 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
PROBLEM 19.58 As we have seen, alkylation of enolates gen-
erally occurs at carbon (see Problem 19.57 for an example).
However, this is not always the case. First, use the following
estimated bond strengths to explain why treatment of an eno-
late with trimethylsilyl chloride leads to substitution at oxygen
( 69 kcal/mol; 109 kcal/mol).
'
Si
O
O
'
Si
O
C
H
3
C
C
C
C
CH
2
CH
3
O
CH
3
H
3
C
(a) (b)
Ph
C
C
C
H
H
O
O
(c)
Ph
C
C
H
O
O
(d)
Ph
C
C
CH
3
H
H
O
O
C
C
PROBLEM 19.55 Perform retrosynthetic analyses on the
following molecules, each of which, in principle, can be synthe-
sized by an aldol condensation–dehydration sequence. Which
syntheses will be practical? Explain why some of your suggested
routes may not work in practice. What are some of the potential
problems in each part?
PROBLEM 19.56 Treatment of compound 1 with an aqueous
solution of bromine and sodium hydroxide affords, after acidifi-
cation, pivalic acid (2) and bromoform. Deduce the structure of
1 and write an arrow formalism mechanism for its conversion
into 2 and bromoform. Use your mechanism to predict the
number of equivalents of bromine and sodium hydroxide neces-
sary for this reaction.
1. NaOH/Br
2
/H
2
O
2. H
2
O/H
3
O
1
+
CHBr
3
(CH
3
)
3
C COOH
+
2
PROBLEM 19.57 Treatment of ketone 1 with LDA in
dimethylformamide (DMF) solvent, followed by addition of
methyl iodide, leads to two methylated ketones. Write arrow
formalisms for their formations and explain why one product is
greatly favored over the other.
O
O
CH
3
2. CH
3
I
–78 ⬚C
1. LDA/DMF
(66%)
O
H
3
C
(3%)
1
+
O
(CH
3
)
3
SiCl
(CH
3
)
3
SiCl
(CH
3
CH
2
)
3
N
(78%)
OSi(CH
3
)
3
OSi(CH
3
)
3
CH
3
CH
3
(22%)
(1%) (99%)
LDA
–78 ⬚C
+
CH
3
Second, explain the different regiochemical results under
the two reaction conditions shown. Hint: Remember that eno-
late formation with LDA is irreversible.
H
2
O/HO
O
O
CH
3
CH
3
–
H
(a)
(b)
O
H
O
H
2
O/H
3
O
+
PROBLEM 19.54 Write mechanisms for the following
isomerizations:
CH
3
(a)
O
(two ways)
Br
(b)
O
(c)
O
O
D
D
D
D
(d)
PROBLEM 19.53 Provide simple synthetic routes from cyclo-
hexanone to the following compounds:
