Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

13.9 Heterobenzenes and Other Heterocyclic Aromatic Compounds 599
carbon are called “heteroatoms,” and pyridine is a heterobenzene, which we
saw in Figure 13.34. It is a member of the general class of compounds called
heteroaromatic compounds, defined as aromatic molecules containing at least
one heteroatom.
The electron counting for pyridine can be confusing at first because of the extra
pair of electrons on nitrogen. Doesn’t pyridine have eight π electrons and thus vio-
late the 4n 2 rule? Put a better way, we have to worry whether antibonding
orbitals are occupied in pyridine. A good orbital drawing shows that the “extra”two
electrons are not in the π system, and pyridine is not in violation of the Hückel
rule (Fig. 13.47).
sp
2
Orbital
perpendicular
to the π system
..
N
The bonds made
from sp
2
/sp
2
overlap
are not in the π system
FIGURE 13.47 Pyridine has only
6 π electrons.The lone-pair electrons
on nitrogen are in an sp
2
orbital
perpendicular to the π system, not
in the π system of the ring.
Other six-membered rings containing one heteroatom are known, but none is
very stable. Oxabenzene must be cationic and the positive charge contributes to mak-
ing it a reactive molecule (Fig. 13.48). Aromaticity must overcome the destabilizing
influences of the charged atom and doesn’t completely succeed in this case, in part
because the electronegative oxygen is forced to carry the positive charge.
H
H
H
H
H
H
H
H
H
H
O
..
+
O
..
+
FIGURE 13.48 Oxabenzene must be
charged and is not very stable.
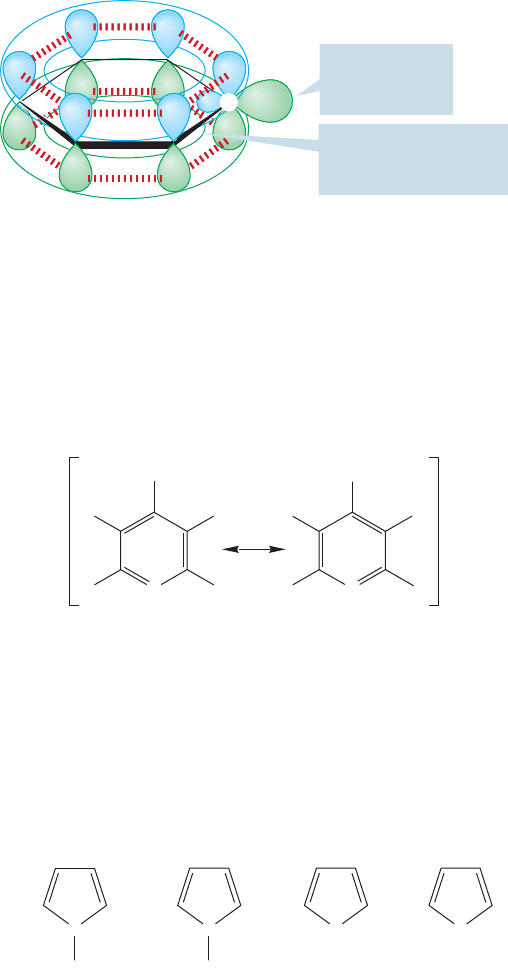
The neutral, five-membered heterocyclic ring compounds, pyrrole and furan,
also show aromatic character. Even thiophene is considered aromatic (Fig. 13.49).
Furan and pyrrole can complete an aromatic sextet of π electrons by using a pair
of nonbonding electrons in a 2p orbital. These molecules have the same number
of π electrons as the cyclopentadienyl anion, but none of the problems induced by
Cyclopentadienyl
anion
C
H
H
..
–
Pyrrole
N
..
Furan
O
..
..
Thiophene
S
..
..
FIGURE 13.49 Four five-membered
aromatic rings.There are 4n 2 π
electrons in the three neutral
molecules, which are isoelectronic
(same number of electrons) with
the cyclopentadienyl anion.
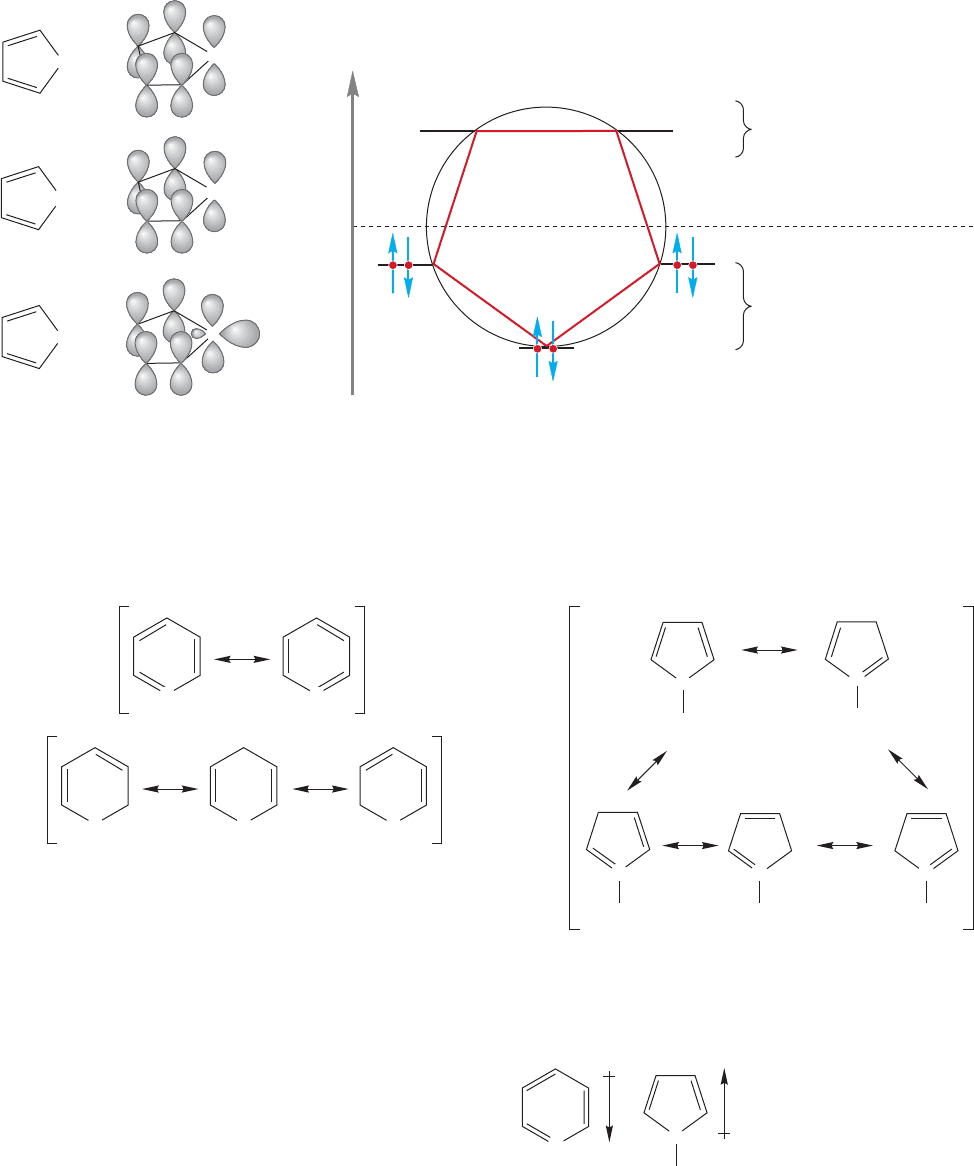
600 CHAPTER 13 Conjugation and Aromaticity
the charge on the all-carbon molecule. In thiophene and furan, there is a second
pair of nonbonding electrons remaining in an sp
2
orbital perpendicular to the
π orbital system (Fig. 13.50).
In pyridine, the electronegative nitrogen is the negative end of the dipole, but in
pyrrole, nitrogen is the positive end (Fig. 13.52).
CH
..
O
..
..
NH
..
..
.
.
.
.
CH
..
.
.
.
.
NH
..
.
.
.
..
.
O
–
–
Energy
Antibonding
molecular orbitals
Bonding
molecular orbitals
Nonbonding
FIGURE 13.50 Orbital pictures of the isoelectronic aromatic compounds that are five-membered rings.
..
..
N
H
N
H
..
N
..
N
–
+
H
+
H
+
H
+
N
..
–
..
N
–
..
–
N
..
..
N
––
+
..
..
N
–
+
..
..
N
+
FIGURE 13.51 Resonance descriptions of pyridine and pyrrole.
Pyridine and pyrrole have contrasting resonance descriptions (Fig. 13.51), and
this difference results in some very different physical properties. For example,
the direction of the dipole moment is different in the two aromatic molecules.
PyrrolePyridine
..
N
..
N
H
1.8 D2.2 D
FIGURE 13.52 The dipole moments
of pyridine and pyrrole are in
different directions.
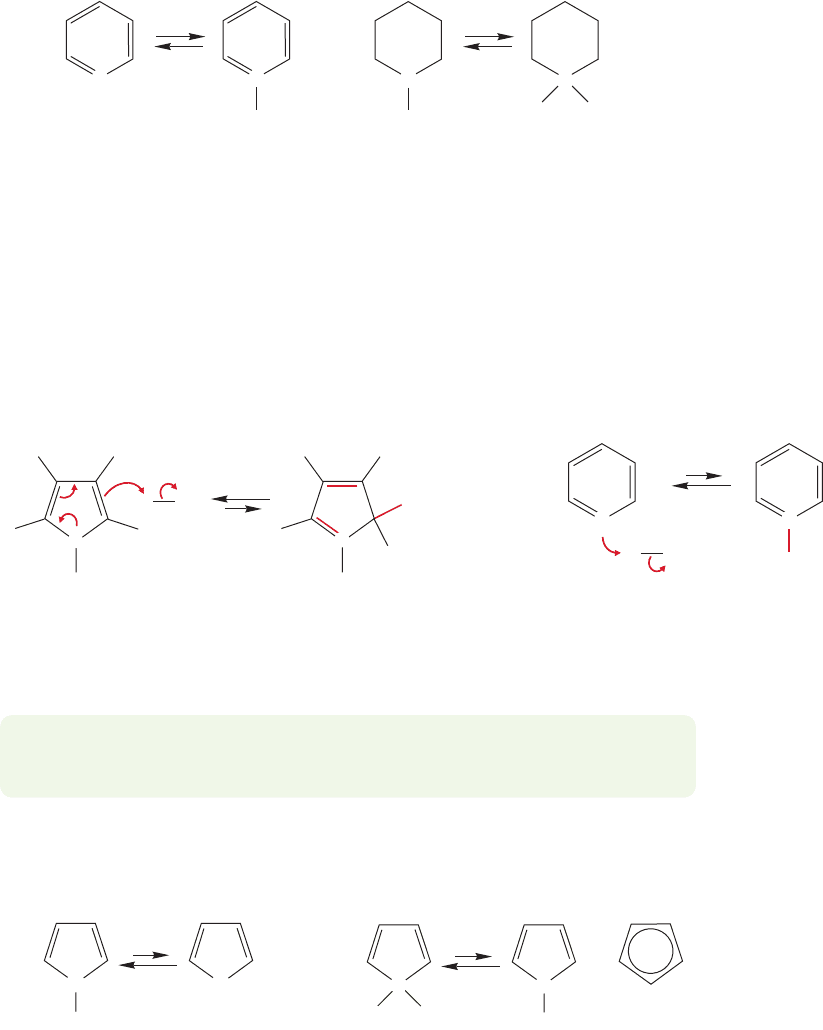
13.9 Heterobenzenes and Other Heterocyclic Aromatic Compounds 601
13.9b Acid and Base Properties Pyridine can be protonated in acid, but
pyridine is a weaker base than a simple cyclic secondary amine such as piperidine
(Fig. 13.53). The pK
a
values of the conjugate acid ammonium ions show this clearly.
Protonated pyridine (the pyridinium ion) is a much stronger acid (pK
a
5.2) than
protonated piperidine, an ammonium ion formed from a typical secondary amine
(pK
a
'
11). The stronger acid corresponds to a weaker conjugate base. Alternatively,
we might make this argument in terms of pK
b
values. The pK
b
of pyridine is 8.8,
whereas that for a simple secondary amine is about 3. These values also tell us that
the secondary amine is a stronger base. Most organic chemists use pK
a
values to ana-
lyze base strength.
N
Pyridine
p
K
b
= 8.8
Pyridinium ion
pK
a
= 5.2
..
N
H
+
Piperidine
p
K
b
~3
Piperidinium ion
pK
a
~11
..
N
HH
+
N
H
FIGURE 13.53 Pyridine is a
weaker base than the similar,
but nonaromatic piperidine.
One reason for the decreased basicity of pyridine is that the lone pair of elec-
trons of the sp
2
hybridized nitrogen of pyridine are held more tightly than those of
the approximately sp
3
hybridized nitrogen of a simple secondary amine like piperi-
dine, and therefore are less basic.
Pyrrole is even less basic than pyridine (Fig. 13.54). One can estimate the pK
a
of the conjugate acid at about 4! When pyrrole is protonated, aromaticity is lost
because the aromatic sextet is disrupted (not true for pyridine).The lone pair of elec-
trons is tied up in the new bond to hydrogen and no longer contributes to the com-
pletion of the aromatic sextet.
H
N
..
..
N
H
+
Aromatic Aromatic
N
H
H
H
H H
H
N
H
Not aromatic
p
K
a
~ –4
Still aromatic
H
H H
H
X
..
..
..
H
X
..
..
..
+
+
X
..
..
..
..
..
–
X
..
..
..
..
–
+
FIGURE 13.54 Pyrrole is a very weak base because protonation on nitrogen destroys aromaticity. Protonation on
carbon also destroys aromaticity, but is better than protonation on nitrogen.
PROBLEM 13.17 Notice in Figure 13.54 that pyrrole is protonated on one of the
carbons, not nitrogen. Explain why.
Aromatic!
H
Not aromatic
p
K
a
= 15
Already aromatic
pK
a
= 23
Still aromatic
=
..
–
–
–
C
H H
C
..
..
N
N
H
..
FIGURE 13.55 Pyrrole is a relatively
weak acid compared to
cyclopentadiene.
For an organic compound, pyrrole is a rather strong Brønsted acid,pK
a
'
23; but
it is much weaker than cyclopentadiene, pK
a
15 (Fig. 13.55).This difference may
seem curious at first, because removal of a proton from pyrrole leaves a negative
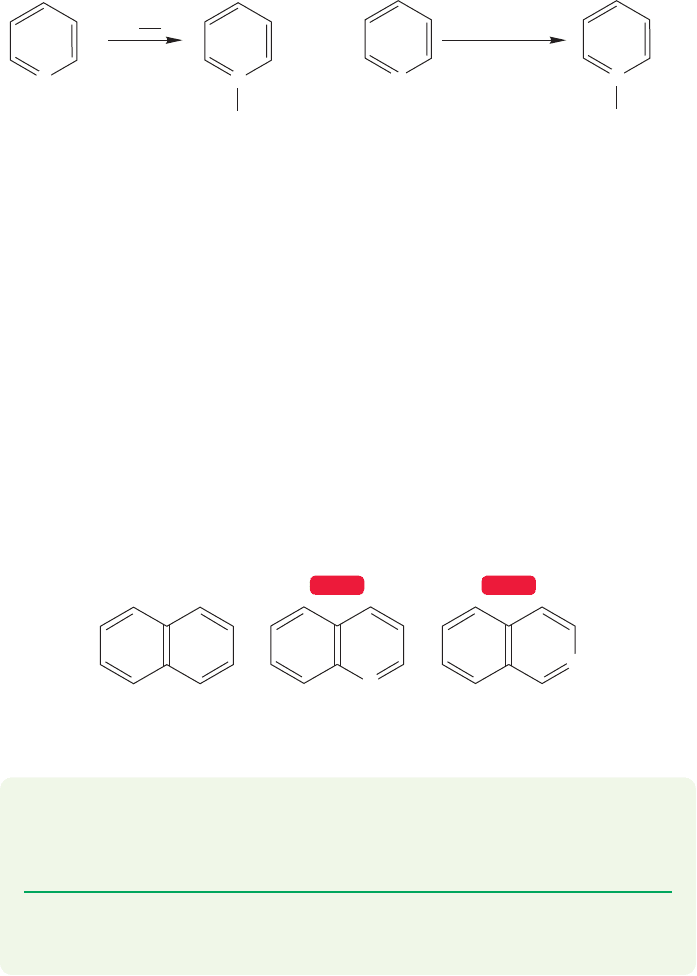
602 CHAPTER 13 Conjugation and Aromaticity
N
..
N
+
+
Pyridine N-oxide
(90%)
Methyl pyridinium
iodide
(>95%)
CH
3
I
CH
3
N
..
N
–
I
–
CH
3
COOH
30% HOOH
75 ⬚C, 12 h
O
..
..
..
FIGURE 13.56 Pyridine can act as
a nucleophile in the formation of
pyridinium salts and pyridine
N-oxides.
charge on a relatively electronegative nitrogen, whereas cyclopentadiene gives a carbon
anion, generally a much less happy event.
However, cyclopentadiene becomes aromatic when a proton is removed, and
pyrrole is already aromatic. Removal of a proton from cyclopentadiene is made
easier by the gain in aromaticity, which does not happen in pyrrole.
13.9c Pyridine as Nucleophile Like other amines, pyridine can be alkylated
in an S
N
2 reaction in which the lone pair electrons on nitrogen displace a leaving
group (p. 317).Treatment of pyridine with a primary or secondary alkyl iodide leads
to alkyl pyridinium ions. Pyridine gives pyridine N-oxide when treated with hydro-
gen peroxide (Fig. 13.56).
Naphthalene Quinoline Isoquinoline
N
..
N
..
WEB 3D WEB 3D
PROBLEM 13.18 Draw schematic orbital pictures of naphthalene and isoquinoline.
Put in the π electrons as dots. It is not necessary to draw the carbon–hydrogen σ
bonds.
PROBLEM 13.19 Can two benzene rings share a single carbon to form a spiro
(p. 211) substituted compound?
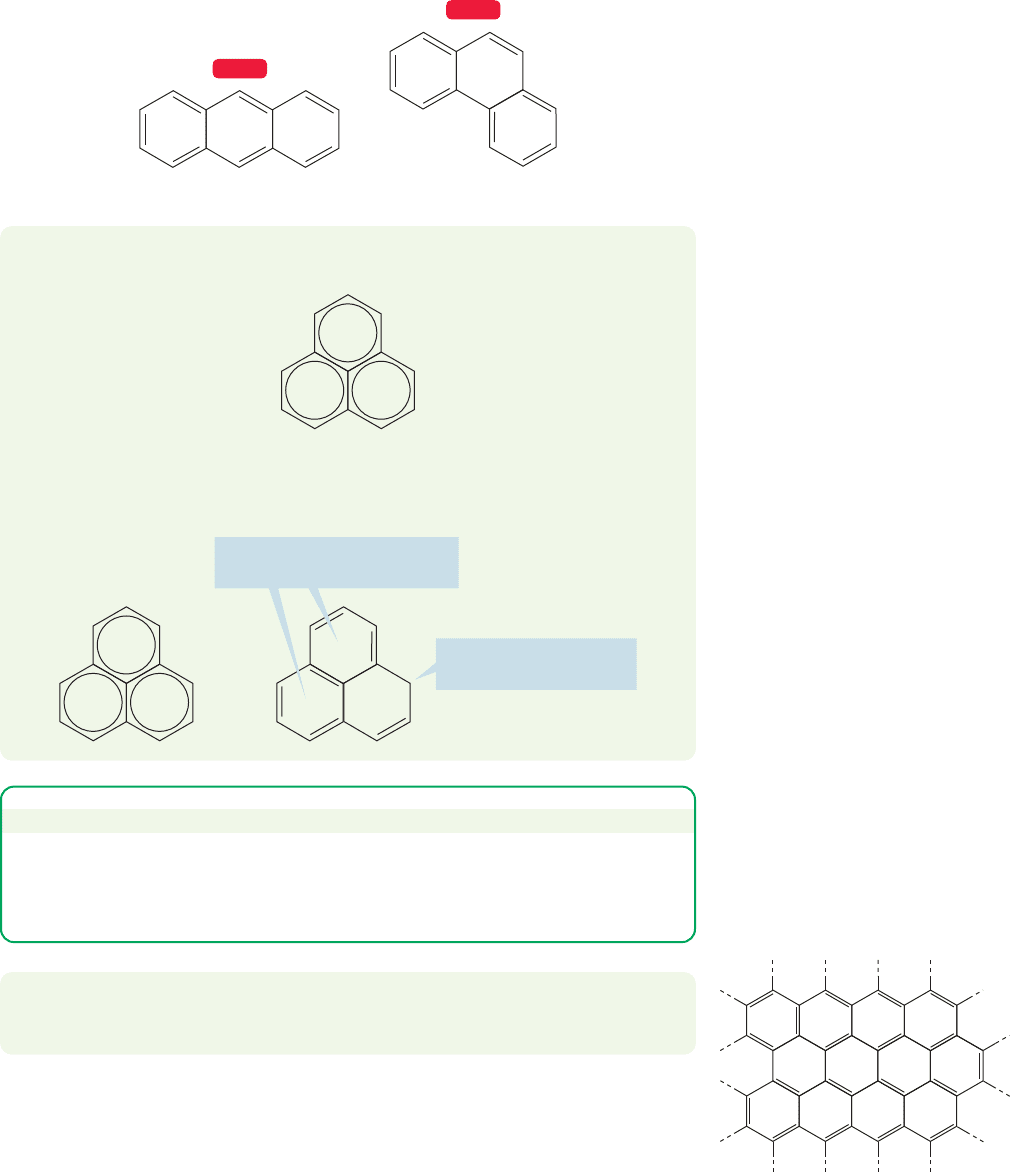
13.10 Polynuclear Aromatic Compounds
13.10a Polynuclear Aromatic Compounds At this point, we can see
benzene as a building block from which more complicated, polycyclic molecules
can be constructed, as we mentioned in Chapter 4 (p. 179). For example, benzene
and other aromatic rings can share edges to form fused, polynuclear aromatic
compounds (also called polyaromatic hydrocarbons or polycyclic aromatic com-
pounds). The simplest of these is naphthalene, in which two benzene rings share
an edge. In quinoline and isoquinoline, it is a benzene ring and a pyridine that
share an edge:
13.10 Polynuclear Aromatic Compounds 603
PROBLEM SOLVING
Watch out for those circles! They evoke the MO description of benzene nicely,
but they can be dangerous, as Problem 13.20 demonstrates. In polycyclic systems,
it is probably a good idea not to use circles. Draw out Kekulé forms instead.
ANSWER The circles are misleading. Drawing the molecule in Kekulé form
allows you to see that there is no way for each carbon to be hybridized sp
2
if each
carbon has four valences.
These two rings are fine: Each
carbon is hybridized sp
2
But what about this ring,
and this carbon?
Clearly,we can continue this building process.Three fused benzene rings produce
either anthracene or phenanthrene:
Anthracene
Phenanthrene
WEB 3D
WEB 3D
WORKED PROBLEM 13.20 Why is the following molecule not stable? Hint: Kekulé
forms are often useful.
FIGURE 13.57 Graphite, an infinitely
extended planar array of fused
benzene rings.
The limit of the ring-fusing business is reached in graphite,a polymer composed
of fused benzene rings extended in what amounts to an infinite plane (Fig. 13.57).
Of course, the plane is not really infinite, but it is large enough so that the edges do
not affect the physical or chemical properties of graphite. Graphite’s lubricating prop-
erties are related to the ease of sliding the planar layers of graphite over each other.
In the mid-1980s, R. F. Curl (b. 1933), H. W. Kroto (b. 1939), R. E. Smalley
(1943–2005),and their co-workers were attempting to explain the striking abundance
PROBLEM 13.21 Draw all the fused polynuclear aromatic compounds made from
four benzene rings. Be careful. This question is much harder than it seems.
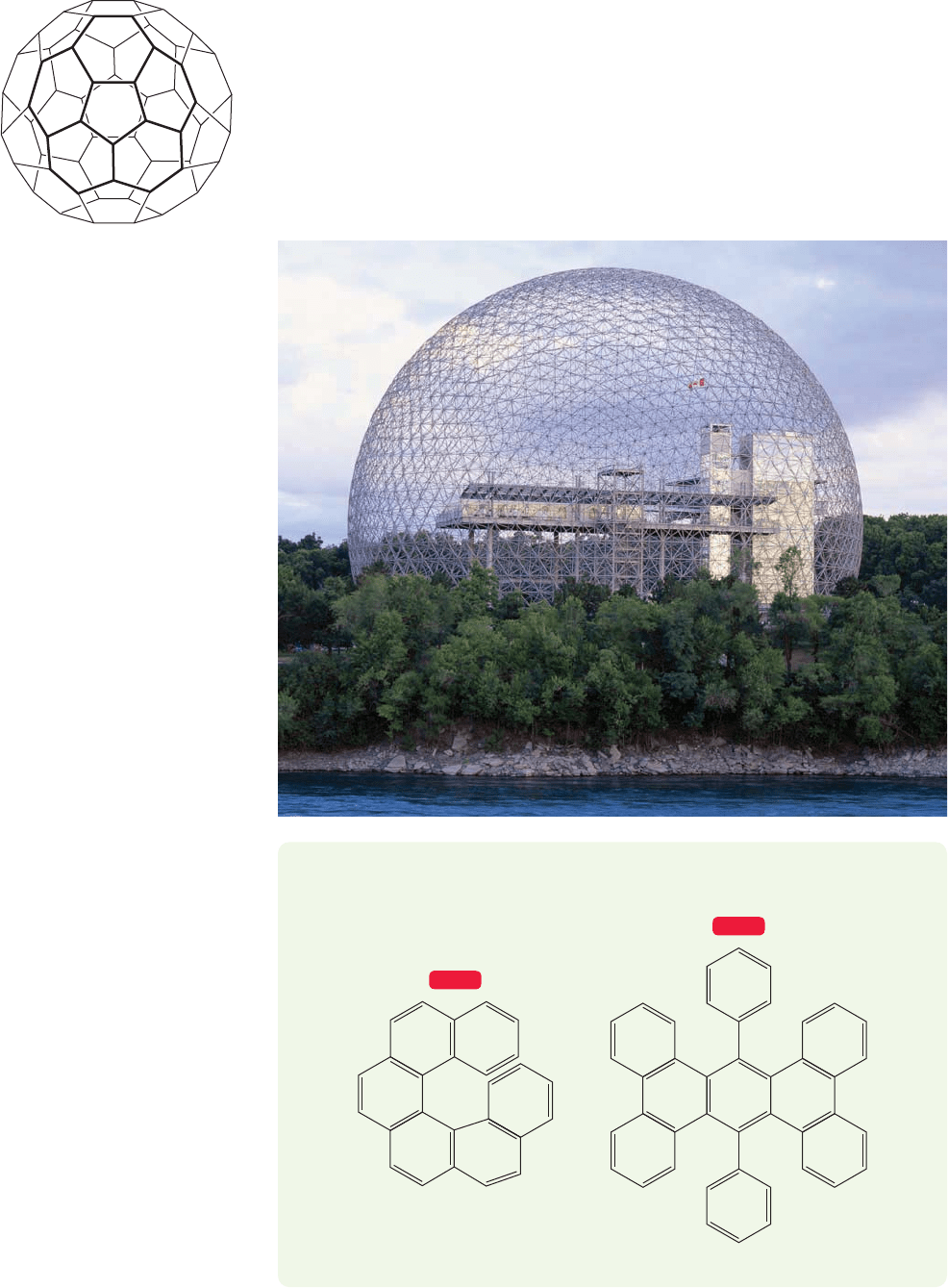
604 CHAPTER 13 Conjugation and Aromaticity
FIGURE 13.58 Buckminsterfullerene,
C
60
, a soccer ball–shaped form of
carbon. The double bonds (or circles)
are left out for clarity.
of certain fragments observed when a carbon rod was vaporized. In the face of much
skepticism in the chemical community, they proposed that the major fragment, C
60
,
had the structure of a soccer ball of pure carbon (Fig. 13.58). They turned out to be
absolutely right, and named this molecule “buckminsterfullerene”after R.Buckminster
Fuller (1895–1983), the inventor of the geodesic dome. This compound, which can
now be made easily in multigram lots through the vaporization of carbon rods, is a
testimony to the proposition that organic chemistry is hardly played out as a provider
of exciting new molecules. Curl, Kroto, and Smalley shared the 1996 Nobel prize in
chemistry for this discovery.
Fuller’s geodesic dome was the
inspiration for the name of C
60
,
buckminsterfullerene.
PROBLEM 13.22 The ring-fusing process produces some interesting molecules along
the way to graphite. “Hexahelicene” and “twistoflex” are chiral, for example. Why?
Hexahelicene
Twistoflex
WEB 3D
WEB 3D
13.10 Polynuclear Aromatic Compounds 605
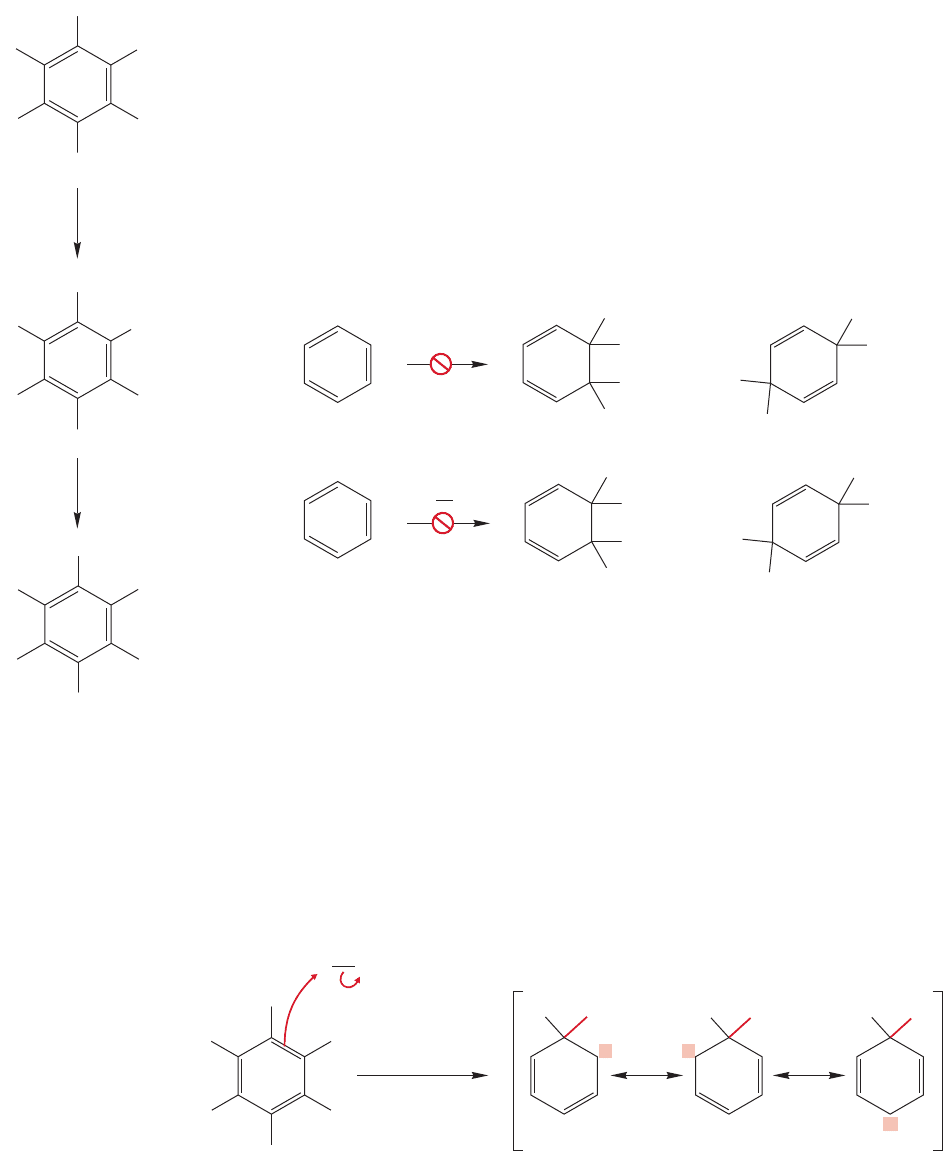
Expanding this building block process to generate ever larger and more varied poly-
cyclic aromatic compounds may be fun, but there is a downside to the process.Many of
the polycyclic aromatic compounds (and some of the simple ones as well) are carcino-
genic. Some of the worst are shown in Figure 13.59. A prudent person avoids exposure
to the known strong carcinogens (it is impossible to avoid exposure to all carcinogens),
and minimizes contact with related aromatic compounds.It is harder to avoid such com-
pounds than it sounds. It is clearly simple to avoid bathing in benzene, and usually (but
not always) possible to substitute other molecules when benzene is called for in a chem-
ical recipe.However,one of the bad carcinogens,benz[a]pyrene,is a product of the inter-
nal combustion engine as well as combustion (cigarettes, charcoal-broiled meat) in
general.The control of such molecules is not only physically difficult,but intersects with
politics and economics as well. This situation creates serious problems of social policy
because, beyond the questions of public health, there are fortunes to be made and elec-
tions to be won. Such matters seem often to complicate the purely scientific questions!
13.10b Related Heterocycles: Indole, Benzofuran, and Benzothiophene
The benzo-fused counterparts of the simple heterocycles pyrrole, furan, and
thiophene are indole, benzofuran, and benzothiophene. These compounds are
related to the two-ring, all-carbon aromatic compound naphthalene:
Naphthalene Indole
N
H
..
Benzofuran
..
..
O
Benzothiophene
..
..
S
WEB 3D
They are important chiefly because of the large variety of natural products contain-
ing these ring systems. Some examples of natural products containing simple and
complex five-membered heterocyclic rings are given in Figure 13.60.
..
..
OCH
3
Reserpine
(antihypertensive)
Murexine
(neuromuscular blocker)
N
H
..
..
..
..
..
..
..
..
..
N
H
H
H
OCH
3
CH
3
O
O
..
..
O
O
C
..
..
..
CH
3
O
..
..
OCH
3
..
..
OCH
3
O
N
H
N
O
N(CH
3
)
3
+
..
..
FIGURE 13.60 Two natural products containing
five-membered heterocyclic rings.
Summary
We have seen that both pyridine and pyrrole qualify as aromatic. However, we
have also seen sharp differences. For example, the lone-pair electrons on nitro-
gen are in the σ system in pyridine, whereas in pyrrole they are in the π system,
completing the aromatic sextet.We have also learned about the Brønsted–Lowry
basicity and nucleophilicity (Lewis basicity) of these heteroaromatic compounds.
Our eyes have been opened to the wonderful, but sometimes hazardous, world
of polyaromatic compounds.
Benz[a ]pyrene
Dibenz[a,h ]anthracene
Cholanthrene
FIGURE 13.59 Some carcinogenic
aromatic hydrocarbons.
606 CHAPTER 13 Conjugation and Aromaticity
13.11 Introduction to the Chemistry of Benzene
We will take a quick look here at reactions. We will first introduce a general reac-
tion that preserves the aromatic character of benzene.It is risky even to suggest that
molecules have desires,but the imperative in benzene chemistry definitely is,Preserve
the Aromatic Sextet! A less flamboyant way of saying this is to point out that ther-
modynamics will surely favor very stable molecules, and therefore benzene chem-
istry is likely to lead to more very low energy aromatic compounds. However,
occasionally there are useful reactions in which the aromatic stabilization is lost,and
we will see one of them in Section 13.11b.
13.11a Aromatic Substitution We have already mentioned that benzene does
not react with Br
2
or HBr, as do almost all alkenes (p. 572), to give addition products
(Fig. 13.61). This apparent lack of reactivity results from the stabilization of
Br
Br
Br
2
+
H
Br
Br
H
H
H
Br
+
H
H
H
H
H
Br
H
HBr
FIGURE 13.61 Benzene does not react with Br
2
or HBr. Neither 1,2- nor
1,4-addition reactions occur.
H
H
H
H
H
H
H
H
H
H
D
H
D
D
D
D
D
D
D
3
O/D
2
O
+
repeat
D
3
O/D
2
O
+
FIGURE 13.62 The hydrogens of
benzene can be exchanged for
deuterium in deuterated acid.
benzene by aromaticity, which produces activation barriers that are too high for the
addition reactions. If we use a deuterated acid we can see that there is a reaction
taking place,at least in strong acid,but the product is merely the exchanged, deuter-
ated benzene, not an addition product (Fig. 13.62).
What is happening in this deuteration? And why is the reaction diverted from
ordinary addition? The answers to these questions will serve to summarize much of
the chemistry of aromatic compounds, which will be encountered in detail in Chapter
14.In deuterio acid,addition of a deuteron gives a resonance-stabilized carbocation,
but aromaticity is lost (Fig. 13.63).
H
H
D
D
3
O/D
2
O
H
H
H
H
+
+
H
D
+
+
+
A cyclohexadienyl cation
(well stabilized but not aromatic)
H
D
DOD
2
H
FIGURE 13.63 Addition of a deuteron to benzene gives a resonance-stabilized, but
nonaromatic, carbocation.
13.11 Introduction to the Chemistry of Benzene 607
~sp
3
Hybridized carbon: No 2p orbital
DH DH DH
C C
This ion is cyclic, could be planar, but is definitely not aromatic,
as the CHD group interrupts the connectivity of 2p orbitals
around the ring; this molecule is not fully conjugated
+
++
C
WORKED PROBLEM 13.23 Be sure you see that the ion in Figure 13.63 is not
aromatic. Why is aromaticity lost?
ANSWER
H
D
H
D
+
–
H
=
D
H
D
+
+
+
+
D Br
..
..
..
Br
..
..
..
..
–
H
D
+
H
D
H
(a)
path b
(b)
Br
..
..
..
..
Br
..
..
..
1,4-addition
..
Br
..
..
D
path a
1,2-addition
FIGURE 13.64 Addition of Br
to
this ion would give two nonaromatic
products.
..
+
Cyclohexadienyl
cation
+
H
D
+
D
–
Br
..
..
..
..
BrD
..
..
..
BrH
..
..
FIGURE 13.65 Loss of a proton or
deuteron from the carbocation
regains aromaticity.
In the normal addition reaction of alkenes or dienes, a nucleophile such as bromide
would attack the carbocation (Fig. 13.64) to give the final product. In this case, we
would anticipate the two products of 1,2- and 1,4-addition.But aromaticity, the more
than 30 kcal/mol of delocalization energy, has been lost in each of these products.
Their formation from benzene is generally endothermic. A far better reaction that
regenerates the benzene ring is possible. Loss of a proton (or deuteron) regenerates
the aromatic sextet of π electrons (Fig. 13.65).

608 CHAPTER 13 Conjugation and Aromaticity
WORKED PROBLEM 13.24 Construct an Energy versus Reaction progress diagram
for the conversion of benzene into deuteriobenzene.
ANSWER
The first step is protonation of the benzene ring (using a deuteron, not a pro-
ton) to give the resonance-stabilized cyclohexadienyl cation. This process is surely
very endothermic, because aromaticity is lost. The second half of this symmetrical
reaction (except for the difference between the isotopes H and D) involves the
deprotonation of the intermediate to produce monodeuterated benzene. If there is
a sufficient source of deuterium, the reaction can continue until all H atoms are
replaced with D atoms.The second step is the reverse of the first, and simply is the
exothermic deprotonation of the intermediate cation to regenerate the aromatic ring.
Energy
Reaction progress
+
+
+
DH DH DH
D
3
O
+
+
D
2
OH
+
+
D
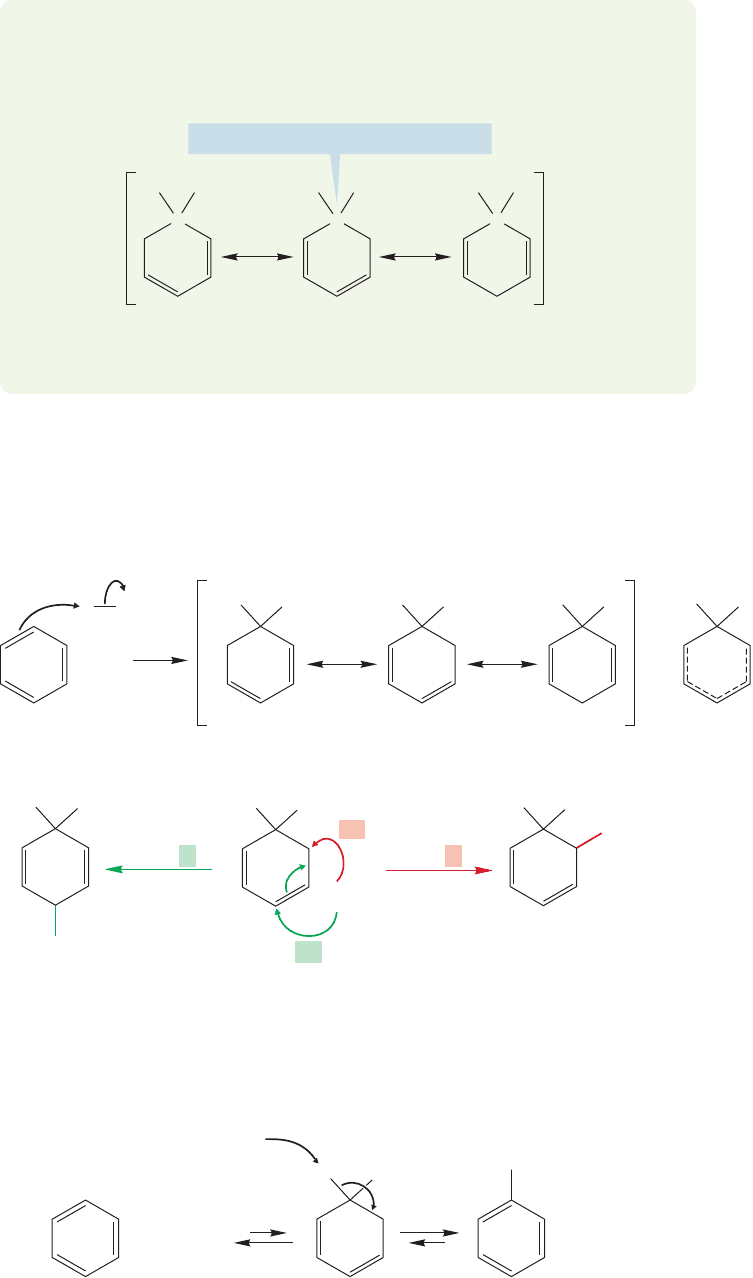
13.11b Birch Reduction of Aromatic Compounds Now we come to a reac-
tion in which aromaticity is lost, the Birch reduction. Remember that alkynes can
be treated with sodium in liquid ammonia to give, ultimately, trans alkenes (p. 452).
Catalytic hydrogenation of benzene is difficult (p. 572), but treatment of benzene
with sodium in liquid ammonia and a little alcohol leads to a reduced product—in
this case, 1,4-cyclohexadiene (Fig. 13.66).The reaction is named after the Australian
chemist Arthur J. Birch (1915–1995).
Immediately note the important difference between this method of reducing the
benzene ring and a typical catalytic reduction in which all carbon–carbon π bonds are
reduced.In this case,the ring is not totally reduced; the reaction stops at the diene stage.
Na/NH
3
1,4-Cyclo-
hexadiene
EtOH
FIGURE 13.66 Birch reduction of
aromatic compounds leads to
1,4-cyclohexadienes.
If a deuteron is lost, we see no reaction, as benzene is simply regenerated. If a
proton is lost, we see the product of an exchange reaction in which deuteriobenzene
is formed (Fig. 13.65). If a large excess of deuterio acid is used, all of the hydrogens
can be exchanged to form hexadeuteriobenzene. We will elaborate on this reaction
in Chapter 14.
