Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


12.16 Additional Problems 569
PROBLEM 12.64 Allylic halides undergo the S
N
2 reaction
much faster than saturated halides (Section 12.11b). There is
another substitution reaction pathway open to allylic halides
called the S
N
2′ reaction.
+
..
Nu
Nu
..
I
S
N
2
..
..
..
I
..
..
–
I
–
..
Nu
–
..
..
..
..
+
Nu
S
N
2ⴕ
–
I
..
..
..
..
As the figure shows, in this simple example it is not possible
to distinguish the two reactions. Devise an experiment that
would allow you to tell if the product were formed through the
S
N
2 or S
N
2′ reaction.
PROBLEM 12.65 Devise an experiment using isotopic labeling
that would determine if the S
N
2 or S
N
2′ reaction were occurring
in the reaction of Problem 12.64.
PROBLEM 12.66 Now here’s a much harder problem. The
stereochemistry of the S
N
2 reaction has been well worked out
and has been described in detail in Section 7.4b. The S
N
2′
reaction has also been investigated, and this problem asks you
to put yourself in the place of R. M. Magid (b. 1938) and his
co-workers. They used the following experiment to determine
the stereochemistry of the S
N
2′ reaction. Use their data to
determine whether the nucleophile approaches the double
bond from the same side as the leaving group or from the
opposite side.
(CH
3
CH
2
)
2
NH
..
(CH
3
CH
2
)
2
N
..
+
+
H
H
H
H
H
H
D
D
Cl
..
..
..
CH
3
CH
3
(CH
3
CH
2
)
2
N
..
H
H
H
D
Major
Minor
(after deprotonation of the initially
formed ammonium ions)
CH
3
PROBLEM 12.67 Write an arrow formalism mechanism for the
following bimolecular process. Be sure to account for the stereo-
chemistry of the product.
CH(CH
3
)
2
CH(CH
3
)
2
OCOPh
NH
N
PROBLEM 12.68 Prostaglandins are a class of very important
natural products found in all animals. They are responsible for a
wide range of physiological events such as dilation of smooth
cells, aggregation of platelets, pain sensation, cell growth,
hormone regulation, and inflammatory mediation. The
central compound in the formation of prostaglandins is called
prostaglandin H
2
(PGH
2
). PGH
2
is an endoperoxide.
Endoperoxides are formed through a Diels–Alder reaction in
which oxygen (O
2
) is the dienophile. Based on the structure of
PGH
2
predict the structure of the organic compound that leads
to PGH
2
. Hint: Find the six-membered ring, then think of the
reverse Diels–Alder-like reaction that would give O
2
.
CO
2
H
HO
O
O
Prostaglandin H
2
(PGH
2
)
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 12.69 Select the reaction “1,2-Hydrohalogenation
of diene” that is on the Semester 1B panel. Observe the reac-
tion several times. When the electrophilic HBr reacts with the
nucleophilic diene, an intermediate carbocation is formed.
The animation shows the end carbon being protonated. Draw
the intermediates that could result from protonation of each of
the other carbons. Convince yourself that protonation at the
end is preferred and that protonation at either end is equivalent
in this case. Now go through this exercise with 2-methyl-
1,3-butadiene. Draw each possible intermediate and decide
which is most stable. Don’t forget resonance structures!
PROBLEM 12.70 Chose the LUMO track of the
“1,2-Hydrohalogenation of diene” and stop the animation at the
intermediate (there is only one intermediate in the energy dia-
gram). Notice the nature of this molecular orbital. It is the same
as the LUMO of an allyl cation. Does there appear to be any
more LUMO density on one carbon than the other?

570 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
PROBLEM 12.71 Look closely at the energy diagrams for the
“1,2-Hydrohalogenation of diene” and “1,4-Halogenation of
diene” reactions. Notice there is a faint pathway in the second
half of the reaction in each case. Why is the product of the
1,4 addition lower in energy than the product of 1,2 addition?
Will the 1,4-addition always be lower? Consider the HBr
addition to 1,3-pentadiene. Is the product of 1,4-addition
lower in this example?
PROBLEM 12.72 Select the “Diels–Alder reaction.” Observe the
reaction several times. Pause the animation just as the bottom
molecule has completely entered the screen. This is the point on
the “Reaction Progress” slide of the energy diagram (the x-axis)
where the reaction is about one-fourth of the way along.The red
ball has not moved. With the reaction paused in this position,
click on LUMO and then HOMO. Based on the information
that you gain from this visualization, which of the two reagents
is the nucleophile? Which is the electrophile? Why?

Conjugation and
Aromaticity
571
13.1 Preview
13.2 The Structure of Benzene
13.3 A Resonance Picture of
Benzene
13.4 The Molecular Orbital Picture
of Benzene
13.5 Quantitative Evaluations of
Resonance Stabilization in
Benzene
13.6 A Generalization of
Aromaticity: Hückel’s 4n ⴙ 2
Rule
13.7 Substituted Benzenes
13.8 Physical Properties of
Substituted Benzenes
13.9 Heterobenzenes and Other
Heterocyclic Aromatic
Compounds
13.10 Polynuclear Aromatic
Compounds
13.11 Introduction to the Chemistry
of Benzene
13.12 The Benzyl Group and Its
Reactivity
13.13 Special Topic:
The Bio-Downside, the
Mechanism of Carcinogenesis
by Polycyclic Aromatic
Compounds
13.14 Summary
13.15 Additional Problems
BAD AIR Forest fires are a significant source of polycyclic aromatic hydrocarbons,
which make up the solid, cancer-causing particulates that come from combustion.
13
572 CHAPTER 13 Conjugation and Aromaticity
CR
2
CR
2
CR
2
CR
2
Br
2
1,2- and
1,4-Additions
excess
1,2- and
1,4-Additions
CR
2
CR
2
Diels–Alder
H
2
/Pt
H
2
/Pt
CR
2
CR
2
Diels–Alder
HBr
Br
2
HBr
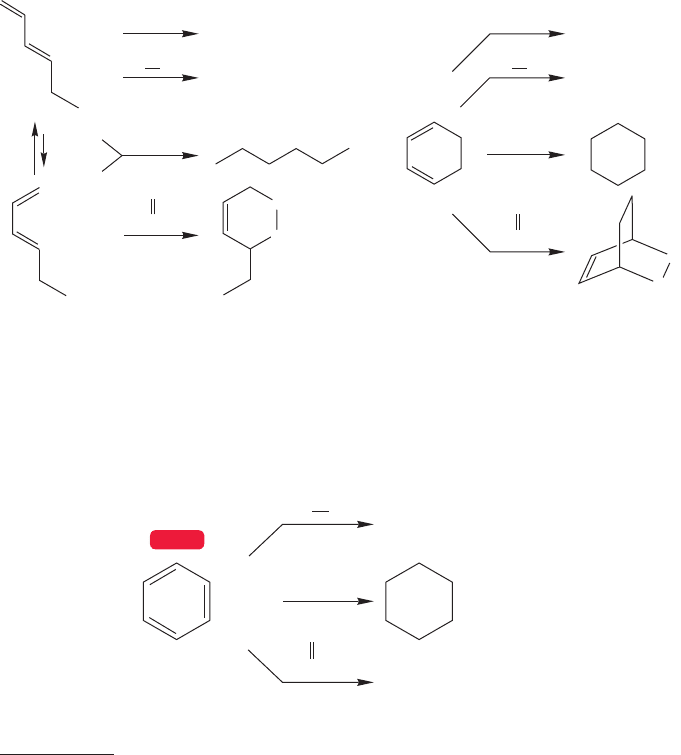
FIGURE 13.1 Acyclic and cyclic
dienes have similar chemical
properties.
H
2
/Pt
Very slowly formed
Benzene
“1,3,5-Cyclohexatriene”
H
Br
No reaction
Diels–Alder
CR
2
CR
2
No reaction
WEB 3D
FIGURE 13.2 Benzene
(“1,3,5-cyclohexatriene”) does not
undergo most of the reactions of
normal alkenes.
1
Vikram Seth was born in Calcutta in 1952 and educated in India, Britain, and the United States. This
brilliant novel gets the MJ 10-star recommendation.
Curious, though, isn’t it, um, Patwardhan, that the number, er, six
should be, um, embodied in one of the most, er, er, beautiful, er, shapes
in all nature: I refer, um, needless to say, to the, er, benzene ring with
its single, and, er, double carbon bonds. But is it, er, truly symmetrical,
Patwardhan, or, um, asymmetrical? Or asymmetrically symmetrical,
perhaps. . . .
—VIKRAM SETH,
1
A SUITABLE BOY
13.1 Preview
Chapter 12 was devoted to the consequences of conjugation—the overlap of 2p
orbitals in acyclic systems. Now we will see what happens when conjugated systems
are cyclized, turned into rings. Nothing much changes if the atoms of the rings do
not maintain orbital overlap throughout the ring—if they are not fully conjugated.
1,3-Cyclohexadiene is not especially different from 1,3-hexadiene, for example
(Fig. 13.1).There are small differences in chemical and physical properties, but the
two molecules clearly belong to the same chemical family. Hydrogenation is easy,
addition reactions abound, the Diels–Alder reaction is common, and so on.
When the conjugation is continued around the 1,3-cyclohexadiene ring to give
what we might call “1,3,5-cyclohexatriene,” matters change (Fig. 13.2). None of
the reactions shown in Figure 13.1 is easy, and this molecule appears to be remark-
ably unreactive. It is clearly in a different class from 1,3-cyclohexadiene and the
acyclic dienes.
13.2 The Structure of Benzene 573
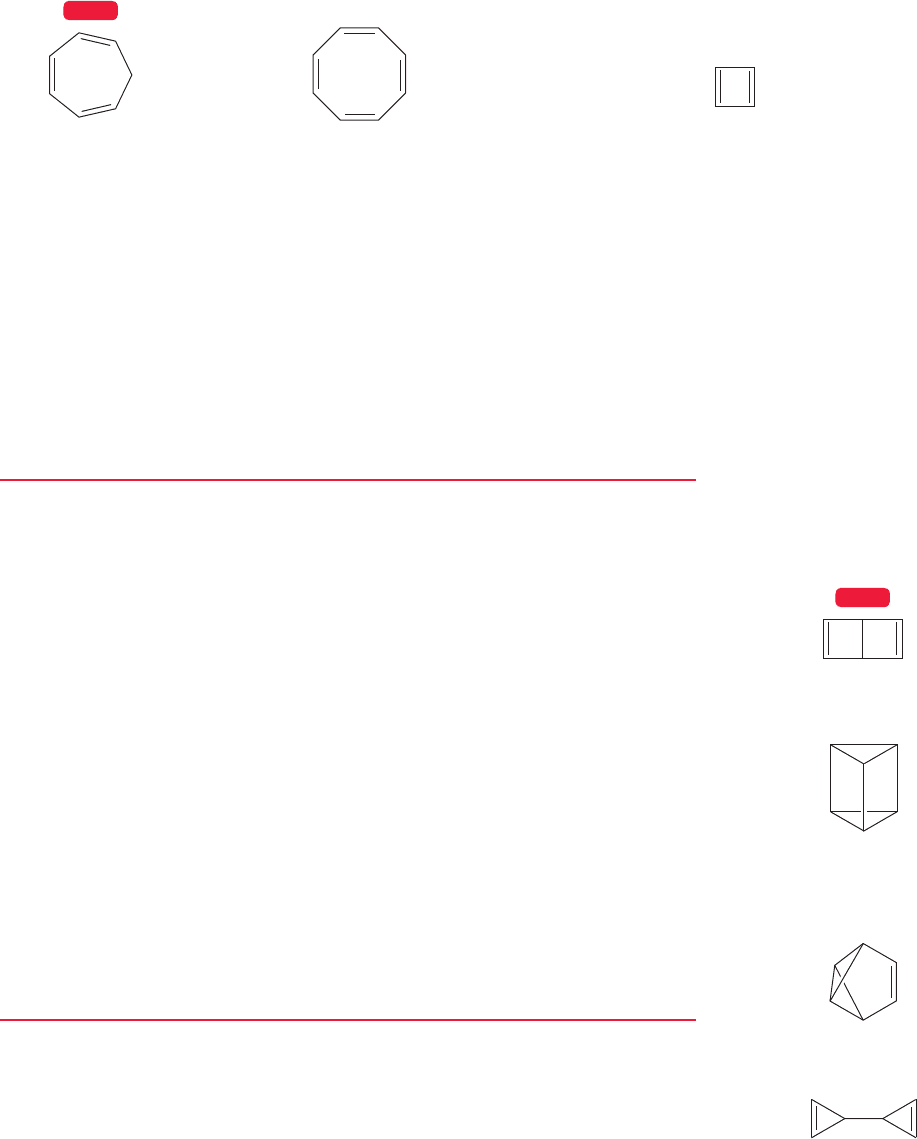
1,3,5-Cycloheptatriene
1,3,5,7-Cyclooctatetraene
Cyclobutadiene
These are normal polyenes (for example, they undergo
addition reactions and hydrogenate easily)
This molecule is extraordinarily unstable—it can
only be detected at very low temperature
WEB 3D
FIGURE 13.3 Many cyclic polyenes are either normal in their chemical properties or exceptionally unstable.
In this chapter, we will uncover the sources of these differences in stability
between cyclic polyenes and begin to see the chemical and physical consequences
of this special kind of conjugative interaction of 2p orbitals. We will encounter the
molecule benzene (Fig.13.2), in which the overlap of six carbon 2p orbitals in a ring
has great consequences for both structure and reactivity. We will also see a general-
ization of the properties that make benzene so stable, and will learn how to predict
which cyclic polyenes should share benzene’s stability and which should not.
But not all ring compounds containing double bonds behave in this way. Some
cycloalkenes react as expected; cycloheptatriene and cyclooctatetraene are examples
of this kind of cyclic molecule. Others are exceptionally unstable; cyclobutadiene is
the archetype of this kind of cyclic polyene (Fig. 13.3).
ESSENTIAL SKILLS AND DETAILS
1. The properties that determine whether or not a molecule is properly termed “aromatic”
are simple to write down but are a bit more difficult to apply. It is very important that
you be able to think about these qualities, to see why they apply to certain molecules
and why they do not apply to others. Understanding the hard-to-define quality called
aromaticity is critical.
Chemists have argued for years about the special stability called aromaticity that
attends cyclic, planar polyenes with the proper numbers of π electrons.The wonderful
quote that begins this chapter is not so far from an accurate representation of remarks
heard at many a chemical conference! However, even though it is not easy to define this
slippery concept precisely, especially in marginal cases, it is not difficult to understand
the simple examples, to see why the number of π electrons is important, and to learn
which molecules are likely to exhibit this property.
2. This chapter introduces the generic aromatic substitution reaction. We will see this
reaction again many, many times in Chapter 14, but it would be a very good idea if you
arrived at that chapter knowing the basics.
3. The Frost circle allows you to determine quickly the relative energies of the π
molecular orbitals for any planar, cyclic, fully conjugated polyene. Remember: The
polygon must be inscribed vertex down.
4. Aromaticity is not magic! It is just another stabilizing effect that can be augmented or
opposed by other effects.
13.2 The Structure of Benzene
The molecule in Figure 13.2 that might be called “1,3,5-cyclohexatriene” was iso-
lated from the thermal rendering of whale blubber in the nineteenth century.
Although the formula was established as C
6
H
6
[or better, (CH)
6
], there remained a
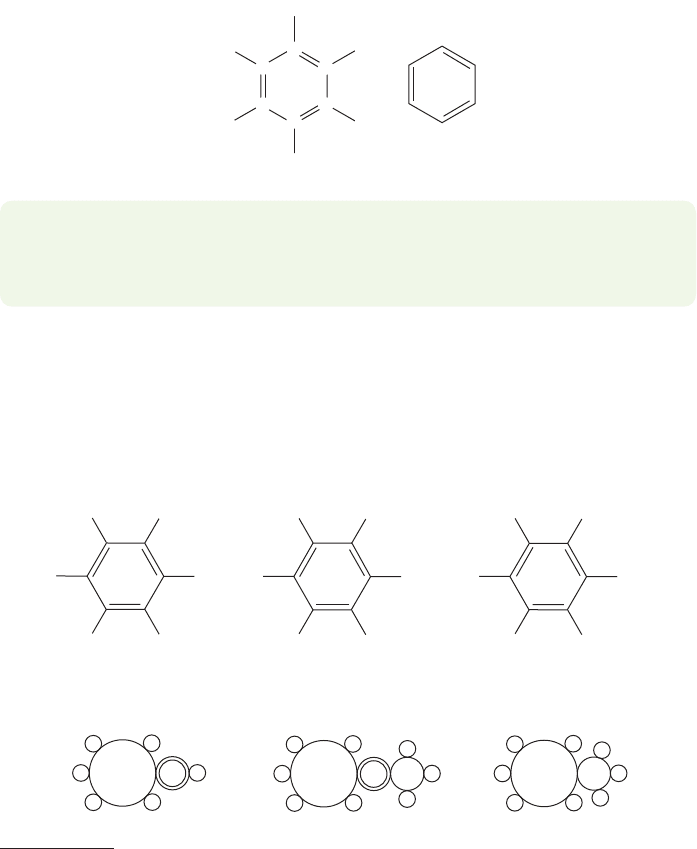
serious problem: What was its structure? There are many possibilities, among them
the four shown in Figure 13.4. Two of these, Dewar benzene and Ladenburg
Bicyclo[2.2.0]hexa-2,5-diene
(Dewar benzene)
Tetracyclo[2.2.0.0
2,6
.0
3,5
]hexane
(Ladenburg benzene or prismane)
3,3'-Bicyclopropenyl
Tricyclo[3.1.0.0
2,6
]hex-3-ene
(benzvalene)
WEB 3D
FIGURE 13.4 Four possible structures
for benzene.
574 CHAPTER 13 Conjugation and Aromaticity
C C
C C
C
C
H
H
H
H
H
H
FIGURE 13.5 The structure
of benzene, drawn as
1,3,5-cyclohexatriene.
OH
H
H
H H
H
OCH
3
H
H
H H
H H
H
H H
H
Traditional, or Kekule
´,
structures
The same structures written by J. J. Loschmidt in 1861
CH
3
FIGURE 13.6 Loschmidt’s
formulations of benzene structures
(see footnote).
benzene, are named for the people who suggested them, Sir James Dewar
(1842–1923) and Albert Ladenburg (1842–1911). The third, benzvalene, is more
cryptically named.The last compound in Figure 13.4, 3,3′-bicyclopropenyl, was first
made only in 1989 by a group headed by W. E. Billups (b. 1939) at Rice University.
The German chemist Friedrich August Kekulé von Stradonitz (1829–1896) sug-
gested the correct ring structure.Legend has it that Kekulé was provoked by a dream
of a snake biting its tail. Kekulé describes dozing and seeing long chains of carbon
atoms twisting and turning until one “gripped its own tail and the picture whirled
scornfully before my eyes.”Kekulé’s suggestion for the structure of benzene is shown
in cyclohexatriene form in Figure 13.5.
2
Kekulé gets the lion’s share of the credit, but in the mid-1800s there were others hot on the trail of structur-
al theory in general and a decent representation of benzene in particular. One such chemist was Alexandr M.
Butlerov (1828–1886) from Kazan, Russia, who may well have independently conceived of the ring structure
for benzene, and who surely contributed greatly, if quite invisibly in the West, to the development of much of
modern chemistry. Another, even more obscure contributor, was the Austrian chemist–physicist Johann Josef
Loschmidt (1821–1895) who, four years before Kekulé’s proposal in 1865, clearly wrote a ring structure for
benzene. Figure 13.6 shows how forward-looking were the structures proposed by this too-little-known
Austrian schoolteacher.
PROBLEM 13.1 Suppose Dewar, Ladenburg, and Kekulé had had a
13
C NMR spec-
trometer. How many signals would the various candidates shown in Figure 13.4
show in their spectra? How many would cyclohexatriene show?
But there were problems with the Kekulé formulation
2
as well as with the four
shown in Figure 13.4. Benzene didn’t react with halogens or hydrogen halides like
any self-respecting polyene, for example. Hydrogenation was much slower than for
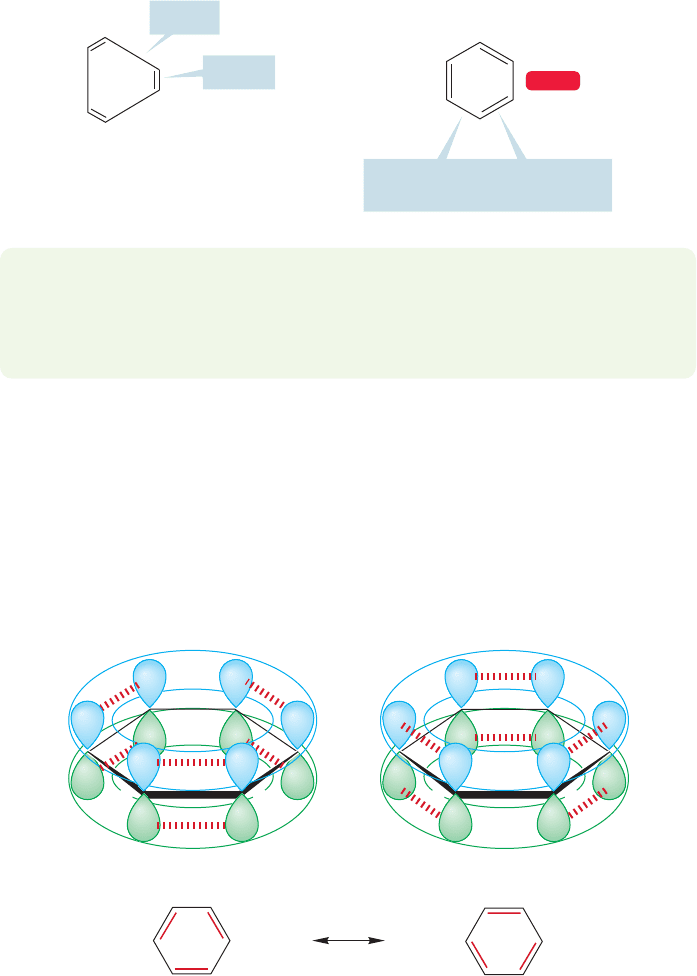
13.3 A Resonance Picture of Benzene 575
other alkenes and required severe conditions. Benzene was clearly a remarkably sta-
ble compound, and the cyclohexatriene structure couldn’t account for this.
Moreover, it eventually became known that the structure of benzene was that of
a regular hexagon (i.e., equal sided). Our 1,3,5-cyclohexatriene is immediately ruled
out as a structural possibility, because it must contain alternating long single bonds
and short double bonds. We might expect normal double bonds of about 1.33 Å and
single bonds of about the length of the bond in 1,3-butadiene: 1.47 Å.
Instead, benzene has a uniform carbon–carbon bond distance of 1.39 Å. Note that
the measured bond distance, 1.39 Å, is just about the average of the double-bond
distance, 1.33 Å, and the single-bond distance, 1.47 Å (Fig. 13.7). At this point, you
can probably see the beginnings of the answer to these structural problems. Kekulé’s
static cyclohexatriene formulation doesn’t take account of resonance stabilization.
C(2)
O
C(3)
1.33 A
⬚
Identical bond lengths (1.39
A
⬚
).
How can this be?
1.47 A
⬚
The structure expected for
1,3,5-cyclohexatriene
(bond length differences
exaggerated)
Real structure
WEB 3D
FIGURE 13.7
Any 1,3,5-cyclohexatriene structure
must contain alternating long single
bonds and short double bonds
(shown here with exaggerated short
and long bonds). Such is not the
case for benzene, which is a
regular hexagon with a uniform
carbon–carbon bond distance
of 1.39 Å.
(a)
23
1
4
(b)
23
1
4
5
5
6
6
FIGURE 13.8 These two Kekulé
forms together provide a real
structure for benzene. The dashed
lines represent the overlap implied in
the line structures.
PROBLEM 13.2 One early problem with the Kekulé structure was that only one
1,2-disubstituted isomer of benzene could be found for any given substituent.
That is, there is only one 1,2-dimethylbenzene (o-xylene). Explain why this
observation would have been hard for Kekulé to explain.
13.3 A Resonance Picture of Benzene
Perhaps the remarkable stability of benzene can be explained just by realizing that
delocalization of electrons is strongly stabilizing.There are really two Kekulé forms
for benzene. They are resonance forms, differing from each other only in the distri-
bution of electrons but not in the positions of atoms. An orbital picture for cyclo-
hexatriene should have influenced us in this direction. Each carbon is hybridized sp
2
and there is, therefore, a 2p orbital on every carbon. The structure in Figure 13.8a
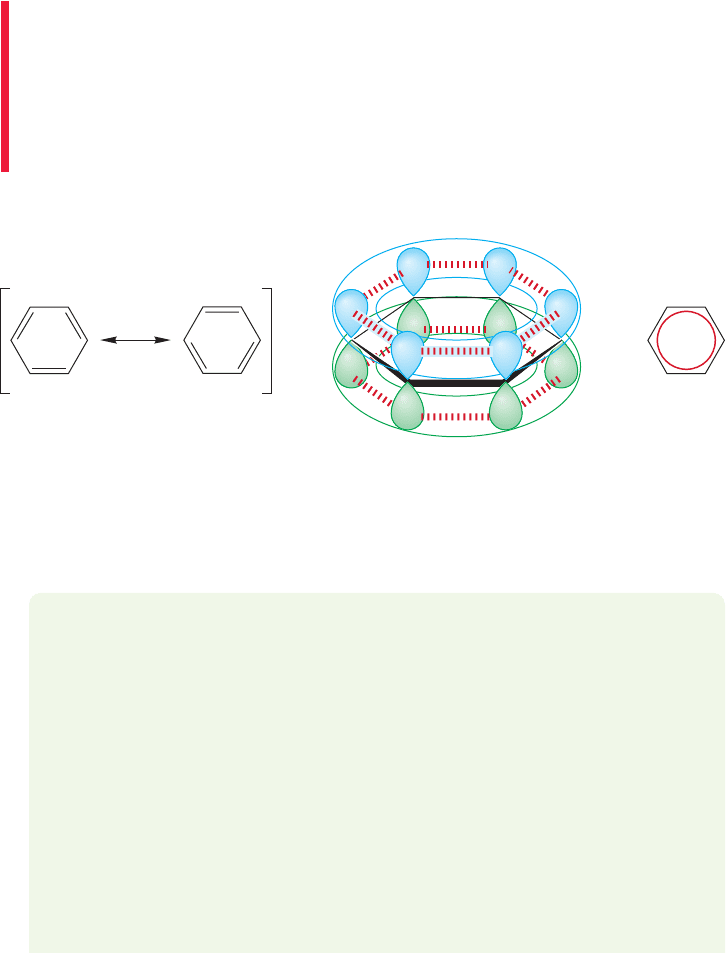
576 CHAPTER 13 Conjugation and Aromaticity
stresses overlap between the 2p orbitals on C(1) and C(2), C(3) and C(4),
and C(5) and C(6), but not between C(2) and C(3), C(4) and C(5), or C(6)
and C(1). This specificity is suspicious, because there is a 2p orbital on every
carbon atom and the drawing of Figure 13.8a takes no account of the symmetry
of the situation. If there is overlap between the orbitals on C(1) and C(2),
then there must be equivalent overlap between the 2p orbitals on C(2) and C(3)
as well, as shown in Figure 13.8b. Drawing both Kekulé forms emphasizes
this, and produces a regular hexagon as the combination of the two Kekulé reso-
nance forms.
So, our picture of benzene has now been elaborated somewhat to show the
orbitals. The 2p orbitals on the six carbons, extending above and below the plane
containing the carbons and their attached hydrogens, overlap to form a circular
cloud of electron density above and below the plane of the ring.
A convention has been adopted that attempts to show the cyclic overlap of
the six 2p orbitals. Instead of drawing out each double bond as in the Kekulé
forms, a circle is inscribed inside the ring (Fig. 13.9). The cyclic overlap of 2p
orbitals is nicely evoked by this formulation, but a price is paid. The Kekulé
forms are easier for bookkeeping purposes: for keeping track of bonds making
and breaking in chemical reactions. We will use the Kekulé form most often in
this book.
=
=
FIGURE 13.9 There is a ring of electron density above and below the six-membered
benzene ring. The circle in the center of the benzene ring is evocative of the cyclic overlap
of the six 2p orbitals.
CONVENTION ALERT
WORKED PROBLEM 13.3 There are three other resonance forms for benzene,
which can be included to get a slightly better electronic description of the mol-
ecule than is provided by the pair of Kekulé forms alone. In these other resonance
forms, overlap between two p orbitals on “across the ring” carbons is taken into
account. Draw these three resonance forms, called Dewar forms, showing the
orbitals involved in the “cross-ring” bond. Be careful! This problem uses bonding
that you probably haven’t seen before.
ANSWER Any resonance form represents one electronic description for a mol-
ecule and ignores all others. For example, in one resonance form for the allyl
cation, overlap between the 2p orbitals on C(1) and C(2) is emphasized, and the
2p orbital on C(3) is written as if it did not overlap. This error is taken into
(continued)
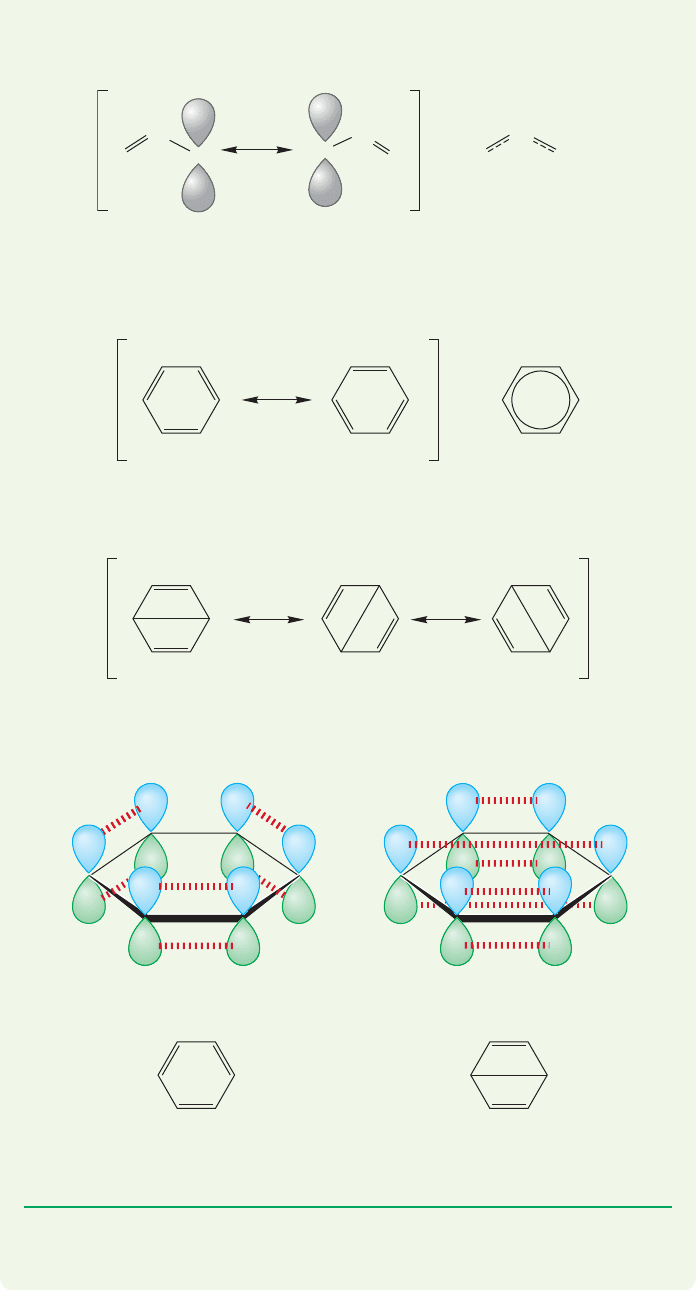
13.3 A Resonance Picture of Benzene 577
For the central bond to be a σ bond, atoms would have to move, and that cannot
be done in resonance forms. Only electrons can move.
PROBLEM 13.4 Do you think the Dewar forms will contribute strongly to the
benzene structure? Why or why not?
C
3
Summary structure
C
3
C
2
C
1
C
1
=
C
2
+
C
3
C
2
C
1
+
+
The two Kekulé forms of benzene shown in Figure 13.8 make similar approx-
imations and do a good job of giving the structure of benzene only when taken in
combination.
=
In the Dewar forms, 1,4-overlap is explicitly emphasized. There are three Dewar
forms that differ in which 1,4-overlap (1,4; 3,6; or 2,5) is counted.
Notice that this business is tricky. By now, your eye is trained to see this central
bond as a
σ bond, and it isn’t! As in the Kekulé forms, it is 2p/2p, π overlap:
14
23
6
3
65
2
5
One Dewar
resonance form
One Kekule´
resonance form
23
1
65
4
23
1
65
4
account in the other resonance form that shows overlap between C(3) and C(2),
and ignores any overlap with the 2p orbital on C(1).
578 CHAPTER 13 Conjugation and Aromaticity
PROBLEM 13.5 There is a real compound (CH)
6
called Dewar benzene containing
a pair of fused (they share an edge) cyclobutene rings (Dewar benzene is correctly
named bicyclo[2.2.0]hexa-2,5-diene). This molecule is not flat, and therefore not
a resonance form of benzene, but a separate molecule potentially in equilibrium
with benzene (see figure below). Make a good three-dimensional drawing of
Dewar benzene.
Energy
Three antibonding
molecular orbitals
Three bonding
molecular orbitals
Nonbonding
FIGURE 13.10 The π molecular
orbitals of benzene.
H
H
WEB 3D
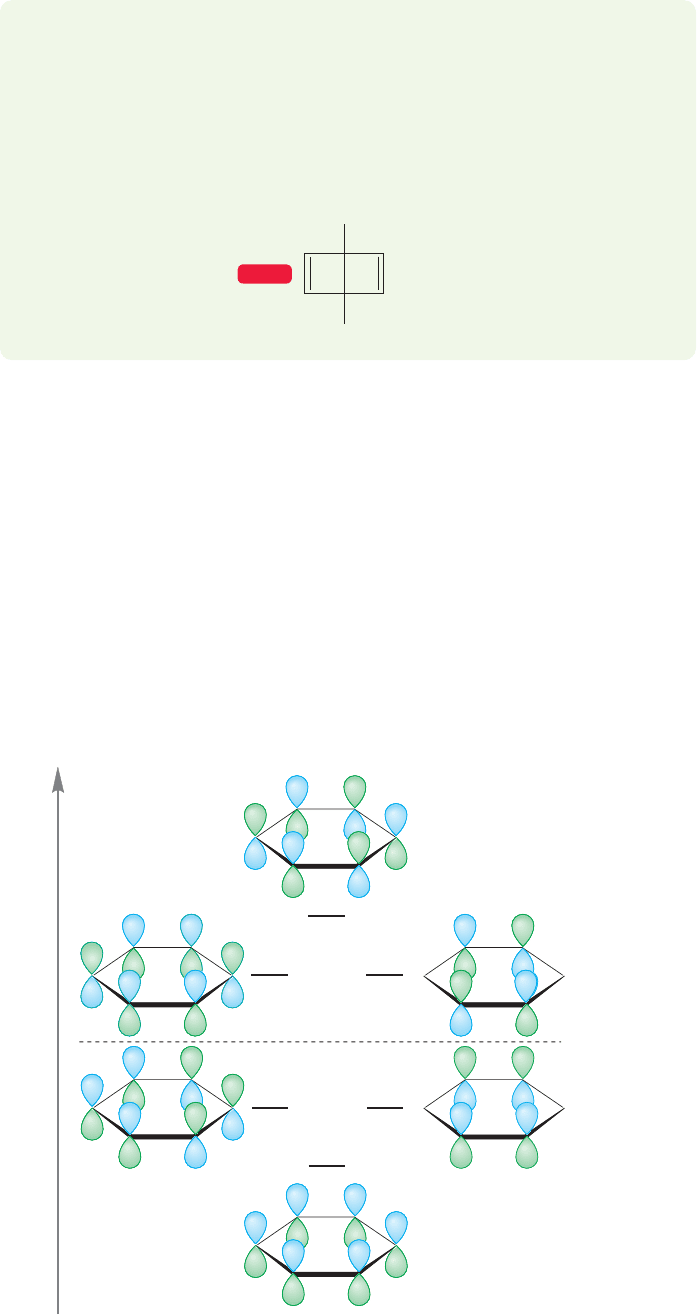
13.4 The Molecular Orbital Picture of Benzene
The raw material for a calculation of the molecular orbitals of benzene is six 2p
orbitals in a ring. Linear combination of these atomic orbitals leads to a set of six
molecular orbitals: three bonding and three antibonding.Their symmetries and rel-
ative energies are shown in Figure 13.10.
There are six electrons to go into the system, one from each carbon, and these
electrons naturally occupy the three lowest-energy, bonding molecular orbitals. The
antibonding molecular orbitals are empty. If we compare benzene to a system of three
double bonds not tied together into a ring (1,3,5-hexatriene is a good model) we
can see that there are substantial energetic savings involved in the ring structure.
