Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

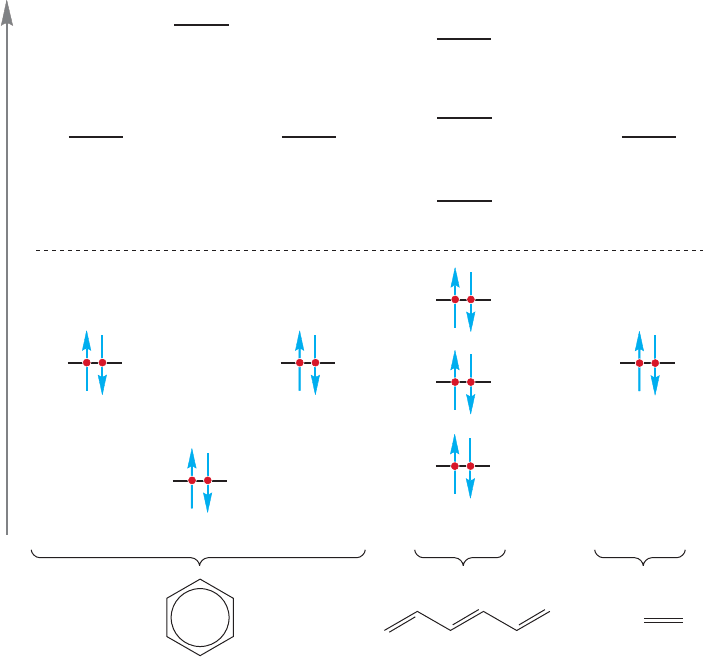
13.4 The Molecular Orbital Picture of Benzene 579
One can calculate the energy difference in terms of a quantity (β) whose value is
approximately −18 kcal/mol (Fig. 13.11). If we sum all the energies of the elec-
trons in Figure 13.11, we find an advantage for benzene over 1,3,5-hexatriene
of 1β. The energetic advantage over three separated ethylenes is even greater: 2β
(Fig. 13.11).
Energy in β
1β
Φ
2
2β
Φ
1
1β
Φ
3
0.45β
1.25β
1.8β
1β
Φ
4
Φ
5
Φ
6
Nonbonding
3
Benzene Three ethylenes1,3,5-Hexatriene
2 2
β = 4β
4 1β = 4β
––––––––––
8
β
2 0.45β = 0.9β
2 1.25β = 2.5β
2 1.80β = 3.6β
––––––––––––––
7.0
β
2 1β = 2β
for 3 molecules = 6β
FIGURE 13.11 A comparison of the total π energy of benzene, 1,3,5-hexatriene, and
three ethylenes.The calculations are based on the number of electrons and the energy of
each π electron.
Look carefully at how well the orbitals are used in benzene. There is maximum
occupancy of bonding molecular orbitals and no electrons are placed in destructive
antibonding molecular orbitals or wasted in nonbonding molecular orbitals. The
molecular orbital picture gives a stronger hint of the special stability of benzene than
does the resonance formulation.The stability comes not from a large number of res-
onance contributors, but from an especially good, especially low-energy, set of
orbitals. We will soon see other examples. Now the question is: Just how good is
this electronic arrangement? How much more stable is benzene than we might have
expected from a cyclohexatriene model?
580 CHAPTER 13 Conjugation and Aromaticity
13.5 Quantitative Evaluations of Resonance
Stabilization in Benzene
Although we can appreciate that delocalization of electrons is an energy-lowering
phenomenon, it is not easy to see intuitively whether this effect is big or small. In
this section, we see two experimental procedures for determining the magnitude of
resonance stabilization.
13.5a Heats of Hydrogenation The heat of hydrogenation of cis-2-butene
is 28.6 kcal/mol (Table 10.1, p. 413). There is no change induced by incorpo-
rating the double bond into a six-membered ring, as the heat of hydrogenation of
cyclohexene is also 28.6 kcal/mol (Fig. 13.12). A reasonable guess about the heat
of hydrogenation of a compound containing two double bonds in a ring would
be twice the value for cyclohexene, or 2 28.6 57.2 kcal/mol.This guess is
very close to being correct; the heat of hydrogenation of 1,3-cyclohexadiene is
55.4 kcal/mol. There is no special effect apparent from confining two double
bonds in a six-membered ring. The difference between the observed heat of
hydrogenation for 1,3-cyclohexadiene (55.4 kcal/mol) and the predicted value
(57.2 kcal/mol) is approximately what one would expect, because our prediction
takes no account of the stabilizing effects of conjugation in 1,3-cyclohexadiene
(p. 522).
ΔH = –28.6 kcal/mol
+ H
2
Pd
+ H
2
Pd
+ 2 H
2
Pd
ΔH = –28.6 kcal/mol
Estimate: 2 –28.6
ΔH = – 57.2 kcal/mol
Actual:
ΔH = – 55.4 kcal/mol
FIGURE 13.12 Heats of hydrogenation for some alkenes
and cycloalkenes.
Now let’s estimate the heat of hydrogenation for 1,3,5-cyclohexatriene, the
hypothetical molecule with three double bonds in a six-membered ring.We might
make this estimate by assuming that the third double bond will increase the heat
of hydrogenation of 1,3-cyclohexadiene by as much as the addition of a second dou-
ble bond did for cyclohexene [55.4 (28.6) 26.8 kcal/mol]. This incre-
ment would give a predicted value of 55.4 (26.8) 82.2 kcal/mol for the
heat of hydrogenation of the hypothetical 1,3,5-cyclohexatriene. Now we can
measure the heat of hydrogenation of the real molecule, benzene. Hydrogenation
is extremely slow under normal circumstances but there are special catalysts that
can speed the reaction up a bit, and eventually even the stable benzene will hydro-
genate to give cyclohexane. You will learn about these catalysts in Chapter 14.
The important point is that the measured heat of hydrogenation for benzene,
49.3 kcal/mol, is much lower than the calculated value for 1,3,5-cyclohexatriene.
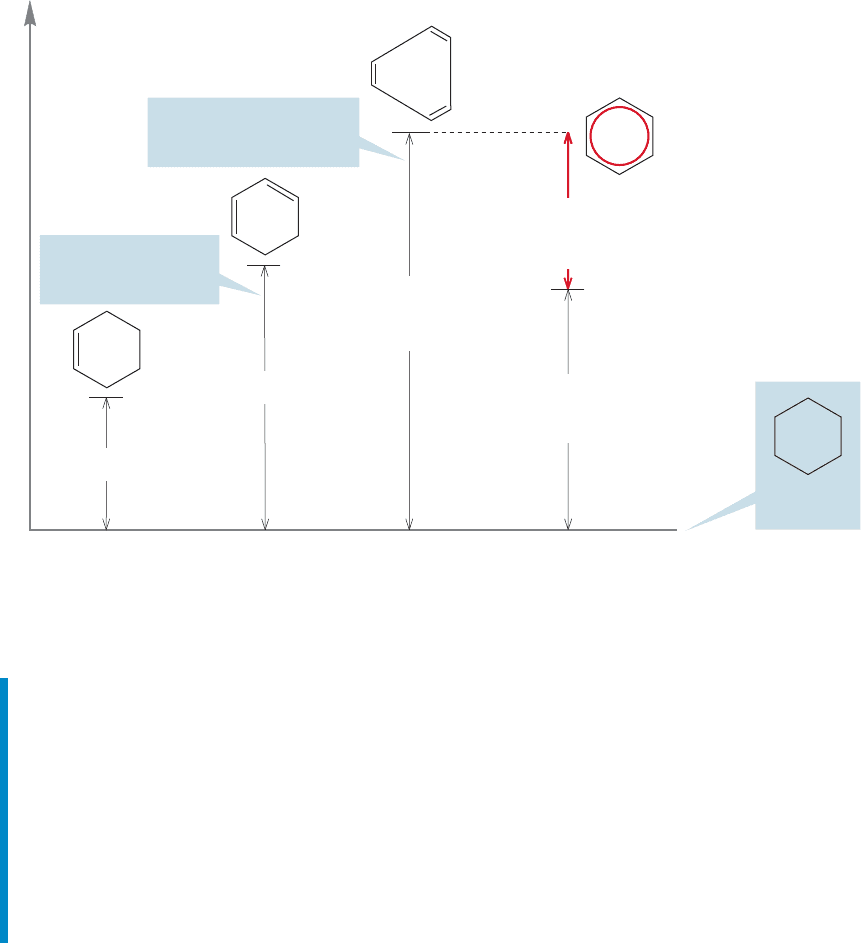
13.5 Quantitative Evaluations of Resonance Stabilization in Benzene 581
Energy
ΔH = –28.6 kcal/mol
ΔH = – 82.2 kcal/mol
estimated for hypothetical
1,3,5-cyclohexatriene
+ 3 H
2
+ 3 H
2
+ 2 H
2
+ H
2
ΔH = –55.4 kcal/mol
Experimental effect
of adding one double
bond (–26.8 kcal/mol)
Predicted effect of adding
one more double bond
(–82.2 kcal/mol)
ΔH = –49.3 kcal/mol
measured for the
real benzene
Energy of
cyclohexane
–32.9 kcal/mol
delocalization energy
of benzene
FIGURE 13.13 The value for the special stability of benzene (red arrow) as derived from
measured and calculated heats of hydrogenation. The 1,3,5-cyclohexatriene is drawn with
exaggerated bond lengths.
Figure 13.13 combines all these numbers and shows graphically how this proce-
dure yields the amount by which benzene is more stable than the hypothetical
1,3,5-cyclohexatriene, 32.9 kcal/mol (Fig. 13.13). This difference represents
the amount of energy the special stabilization of benzene is worth. It is more
than 30 kcal/mol and is called the resonance energy or delocalization energy
of benzene.
Summary
We calculated the heat of hydrogenation of the unreal, unknown, hypothetical
molecule 1,3,5-cyclohexatriene by using values for the heats of hydrogenation of
the real molecules cyclohexene and 1,3-cyclohexadiene.The heat of hydrogena-
tion of 1,3-cyclohexadiene was calculated quite accurately, so we can be rather
confident that our estimate for 1,3,5-cyclohexatriene won’t be far off. That’s
important, because we can never make the cyclic triene to check the calculation.
Any synthetic route will inevitably produce the much more stable real molecule,
benzene. The heat of hydrogenation for the real molecule, benzene, is far lower
than our estimate.
13.5b Heats of Formation The delocalization energy of benzene can be
estimated in another way, using heats of formation (ΔH
f
°). The chemistry of
1,3,5,7-cyclooctatetraene tells us there is no special stabilization in this mol-
ecule. Hydrogenation is fast and addition reactions occur with ease. The ΔH
f
°
for cyclooctatetraene is 71.23 kcal/mol, or 8.9 kcal/mol per CH unit.

582 CHAPTER 13 Conjugation and Aromaticity
VANILLIN
Vanillin
Vanilla beans
OCH
3
CHO
OH
Eugenol
H
3
CO
HO
seed pods of the vanilla orchid, Vanilla fragrens.
It appears as well in many other natural sources found in
the tropical world. It is now made synthetically from
eugenol, which, itself has a pleasant clove-like smell.
The term “aromatic” was coined because many such
compounds were odoriferous. Vanillin is a good example,
although there are a great many more. Vanillin is found
in nature attached to a sugar molecule, glucose, in the
ΔH ⬚
f
= +71.23 kcal/mol
8.9 kcal/mol per CH unit
ΔH ⬚
f
= +19.82 kcal/mol
3.3 kcal/mol per CH unit
6
⫻ (8.9 – 3.3) = 33.6 kcal/mol delocalization energy—this value compares
well with that calculated from heat of hydrogenation data (32.9 kcal/mol)
FIGURE 13.14 The delocalization
energy of benzene as calculated from
heats of formation.
The ΔH
f
° for benzene is 19.82 kcal/mol, or 3.3 kcal/mol per CH unit (Fig. 13.14).
Therefore, benzene is about 5.6 kcal/mol more stable per CH unit than a cyclic poly-
ene with no special stability (8.9 3.3 5.6 kcal/mol). Because there are six CH
units in benzene we can estimate the special stability (resonance or delocalization
energy) to be 6 5.6 33.6 kcal/mol. Note that the two methods, which use the
related heats of hydrogenation and heats of formation, are in rather good agreement.
This special stability has come to be known as benzene’s aromatic character or
aromaticity, a name derived in an obvious way from the pleasant (?) odor of many
benzene derivatives. In the next few pages, we will expand on the theme of aromaticity,
eventually ending up with a general method for identifying aromatic compounds.
13.6 A Generalization of Aromaticity:
Hückel’s 4n ⴙ 2 Rule
It would certainly be surprising if there were not other compounds sharing the spe-
cial stability we have just called aromaticity. To begin our search for other aromatic
compounds,and, ultimately,for a general way of finding such compounds,let’s review
the structural features of the prototype, benzene, that lead to special stability.
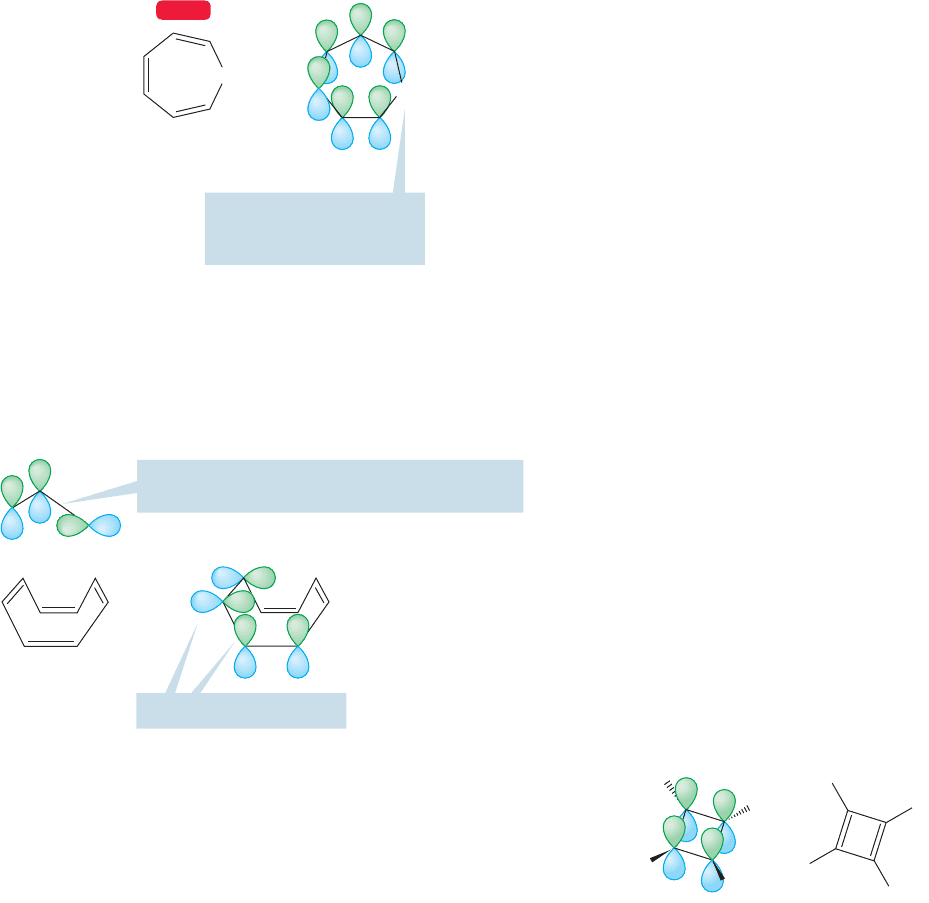
13.6 A Generalization of Aromaticity: Hückel’s 4
n
ⴙ 2 Rule 583
CH
2
CH
2
1,3,5-Cycloheptatriene
2p Orbital connectivity
at C(7) broken here by the
CH
2
group (no 2p orbital)
WEB 3D
FIGURE 13.15 In 1,3,5-
cycloheptatriene, 2p orbital
connectivity is broken by the CH
2
group. This molecule does not have
benzene’s aromaticity.
1. The molecule is cyclic.Aromaticity is a property of ring compounds. Recall the dis-
cussion of the difference between the molecular orbitals for the cyclic molecule,
benzene, and the acyclic molecule, 1,3,5-hexatriene (Fig. 13.11).
2. The molecule is fully conjugated, which simply means that there is a 2p orbital on
every atom in the ring, and that connectivity between adjacent 2p orbitals is main-
tained throughout. 1,3,5-Cycloheptatriene, for instance, is not aromatic, because
the 2p orbitals of the complete cycle do not overlap. Its properties resemble those
of the acyclic 1,3,5-hexatriene (Fig. 13.15).
If the shape of the molecule uncouples the 2p
orbitals, the molecule is effectively unconjugated
=
The tub-shaped
cyclooctatetraene
is an example of
this decoupling
phenomenon
Poor 2p connectivity here
FIGURE 13.16 The nonplanar
cyclooctatetraene is not aromatic.
A most unstable molecule, even
though it is planar, cyclic, and
fully conjugated
=
H
H
H
H
H
H
H
H
FIGURE 13.17 Cyclobutadiene is
cyclic, planar, and fully conjugated,
but it is not aromatic and it is very
unstable.
3. The molecule is planar. Why should this make a difference? It ensures optimal
overlap of the 2p orbitals. If the ring is not planar, or at least quite close to planar,
the 2p orbitals do not overlap well and some connectivity between orbitals is lost.
Large deviations from planarity can nearly or completely uncouple the cycle of
orbitals. An example is the tub-shaped molecule cyclooctatetraene (Fig. 13.16).
One might now be tempted to try a generalization. Might not all molecules that are
cyclic, planar, and fully conjugated share benzene’s aromatic character and stability?
Apparently not. Recall from Figure 13.3 that cyclobutadiene, a molecule that meets
all three criteria mentioned above, is most unstable (Fig. 13.17).
There must be more to it, and in our search for further criteria, we must take
into account the difference between benzene and cyclobutadiene. The crucial gen-
eralization was made by the German Erich Hückel (1896–1980) in the early 1930s.
His observation can be added to our previous list of three criteria (also recognized
by Hückel). It is called Hückel’s rule:
4. Aromatic systems will contain 4n 2 π electrons, where n is an integer, 0, 1, 2,
3,...Please note carefully that “n” has nothing to do with the number of atoms in the
ring. We will now discuss the significance of “n.”
We are now faced with the most important question of this section: Why should
the number of π electrons make a difference? Why does Hückel’s rule work? Briefly
stated,it works because it is only those molecules containing 4n 2 π electrons (and
satisfying the other three criteria for aromaticity) that will have molecular orbitals
arranged like those of benzene.These molecules will have a single orbital at the low-
est energy and equal energy pairs of orbitals above it. Orbitals that are equal in ener-
gy are called degenerate. When the lowest molecular orbital and all the bonding
degenerate pair(s) above it are filled, the molecule is aromatic. In “4n 2”, the “2”
is that lowest molecular orbital, and the “4n”refers to the electrons filling the degen-
erate pairs. The value of n is simply the number of filled degenerate pairs. An aro-
matic molecule is stable as a result of the filled molecular orbital system much as
the noble gases are stable as a result of their filled shells. It’s best to look at some
examples now.
cis-1,3,5-Hexatriene can serve as an example for all acyclic molecules. Although
it is fully conjugated (a 2p orbital on every carbon), and can be planar without induc-
ing great strain, it most certainly is not cyclic. The lack of a ring means that there
is no chance of strong overlap between the 2p orbitals on C(1) and C(6) and, there-
fore,no chance of aromatic character (Fig. 13.18).1,3,5-Cycloheptatriene,which we
saw in Figure 13.15, is a cyclic molecule that is not fully conjugated. The connec-
tivity of the 2p orbitals is broken by the methylene group at C(7). This molecule
cannot be aromatic. Cyclobutadiene is a planar, cyclic molecule that is fully conju-
gated. It fails only the fourth criterion because it has 4 π electrons, not a “Hückel
number” of 4n 2.
Before we can go on,we need a reliable way of finding the molecular orbital pat-
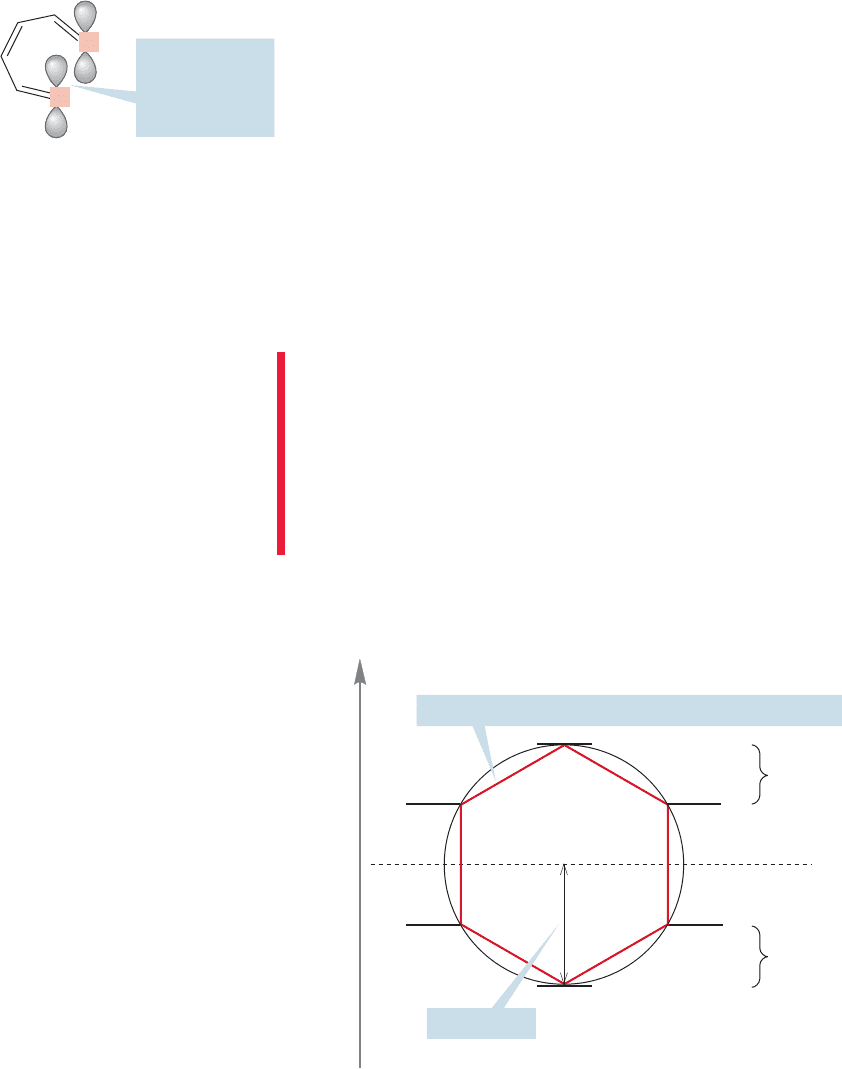
terns for these molecules. Consider the device called a Frost circle. It is named for
the American Arthur A. Frost (1909–2002) who devised it in 1953. It is a method
of finding, mercifully without the use of mathematics, the relative energies of the
molecular orbitals for planar,cyclic,fully conjugated molecules. One merely inscribes
a regular polygon, having the same number of sides as the cyclic molecule, into a
circle of radius 2β, vertex down, and the intersections of the ring with the circle will
mark the positions of the molecular orbitals.
For example, to locate the molecular orbitals of benzene, inscribe a hexagon in
a circle, vertex down as shown in Figure 13.19, with the nonbonding line dividing
the circle exactly in half.
584 CHAPTER 13 Conjugation and Aromaticity
Energy
1β
2β
1β
Hexagon inscribed inside the Frost circle, vertex down
Radius = 2β
Three antibonding orbitals
Three bonding orbitals
will hold the six available
π electrons
Nonbonding
FIGURE 13.19 The relative energies
of the molecular orbitals of benzene
derived from a Frost circle.
No strong
overlap here;
the molecule is
not aromatic
cis-1,3,5-Hexatriene
C
1
C
6
FIGURE 13.18 cis-1,3,5-Hexatriene is
fully conjugated and can be planar,
but the lack of a ring structure means
that overlap between the 2p orbitals
on C(1) and C(6) is essentially zero.
CONVENTION ALERT
13.6 A Generalization of Aromaticity: Hückel’s 4
n
ⴙ 2 Rule 585
Energy
A square inscribed
in a circle
(vertex down)
Nonbonding
molecular orbital
Antibonding molecular orbital
Nonbonding
molecular orbital
Bonding molecular orbital
FIGURE 13.20 The relative energies
of the molecular orbitals of cyclobu-
tadiene derived from a Frost circle.
Electrons have been added to this
construct after the energies of the
orbitals have been determined.
Square, delocalized cyclobutadiene, not observed
=
Rectangular
cyclobutadiene
FIGURE 13.21 In rectangular
cyclobutadiene, shown here with
bond lengths exaggerated, the orbital
connectivity is minimized. Square
cyclobutadiene has never been
observed.
The familiar (p. 578) arrangement of molecular orbitals appears, and with some
plane geometry we can accurately get the energies of the molecular orbitals in terms
of β. For benzene, the available six π electrons completely fill the three available
bonding molecular orbitals with no offending electrons remaining to be placed in
the antibonding set. There are no nonbonding molecular orbitals. Most important,
the degenerate pair of bonding orbitals is filled.
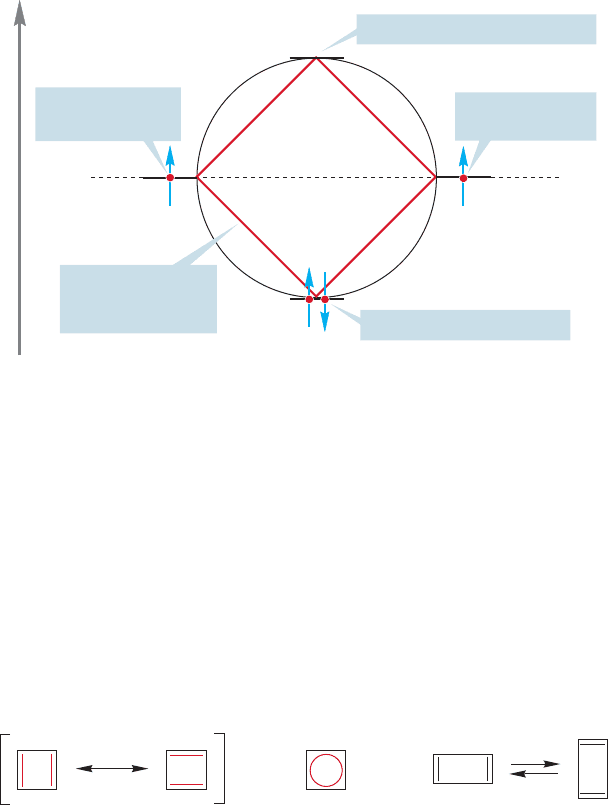
If we follow this procedure for cyclobutadiene, a set of four molecular orbitals
appears (Fig. 13.20). One is bonding, one is antibonding, and two are degenerate,
nonbonding molecular orbitals. Four π electrons are available and two of them will
surely go into the bonding molecular orbital.The remaining two electrons will have
to occupy the nonbonding degenerate pair, and will be most stable in this square
arrangement if the spins are unpaired and a single electron is put into each orbital
of the pair (Fig. 13.20). This arrangement is surely not particularly stable, and we
would not expect the properties of cyclobutadiene to resemble those of benzene, as
they most emphatically do not. It is also true that cyclobutadiene must pay a price
in ring strain as the internal angles are constrained at 90° but “want to be” 120°.
But it is not this strain that makes cyclobutadiene so especially unstable.There are
many compounds with considerable ring strain that are isolable and stable at room
temperature. Its instability is due to the fact that it has 4n π electrons. The degen-
erate orbitals have a single electron each, essentially making square cyclobutadiene
a diradical species. Remember from Chapter 11 that radicals are very reactive.
Cyclobutadiene can be isolated and observed at very low temperatures (its IR spec-
trum was measured at 8 K 265 °C), as long as it is segregated from other reac-
tive molecules, including itself. Theory and experiment agree that cyclobutadiene
distorts from the square arrangement to form a rectangle with short carbon–carbon
double bonds and longer carbon–carbon single bonds. This distortion to a rectan-
gle minimizes the conjugation of the p orbitals (Fig. 13.21).
Energy
17 kcal/mol
FIGURE 13.24 The tub-shaped
cyclooctatetraene is 17 kcal/mol more
stable than the planar, delocalized
structure.
586 CHAPTER 13 Conjugation and Aromaticity
Energy
Antibonding
molecular
orbitals
Bonding
molecular
orbitals
An octagon inscribed
in a circle (vertex down)
Nonbonding
molecular orbital
Nonbonding
molecular
orbital
FIGURE 13.22 The molecular orbitals
of planar cyclooctatetraene. Because
there are eight π electrons, two must
occupy nonbonding orbitals.
Angle strain
relieved 126°
(calculated)
Angle strain 135°
FIGURE 13.23 In planar
cyclooctatetraene, there is both
angle strain and diradical reactivity.
Both can be avoided by distorting
into a tub shape.
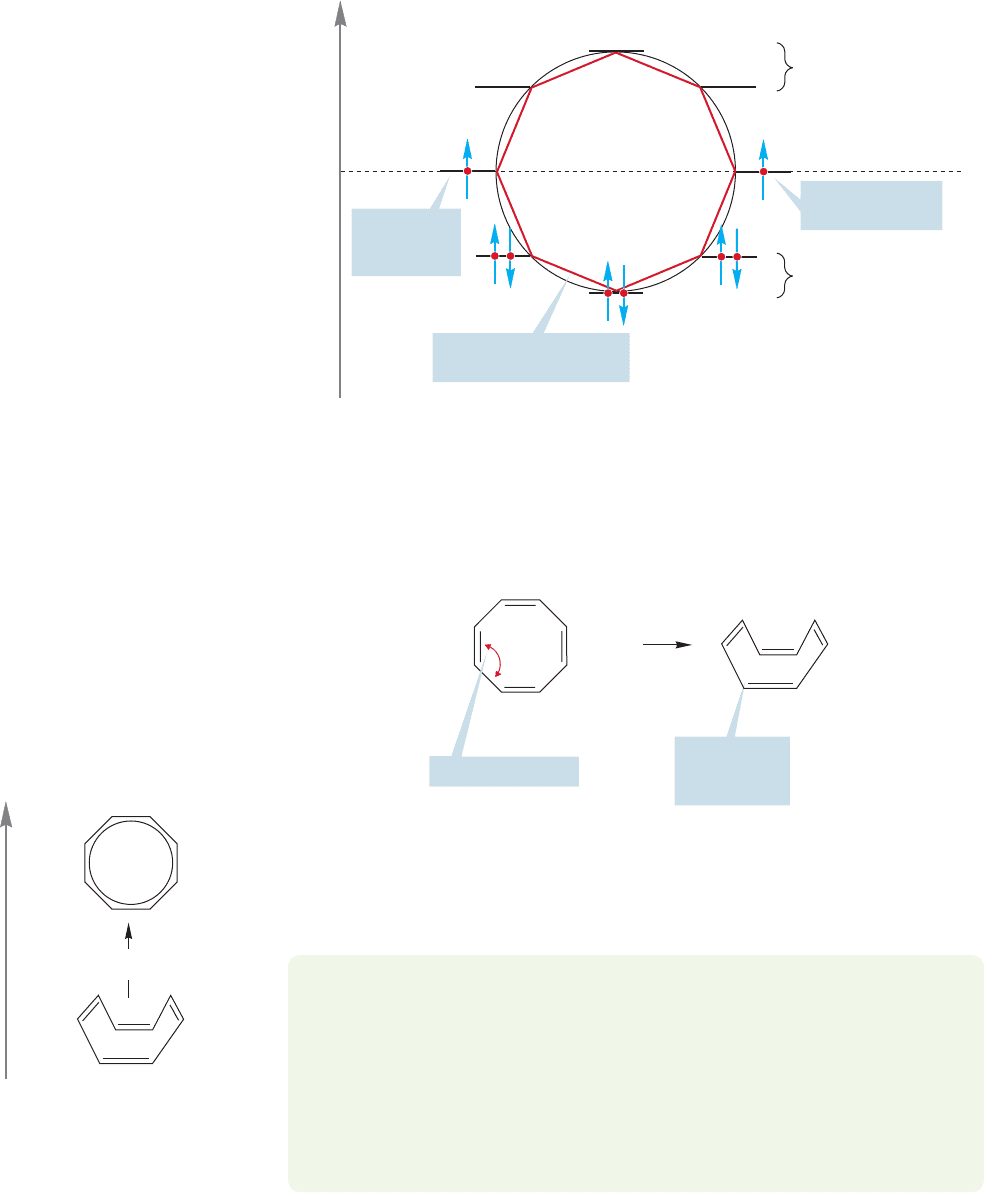
1,3,5,7-Cyclooctatetraene is another example of a 4n molecule.But here we find
neither the special stability of benzene,nor the special instability of cyclobutadiene.
The molecule behaves like four separated, normal alkenes. Let’s first examine pla-
nar cyclooctatetraene.The Frost circle again determines the relative positions of the
molecular orbitals (Fig. 13.22).
Like cyclobutadiene, planar cyclooctatetraene must have one electron in each of
the degenerate nonbonding molecular orbitals, making it a diradical. Unlike cyclobu-
tadiene, however, there is a way for cyclooctatetraene to get out of its energetic mis-
ery. Cyclobutadiene must be planar, or very nearly so. By contrast, cyclooctatetraene
can distort easily into a tublike form in which most of the angle strain and the dirad-
ical nature of the planar form is avoided (Fig. 13.23).
Kinetic measurements reveal that the energy difference between the tub and the
planar, delocalized species is 17 kcal/mol. The tub is more stable than the planar,
symmetrical, fully conjugated octagon (Fig. 13.24). This example is a rare case of
conjugation being avoided because it destabilizes the molecule.
PROBLEM 13.6 The interconversion of cyclooctatetraene forms is a bit more compli-
cated.There are actually two tub forms that interconvert rather easily.The two local-
ized tubs (alternating single and double bonds, not conjugated) equilibrate without
the double bonds changing position. The activation energy for this process is
14.6 kcal/mol. The conversion into the planar, delocalized form requires an addi-
tional 2.4 kcal/mol. Neither the localized planar form nor the delocalized planar
form is an energy minimum. In other words, they can’t be isolated. Based on this
information, sketch an Energy versus Reaction progress diagram for these processes.
PROBLEM 13.7 Which of the following molecules are properly termed “aromatic”
and which are not? For those that are not, explain why not.
13.6 A Generalization of Aromaticity: Hückel’s 4
n
ⴙ 2 Rule 587
(a)
(b)
(c)
CH
2
WEB 3D
(d)
(e)
C
H
1,3,5-Cycloheptatriene
(tropilidine)
–
BF
4
+
C
C
+
H
+
C
Trityl cation TriphenylmethaneTropylium
fluoborate
–
BF
4
H
H
FIGURE 13.25 The tropylium ion (C
7
H
7
) can be made through transfer of hydride ( )
from 1,3,5-cycloheptatriene to the trityl cation.
H
:
-
We have now discussed the lack of aromaticity in 4n molecules and we have
seen a few examples of aromaticity (benzene and some of the molecules in Problem
13.7); it would seem to be time to look at some other potentially aromatic 4n 2
molecules. It is in the stability of certain ions that the most spectacular examples
have appeared.As we have stressed over and over, small carbocations are most unsta-
ble. But there are a few exceptions to this generality. One of them is the cyclohep-
tatrienylium ion, or tropylium ion (C
7
H
7
), the ion derived from the loss of
hydride ( ) from 1,3,5-cycloheptatriene, also called tropilidene (Fig. 13.25).
Hydride cannot be simply lost from cycloheptatriene, but it can be transferred to
the trityl cation, a carbocation attached to three benzenes, which is itself a quite
stable carbocation.
H
:
-
Tropylium fluoborate appears to be stable indefinitely under normal conditions.
It sits uncomplaining on the desktop, for all the world resembling table salt. This
stuff is no ordinary carbocation!
PROBLEM 13.8 Why is the trityl cation relatively stable?
588 CHAPTER 13 Conjugation and Aromaticity
Energy
Nonbonding
+
Tropylium ion
Benzene
Antibonding
molecular
orbitals
Bonding
molecular
orbitals
A heptagon inscribed in a circle (vertex down)
FIGURE 13.27 In the tropylium ion, the lowest single orbital and the bonding degenerate molecular orbitals are fully occupied.
The molecular orbital picture of the tropylium ion closely resembles that of benzene.
The cycloheptatrienylium
(tropylium) ion is fully conjugated
+
CH
2
CH
2
CH
1,3,5-Cycloheptatriene is
not fully conjugated
C(7) C(7)
+
FIGURE 13.26 In the tropylium ion
(the cycloheptatrienylium ion)
orbital connectivity is continuous
around the ring.
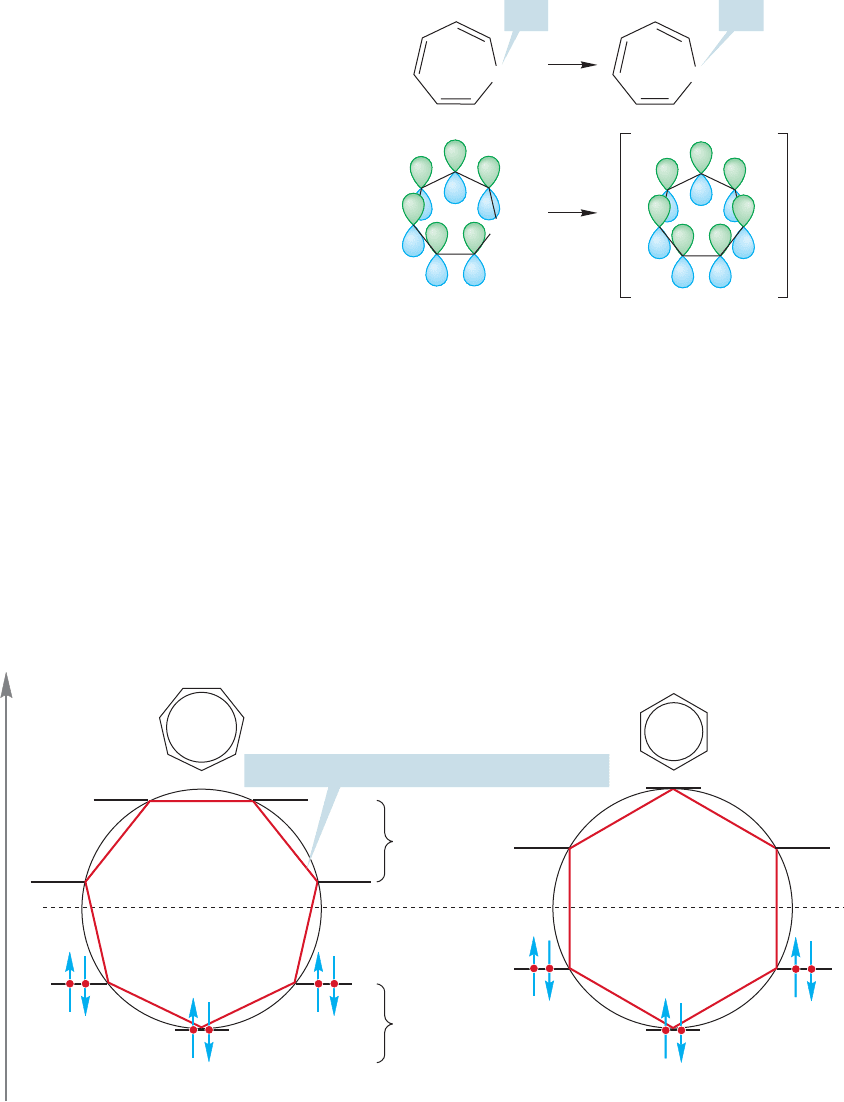
Note first that the tropylium ion has one important difference from its parent,
1,3,5-cycloheptatriene. The ion is fully conjugated (Fig. 13.26). The removal of a
hydride from the cyclic triene generates a 2p orbital at the 7-position, which once
insulated the two ends of the π system from each other. In the cation they are now
connected and this molecule is fully conjugated.
The Frost circle analysis lets us see the relative energies of the molecular orbitals
for this system (Fig. 13.27). Notice the degenerate pairs of orbitals. How many elec-
trons must go into the π system? In the cation, there is an empty 2p orbital on C(7)
and the only π electrons are the six from the three original double bonds. These elec-
trons can fit nicely into the three bonding molecular orbitals of the π system.Note how
this system resembles the arrangement of benzene—the occupied degenerate molec-
ular orbitals are full (Fig. 13.27).This ion qualifies as aromatic, and the increased sta-
bility relative to other carbocations is dramatic indeed. Notice that the number of atoms
involved in the ring—here seven—is independent of the number of π electrons—here
