Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

6.5 Solvents in Organic Chemistry 239
aprotic; hydrocarbons are obvious examples you know. Table 6.7 collects a few
common polar solvents and their descriptions.
TABLE 6.7 Some Common Polar Solvents
Dielectric
Compound Name Constant (ε) Description
H
2
O Water 78 Protic, polar
HCOOH Formic acid 59 Protic, polar
CH
3
OH Methyl alcohol 33 Protic, polar
CH
3
CH
2
OH Ethyl alcohol 25 Protic, polar
(CH
3
)
2
SO Dimethyl sulfoxide (DMSO) 47 Aprotic, polar
CH
3
CN Acetonitrile 38 Aprotic, polar
(CH
3
)
2
NCHO Dimethylformamide (DMF) 37 Aprotic, polar
CH
3
NO
2
Nitromethane 36 Aprotic, polar
6.5b Solubility: “Like Dissolves Like” Oil spreads on water, but very little
dissolves.Two solids, salt (the prototypal ionic solid sodium chloride) and the sugar
sucrose (C
12
H
22
O
11
, an organic material bristling with OH groups), both dissolve
completely in water (Fig. 6.24). What is going on?
Sodium chloride, Na
Cl
, dissolves in the polar solvent water because both the
positive sodium ion and the negative chloride ion are well solvated by the protic,
polar solvent water (Fig. 6.25). Nonpolar solvents cannot solvate ions efficiently and
do not dissolve sodium chloride.
Sodium chloride
Na
+–
Cl
Sucrose
CH
2
OH
HO
HO
HO
O
O
O
OH
OH
CH
2
OH
HOCH
2
FIGURE 6.24 Two very different
solids that dissolve easily in water.
(Only two
hydrogen
bonds shown)
O
HH
Na
+
Cl
–
O
H
O
HH
H
O
H
H
O
H
H
O
H
H
FIGURE 6.25 Solvation of the positive
sodium cation, and hydrogen
bonding to the negative chloride ion
(only two hydrogen bonds shown).
Protic solvents are particularly good at dissolving molecules capable of hydro-
gen bonding. Formation of hydrogen bonds in solution is highly stabilizing. Thus
sucrose, a molecule with many OH groups, dissolves easily in water. The polar sol-
vent water stabilizes sucrose both through formation of many hydrogen bonds and
through stabilization of the polarized groups of sucrose. Figure 6.26 shows
a schematic picture of hydrogen bonding (only one of many shown) and dipolar
stabilization of the polyhydroxy compound sucrose by water.
O
O
H
Sucrose
CH
2
H
O
HH
O
Sucrose
Dipole–dipole
stabilization
Hydrogen
bonding
CH
2
δ
–
δ
+
H
O
H
H
O
δ
–
δ
+
FIGURE 6.26 Solvation of an
bond by water.
O
O
H
240 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
Polar aprotic solvents can help disperse the partial charges in other polar apro-
tic molecules and thus dissolve them well. Although they cannot act as proton
donors, such solvents can align their negative ends toward the positive charges in a
polar molecule and their positive ends toward the negative charges (Fig. 6.27). For
example, Figure 6.27 shows the polar solvent THF solvating a water molecule by
aligning dipoles in a stabilizing way. Water and THF are miscible.
By contrast, hydrocarbons cannot form hydrogen bonds (they are neither proton
donors nor acceptors) and are not very polar. Typical ε values for hydrocarbons are
around 2.The more nonpolar and “greasy”the solvent,the more easily the similarly non-
polar hydrocarbon molecules dissolve in it.Thus,hydrocarbons (oil is a mixture of many
hydrocarbons) are almost completely insoluble in water,but very soluble in other hydro-
carbons.The polar molecule acetone [dimethyl ketone, (CH
3
)
2
CO],with an ε value of
21, is completely soluble in water, but the alkene isobutene, with the same number of
nonhydrogen atoms, is almost completely insoluble in water. Like does dissolve like.
H
H
O
THF
δ
–
δ
–
δ
+
δ
+
O
FIGURE 6.27 Stabilization (solvation)
of water by tetrahydrofuran, THF.
Summary
In the practical world of making chemical reactions work, we need to allow
molecules to “find each other”and react. Dissolving molecules in a fluid medium
provides an opportunity for molecules to move around and do just that—find
other reactive molecules. The choice of solvent is critical because we need to
dissolve the molecules we want to react together. In making that choice, the
rather simple notion that “like dissolves like” really works! As we work through
chemical reactions in the next few chapters, we will have to take account of
solvents and solvation.
6.6 Diols (Glycols)
Although molecules containing two OH groups are logically enough called diols,
there is an often-used common name as well, glycol.
Some 1,2-diols or 1,2-glycols are familiar and are often important molecules.
1,2-Ethanediol, or ethylene glycol, is the commonly used antifreeze, for example.
Note that in naming these compounds, the final “e”of the alkane parent compound
is not dropped, as it is in naming simple alcohols, and that the almost universally
used common name can be very misleading. Ethylene glycol implies the presence of
an alkene, and propylene glycol (1,2-propanediol) suggests a three-carbon unsaturat-
ed chain. In neither case is the implied unsaturation present (Fig. 6.28). Be aware
that the real structures are based on saturated hydrocarbon chains.
CH
2
H
2
C
HO OH
1,2-Ethanediol
or ethylene glycol
CH
CH
3
H
2
C
OH
1,2-Propanediol
or propylene glycol
HO
WEB 3D
FIGURE 6.28 1,2-Ethanediol and
1,2-propanediol are called ethylene
glycol and propylene glycol,
respectively.
6.7 Amines
6.7a Nomenclature We first saw amines in Chapter 1, albeit very briefly.
Amines are derivatives of ammonia,NH
3
(Fig. 6.29). Successive replacement
of the hydrogens of ammonia leads to primary, secondary, and tertiary amines,
NRH
2
,NR
2
H, and NR
3
. Quaternary nitrogen compounds are positively
charged and are called ammonium ions. Many systems for naming amines exist,
and the chemical world seems to be resisting attempts to bring order out of this
minichaos by keeping to the old common names.
:::
:
NH
3
NH
2
R NHR
2
A
mmonia Primary amine Secondary amine Tertiary amine Ammonium ion
NR
3
NR
4
+
FIGURE 6.29 Substituted amines.
6.7 Amines 241
Primary amines are commonly named by using the name of the substituent, R,
and appending the suffix “amine.” Secondary and tertiary amines in which the R
groups are all the same are simply named as di- and trialkylamines. Amines
bearing different R groups are named by ordering the groups alphabetically.
Ethylmethylamine is correct, methylethylamine is not, although both are surely
intelligible (Fig. 6.30).
CH
3
NH
2
Methylamine
(CH
3
)
3
CNH
2
tert-Butylamine
Propylamine
Cyclohexylamine
Dicyclopentylamine
CH
3
CH
2
NH
2
Ethylamine
PhCH
2
NH
2
Benzylamine
(CH
3
)
2
NH
Dimethylamine
Benzylethyl-
methylamine
(CH
3
CH
2
)
3
N
CH
2
CH
3
CH
3
N
H
Triethylamine
Ethylmethylamine,
(not methylethylamine)
CH
2
CH
3
PhCH
2
CH
3
N
CH
3
CH
2
CH
2
NH
2
NH
2
NH
WEB 3D
WEB 3D
FIGURE 6.30 A naming scheme for primary, secondary, and tertiary amines.
The IUPAC system names amines analogously to alcohols. If CH
3
OH is off-
cially known as methanol
, then CH
3
NH
2
is methanamine. Figure 6.31 gives two
examples.
Still another method names amines as substituted alkanes. Now CH
3
NH
2
becomes aminomethane. Figure 6.32 gives some examples of amines named
this way.
CH
3
NH
2
Methanamine
Cyclohexanamine
NH
2
FIGURE 6.31 Two systematically
named amines.
CH
3
NH
2
A
minomethane
..
Aminocyclohexan
e
..
CH
3
CH
2
CH
2
NH
2
H
3
C
CH
3
CH
NH
2
1-Aminopropane
2-Aminopropane
..
NH
2
..
FIGURE 6.32 Still another method for naming amines.
The amino group is just below the alcohol group in the naming protocol prior-
ity system. Figure 6.33 gives some correct and incorrect names of polyfunctional
compounds containing amino groups.
..
2
1
NH
2
..
3
2
1
NH
2
Cl
..
..
..
..
3
3
2
2
1
1
NH
2
..
NH
2
OH
..
..
Br
..
..
..
3
2
1
..
NH
2
HO
..
..
2-Methyl-1-propanamine
(1-amino-2-methylpropane)
3-Chloro-2-butanamine
(not 3-amino-2-chlorobutane)
3-Amino-2-butanol
(or 3-amino-2-hydroxybutane,
not 2-amino-3-butanol
or 2-amino-3-hydroxybutane)
3-Bromocyclopentanamine
(neither 3-aminobromocyclopentane
nor 4-bromoaminocyclopentane)
3-Aminocyclopentanol
(not 3-hydroxycyclopentylamine)
FIGURE 6.33 Some polyfunctional
amines and their names.
242 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
Simple, cyclic amino compounds are also widely seen in nature, and are almost
invariably known by their common names. These compounds are often named as
“aza” analogues of an all-carbon system. Thus aziridine becomes azacyclopropane.
Methylamine could be called azaethane, although in point of fact this system is
almost never used for acyclic compounds.Unless there is an oxygen atom in the ring,
the numbering scheme places the nitrogen at 1 and proceeds so as to minimize the
numbering of any substituents. Figure 6.34 gives a few important examples. Once
again there is no way out other than to learn these names.
WEB 3D
WEB 3D
WEB 3D
..
N
..
N
..
N
H
..
N
H
H H
H
H
..
..
N
N
CH
3
CH
2
Ph
2
2
2
3
3
3
4
1
1
1
Br
..
..
..
..
N
O
..
..
Aziridine
(azacyclopropane)
..
N
H
O
..
..
Morpholine 3-Methylpiperidine
(
not 5-meth
y
l
p
i
p
eridine
)
3-Bromopyrrolidine
(
not 4-bromo
py
rrolidine
)
4-Benzylmorpholine
(
N-benz
y
lmor
p
holine
)
Azetidine
(azacyclobutane)
Pyrrolidine
(azacyclopentane)
Piperidine
(azacyclohexane)
..
N
Pyridine
(azabenzene)
..
N
H
Pyrrole
FIGURE 6.34 Cyclic amines
and their names.
Despite this seeming excess of nomenclature, another aspect of naming amines
must be mentioned.Substituted amines can also be named as N-substituted amines.
In this system, the substituent on nitrogen is designated with the locating prefix N.
Some examples are shown in Figure 6.35.
..
..
N
N
..
N
H
2
C
CH
2
CH
3
N
-Benzylaziridine N-Ethylpiperidine N-Methylazacyclooctane
(heptamethylenemethylamine)
CH
3
FIGURE 6.35 Still another naming
protocol for amines.
WEB 3D
N
Tetraethylammonium bromide Butylethylmethylpropylammonium iodide
+
N(CH
2
CH
3
)
4
+
CH
3
CH
2
CH
2
CH
2
CH
3
CH
2
CH
2
CH
2
CH
3
CH
3
I
–
N
Ethylmethylammonium chloride
+
CH
3
CH
2
H
CH
3
H
Cl
–
Br
–
FIGURE 6.36 Names for three ammonium ions.
Ammonium ions are named by alphabetically attaching the names of the substituents.
Don’t forget to append the name of the negatively charged counterion (Fig. 6.36).
6.7 Amines 243
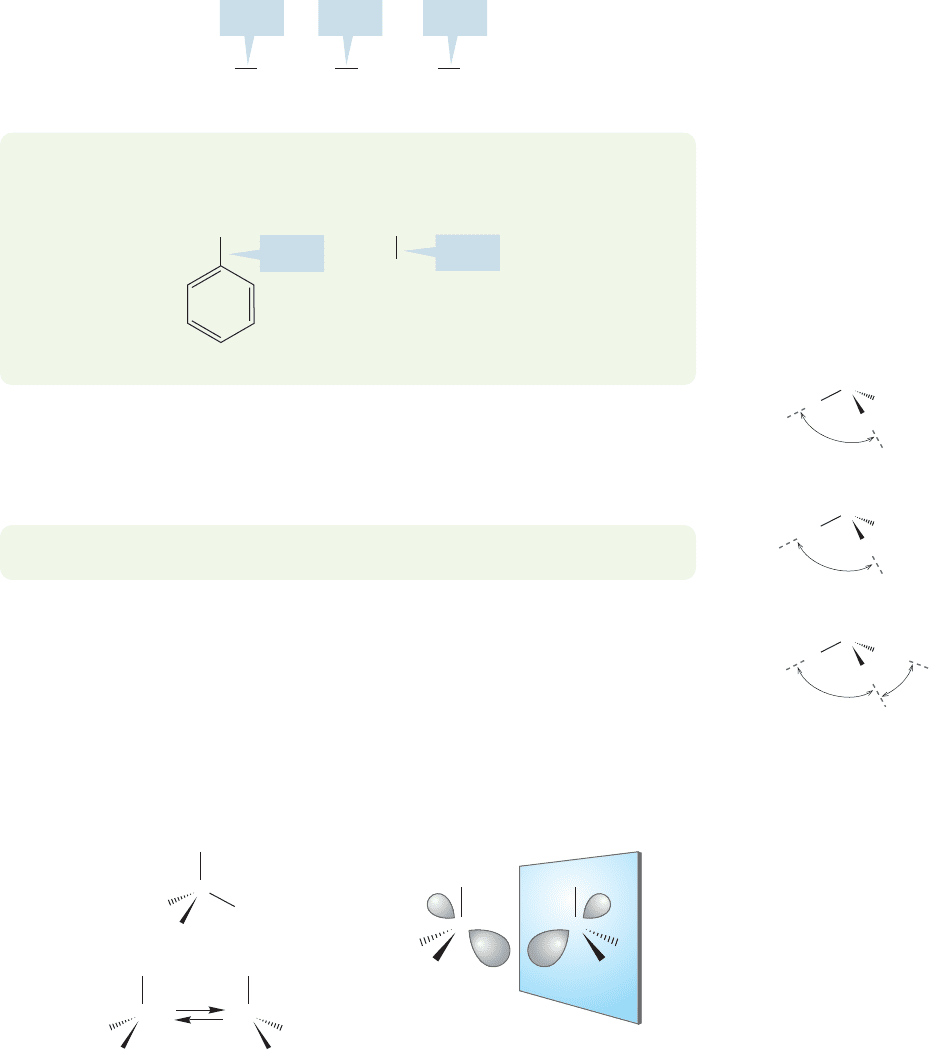
6.7b Structure and Physical Properties of Amines In simple amines, the
carbon–nitrogen bond distance is a little shorter than the corresponding
carbon–carbon bond distance in alkanes.This phenomenon is similar to that found
in the alcohols and ethers (Section 6.8) (Fig. 6.37).
H
3
COH
Alcohol Amine Alkane
H
3
CNH
2
H
3
CCH
3
1.43 A
⬚
1.47 A
⬚
1.54 A
⬚
FIGURE 6.37 The carbon–nitrogen
bond in amines is shorter than the
carbon–carbon bond in alkanes.
PROBLEM 6.11 Why is the carbon–nitrogen bond distance in aniline slightly
shorter than that in methylamine?
NH
2
CH
3
1.47 A
⬚
1.40 A
⬚
NH
2
Aniline
Methylamine
Simple amines are hybridized approximately sp
3
at nitrogen and thus are
pyramidal. The bond angles are close to the tetrahedral angle of 109.5° but, of
course, cannot be exactly the tetrahedral angle.
PROBLEM 6.12 Why not?
For amines in which all three R groups are identical, there will be only a single
angle,but for less symmetrical amines,the different angles
cannot be the same (Fig. 6.38).
The most important structural feature of amines comes from the presence of the
pair of nonbonding electrons acting as the fourth substituent on the pyramid. In
alkanes, where there are four substituents, there is nothing more to consider once
the pyramid is described. In amines there is, because the pyramid can undergo an
“umbrella flip,”amine inversion, forming a new, mirror-image pyramid (Fig. 6.39).
R
O
N
O
RR
O
N
O
R
CH
3
CH
3
H
3
C
N
..
108.4⬚
H
H
CH
3
N
..
112.9⬚
105.9⬚
H
H
H
N
..
107.3⬚
FIGURE 6.38 Some bond angles in
simple amines.
..
A carbon-based pyramid is static
The nitrogen-based pyramid can invert
Inversion produces the
mirror-image pyramid
C
N
N
N
N
..
A
C
C
B
B
A
Mirror
.. ..
FIGURE 6.39 Amine inversion
interconverts enantiomeric amines.
This inversion process is sometimes confusing when first encountered,so let’s work
through it, making comparisons with the alkanes as we go along. At the beginning
of the process,the pyramid begins to flatten and the bond angles increase.R
O
N
O
R
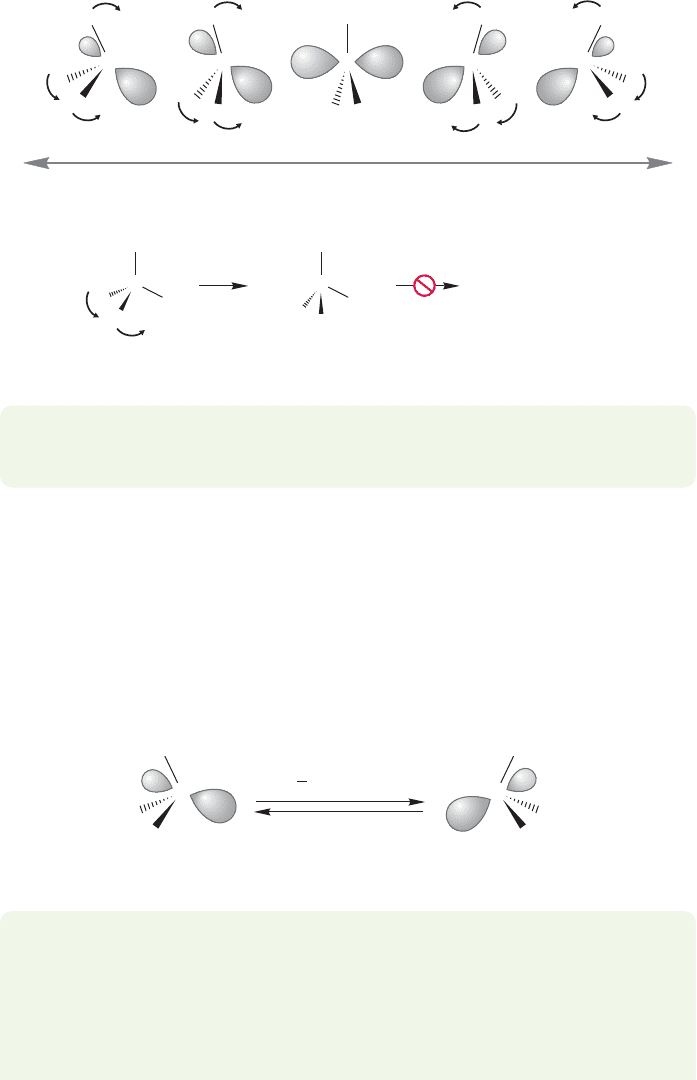
244 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
At the halfway point along the path to the inverted pyramid, the transition state is
reached. Here the molecule is planar, with 120° bond angles. At this
point, the hybridization of the central nitrogen is sp
2
! The inversion process main-
tains a mirror plane of symmetry. In the alkanes, we could certainly imagine the
beginning of the flattening process,but there is no way to force the fourth substituent
through the molecule to the other side (Fig. 6.40).
R
O
N
O
R
Inversion progress
This flattening
will cost energy
This process can't
go further
Planar transition state
N is
sp
2
N
R
R
R
R
R
R
R
R
R
R
N
..
..
..
..
N
NN
R
R
R
R
R
R
R
R
R
..
C
R
R
R
R
C
FIGURE 6.40 The transition state for
amine inversion is planar.
H
H
N
One enantiomer The other enantiomer
..
..
N
H
3
C
CH
3
CH
2
CH
2
CH
3
CH
3
ΔG
†
~ 5 kcal/mol
FIGURE 6.41 Rapid inversion
racemizes optically active amines.
PROBLEM 6.13 In general terms, what is the hybridization of nitrogen at some
point between the starting pyramidal amine and the transition state?
An important concern is the rate of this inversion process. If it is slow at room
temperature, then we should be able to isolate appropriately substituted optically
active amines. If inversion is fast, however, racemization will also be fast as the
inversion process interconverts enantiomers. In practice, this is what happens.
Barriers to inversion in simple amines are very low, about 5–6 kcal/mol, and
isolation of one enantiomer, even at very low temperature, is difficult. Inversion
is simply too facile, and the rapid inversion interconverts enantiomeric amines
(Fig. 6.41).
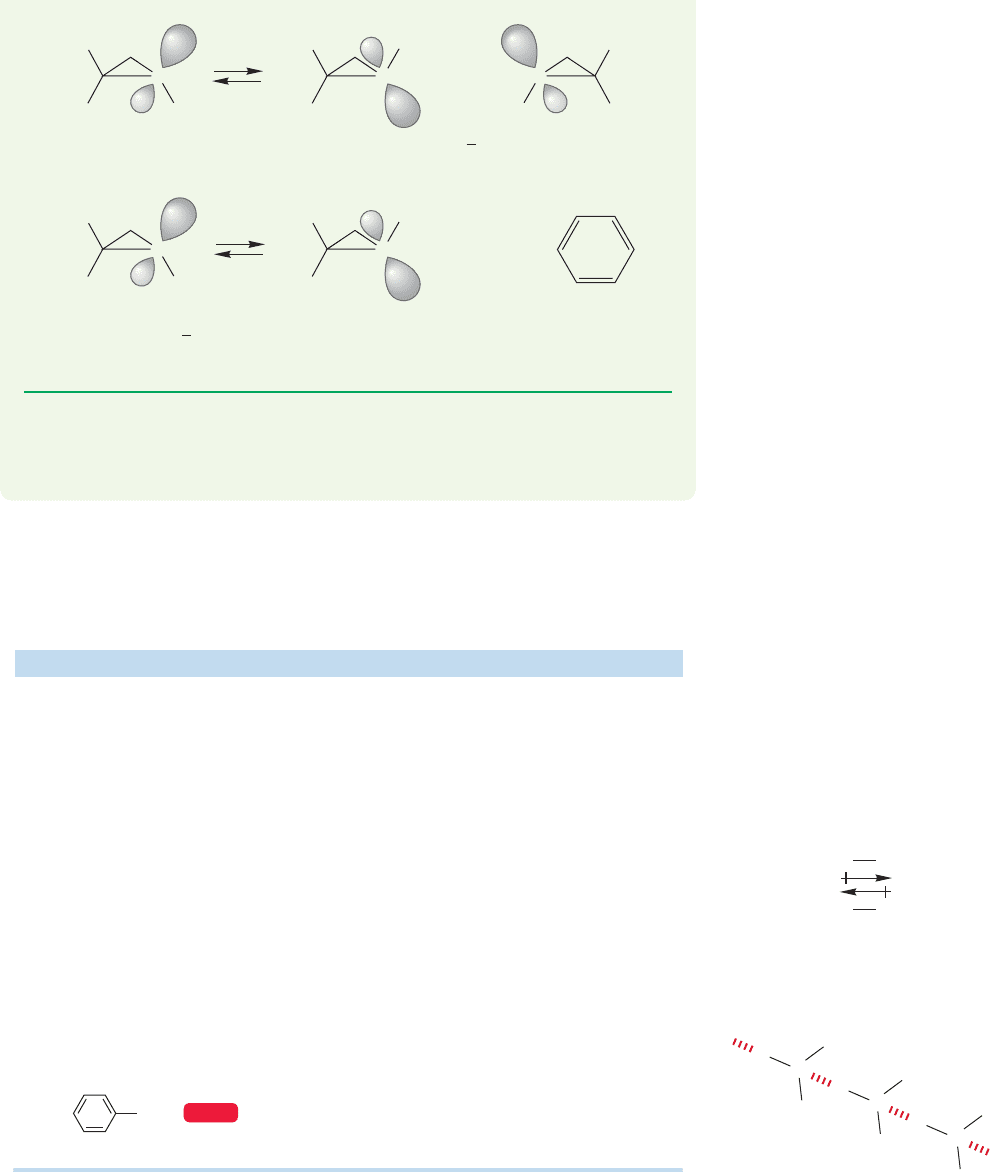
PROBLEM 6.14 In contrast to simple amines, aziridines (three-membered
rings containing a nitrogen) can often be separated into enantiomers.
For example, the activation energy (ΔG
‡
) for the inversion of 1,2,2-
trimethylaziridine is about 18.5 kcal/mol,much higher than that for simple amines.
Explain.
(continued)
6.7 Amines 245
When the methyl on nitrogen is replaced
by phenyl, ΔG
†
decreases to 11.2 kcal/mol
..
H
3
C
H
3
C
CH
3
N
..
H
3
C
CH
3
CH
3
..
H
3
C
H
3
C
CH
3
N
..
H
3
C
H
3
C
Ph
Ph
N
..
H
3
C
H
3
C
N
=
Ph =
N
The barrier to this inversion is unusually high, ΔG
†
= 18.5 kcal/mol
PROBLEM 6.15 If a phenyl group (Ph in the figure above) is attached to the
nitrogen atom of the aziridine, the barrier to inversion decreases (see figure in
Problem 6.14). Explain.
Like alcohols, amines are much higher boiling than hydrocarbons of similar
molecular weight, although the effect is not as great as with alcohols (Table 6.8;
compare to Table 6.3). The reasons for the increased boiling points are the same as
TABLE 6.8 Some Physical Properties of Amines and a Few Related Alkanes
Compound bp (°C) mp (°C)
NH
3
Ammonia
–33.4 –77.7
–6.3 –93.5
CH
3
CH
3
–88.6 –183.3
Ethane
16.6 –81
Density (g/cm
3
)
0.77
0.70
0.57
0.68
CH
3
NH
2
CH
3
CH
2
NH
2
Ethylamine
Methylamine
CH
3
CH
2
CH
3
–189.7
–93
–43.1
7.4
Dimethylamine
(CH
3
)
3
N 2.9 –117.2
184 –6.3
0.59
0.68
0.64
1.02
Propane
Trimethylamine
NH
2
Aniline
(CH
3
)
2
NH
WEB 3D
for alcohols. Amines are polar compounds and aggregate in solution. Like alcohols,
small amines are miscible with water. Boiling requires overcoming the intermolecular
attractive forces between the dipoles. Moreover, amines form hydrogen-bonded
oligomers in solution,effectively increasing the molecular weight and further increas-
ing the boiling point (Fig. 6.42).
H
3
C
NH
2
CH
3
Association because of favorable
dipole alignment
NH
2
Oligomer formation through
h
y
dro
g
en bondin
g
CH
3
N
H
..
..
..
CH
3
N
H
CH
3
N
H
H
H
H
FIGURE 6.42 The effective molecular
weight of amines is increased through
association and hydrogen bonding.

246 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
When hydrogen bonding is impossible, as with tertiary amines, boiling points
decrease (Table 6.8).
It is impossible to discuss the properties of amines without mentioning smell!
The smell of amines ranges from the merely fishy to the truly vile. Indeed, the odor
of decomposing fish owes its characteristic unpleasant nature to amines.
Diamines are even worse. Some indication of how bad-smelling they often are
can be gained from their names. 1,4-Butanediamine is called putrescine, and
1,5-pentanediamine is called cadaverine.
PROBLEM 6.16 Many people use lemon when eating fish. This custom is a carry-
over from the days when it was difficult to preserve fish, and the lemon acted to
diminish the unpleasant odor (if not the decomposition). Lemons contain 5–8%
citric acid, and this acidity contributes to their sour taste. Explain why lemon juice
is effective at reducing the odor of fish.
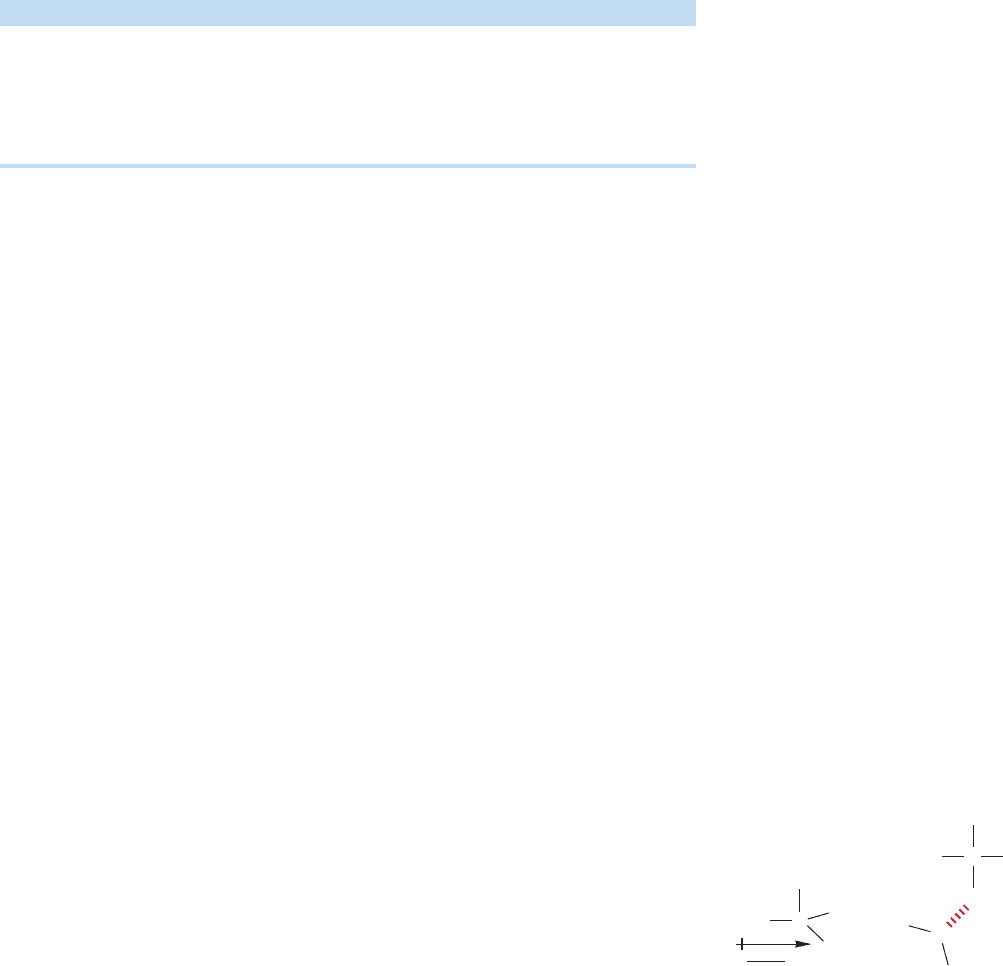
6.7c Acid and Base Properties of Amines Like water and alcohols, amines
are Brønsted bases (proton acceptors). Primary and secondary amines are weak
Brønsted acids (proton donors) as well. Brønsted basicity results in the formation
of ammonium ions through proton transfer (Fig. 6.43).
H
3
N
Cl
H
H
–
..
..
..
..
H
3
N
Ammonium
ion
A
mmonia
+
Cl
..
..
..
..
FIGURE 6.43 Ammonia acting as a
Brønsted base in proton transfer.
NH
2
A weakly basic amine is a less effective competitor for
H
+
and produces an ammonium ion that is a relatively
strong Brønsted acid (low pK
a
)
R
X
H
R
++
..
NH
3
+
X
–
..
… an ammonium ion that is a relatively
weak Brønsted acid (high pK
a
)
A strongly basic amine is a good
competitor for H
+
and produces…
FIGURE 6.44 Ammonium ions with
high pK
a
values are related to
strongly basic amines, and
ammonium ions with low pK
a
values
are related to weakly basic amines.
The better competitor an amine is in the proton-transfer reaction, the stronger
is its Brønsted basicity. A way of turning this statement around is to focus not on
the Brønsted basicity of the amine, but on the Brønsted acidity of its conjugate acid,
the ammonium ion. If the ammonium ion is a strong Brønsted acid, the related
amine must be a weak Brønsted base. If it is easy to remove a proton from the
ammonium ion to give the amine, the amine itself must be a poor competitor in
the proton-transfer reaction, which is exactly what we mean by a weak Brønsted
base.Strongly basic amines give ammonium ions from which it is difficult to remove
a proton, ammonium ions with high pK
a
values. So the pK
a
of the ammonium ion
has come to be a common measure of the basicity of the related amine.Ammonium
ions with high pK
a
values (weak acids) are related to strongly basic amines, and
ammonium ions with low pK
a
values (strong acids) are related to weakly basic
amines (Fig. 6.44).
6.7 Amines 247
Table 6.9 relates the basicity of ammonia and some very simple alkylamines by
tabulating the pK
a
values of the related ammonium ions.
TABLE 6.9 Some pK
a
Values for Simple Ammonium Ions in Solution
Amine Formula Ammonium Ion pK
a
(in aqueous solution)
Ammonia NH
3
NH
4
9.24
Methylamine CH
3
NH
2
10.63
Dimethylamine (CH
3
)
2
NH 10.78
Trimethylamine (CH
3
)
3
N9.80(CH
3
)
3
N
+
H
(CH
3
)
2
N
+
H
2
CH
3
N
+
H
3
The general trend of the first three entries in Table 6.9 is understandable if we
make the analogy between ammonium ions and carbocations. Remember (Chapter 3,
p. 138): The more substituted a carbocation, the more stable it is. Similarly, the
more substituted an ammonium ion,the more stable it is.The more stable the ammo-
nium ion, the less readily it loses a proton and the higher its pK
a
. At least for the
first three entries of Table 6.9, increasing substitution of the ammonium ion carries
with it a decreasing ease of proton loss.
Actually, even the first three entries in Table 6.9 should make you suspicious.
Why is there a much bigger change between ammonia and methylamine (1.4 pK
a
units) than between methylamine and dimethylamine (only 0.15 pK
a
units)? The
structural change is the same in each case, the replacement of a hydrogen with a
methyl group. When we get to the fourth entry, we find that things have truly gone
awry; trimethylammonium ion is a stronger acid than the dimethylammonium ion
and the methylammonium ion, and almost as strong as the ammonium ion itself,
which implies that trimethylamine is a weaker base than dimethylamine or methyl-
amine. There seems to be no continuous trend in these data.
Indeed there isn’t,and it takes a look at matters in the gas phase to straighten things
out. In the gas phase, the trend is regular. The basicity of amines increases with sub-
stitution, and the acidity of ammonium ions decreases with substitution (Fig. 6.45).
There must be some effect present in solution that disappears in the gas phase,
and this effect must be responsible for the irregularities in Table 6.9. The culprit is
the solvent itself. Ions in solution are strongly stabilized by solvation, by interaction
of the solvent molecules with the ion. These interactions can take the form of elec-
trostatic stabilization or of partial covalent bond formation, as in hydrogen bonding
(Fig. 6.46).
In solution, an alkyl group has two effects on the stability of an ammonium ion.
It stabilizes by helping the ammonium ion to disperse the charge, but it destabilizes
the ion by interfering with solvation, by making intermolecular solvation (charge
dispersal) more difficult. These two effects operate in opposite directions! There is
little steric interference with solvation when we replace one hydrogen of the ammo-
nium ion with a methyl group, and the pK
a
change is more than a full pK
a
unit.This
change reflects the stabilizing effect of the methyl group on the ammonium ion.
However, when a second hydrogen is replaced by a methyl, the stabilization and
interference with solvation nearly balance.The result is practically no change in the
overall stability of the ammonium ion. When a third hydrogen is replaced with
methyl, the destabilizing effects outweigh the stabilizing forces, and the result is a
less stable, more acidic ammonium ion. In the gas phase, where there can be no
solvation, only the stabilizing effects remain, and each replacement of a hydrogen
with a methyl is stabilizing.
Order of increasing gas-phase pK
a
Lowest pK
a
Highest pK
a
+
NH
4
<
+
NRH
3
<
+
NR
2
H
2
+
NR
3
H<
FIGURE 6.45 The gas-phase acidity
of ammonium ions.
H
3
C
Stabilization through
dipole–dipole
interaction with a
polar solvent
Stabilization
through hydrogen
bonding
N
H
OH
H
H
H
H
3
C
H
H
+
+
..
..
H
O
H
H
N
FIGURE 6.46 Stabilization of amines
in solution.
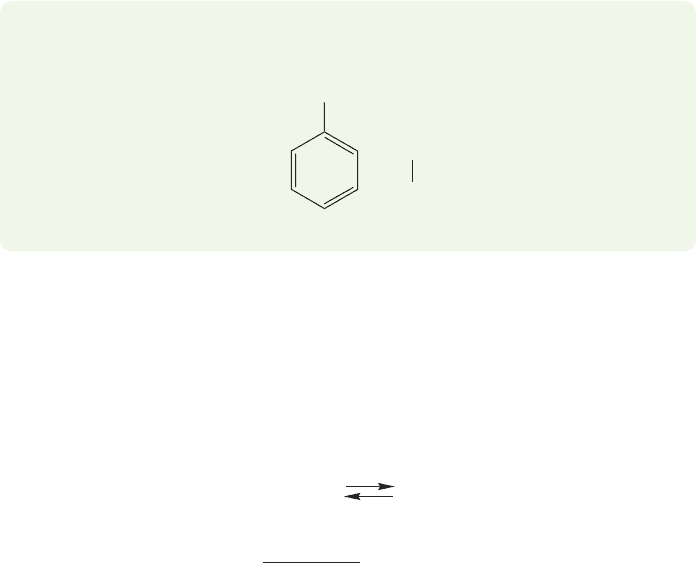
248 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
PROBLEM 6.17 Amines can be stabilized by other factors. Explain the following
pK
a
data.
4.6pK
a
10.6
CH
2
CH
3
+
NH
3
+
NH
3
It must be admitted that this traditional discussion of base strength of amines
in terms of the pK
a
of the related ammonium ion is indirect. There is another,
less common but undeniably more direct method. That is to frame the discus-
sion in terms of the pK
b
(log K
b
), where K
b
is the basicity constant of the amine
(Fig. 6.47).
K
b
=
K
For
RNH
2
RNH
3
+
[RNH
3
] [HO
–
]
+
[RNH
2
]
++
H
2
O
HO
–
FIGURE 6.47 The expression for pK
b
.
The higher the pK
b
, the weaker is the base, just as the higher the pK
a
, the weaker
is the acid. Typical amines have pK
b
values of about 4, which makes them quite
strong bases.
Amines are much stronger bases than alcohols are. For example, it is much
harder to deprotonate
NH
4
than
OH
3
.The pK
a
value for the ammonium ion (9.2)
is much higher than that for the oxonium ion (–1.7) (Fig. 6.48).
pK
a
= 9.2
+
NH
4
+
N(CH
3
)
4
Ammonium ion
Tetramethylammonium
chloride, a stable solid,
easily isolable
Trimethyloxonium
fluoborate, not easily
isolated, and very reactiv
e
pK
a
= –1.7
+
OH
3
Oxonium ion
Cl
–
+
O(CH
3
)
3
BF
4
–
FIGURE 6.48 Ammonium ions are
weaker acids than oxonium ions.
Why is the oxonium ion much more acidic than the ammonium ion? Oxygen
is a more electronegative atom than nitrogen and bears the positive charge less well.
We can see the results of the increased stability of the ammonium ion in practical
terms. Stable ammonium salts are common, but salts of oxonium ions, though
known, are rare and usually unstable (Fig. 6.48).
In addition, as we progress along the series
NH
4
,
NRH
3
,
NR
2
H
2
,
NR
3
H,
NR
4
, there are fewer hydrogens available for hydrogen bonding and therefore less
stabilization of the ammonium ion in solution.
