Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

13.6 A Generalization of Aromaticity: Hückel’s 4
n
ⴙ 2 Rule 589
CH
2
H
H
2
C
H
C
..
CH
2
H
2
C
H
C
CH
2
H
2
C
H
C
CH
2
H
H
3
C
CH
2
pK
a
= 43
pK
a
~ 50–60
–
..
–
..
CH
2
–
H
3
C
CH
2
FIGURE 13.28 Resonance stabilization of its conjugate base makes propene far more acidic than propane.
..
–
base, B
Energy
Antibonding
molecular
orbitals
Bonding
molecular
orbitals
Nonbonding
A pentagon inscribed in a circle (vertex down)
BH
+
pK
a
= 15
The cyclopentadienyl
anion is easily formed
CH
2
..
CH
–
FIGURE 13.29 Cyclopentadiene is
quite a strong acid.The cyclopen-
tadienyl anion is an aromatic species.
Notice the filled degenerate orbitals.
For an anion, it is extremely stable.
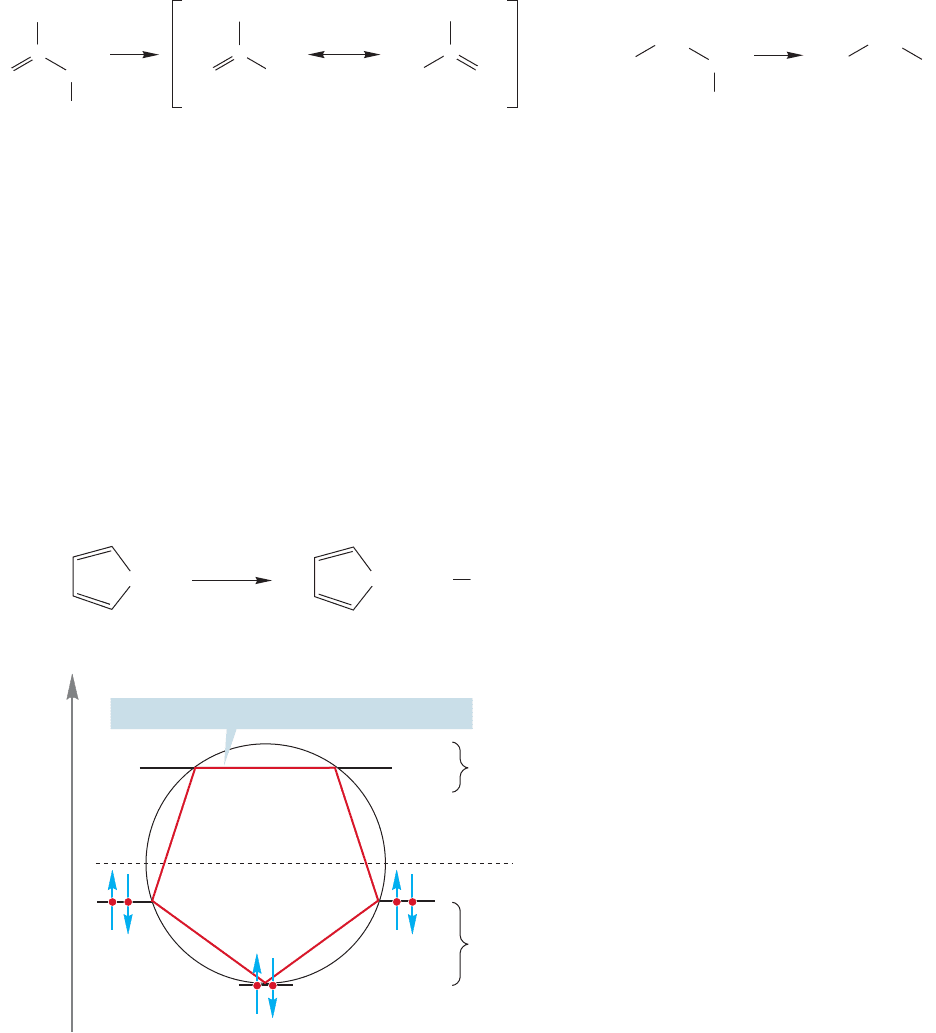
six (4n 2), n 1.The value of n is simply the number of filled degenerate pairs.
Hückel’s rule is 4n 2 because aromaticity requires a filled degenerate pair of orbitals
(the 4n part) and the lowest single orbital is filled first (the 2 part).
There is an anionic counterpart to the stable tropylium cation, the cyclopenta-
dienyl anion.The pK
a
of propene is 43,and the pK
a
of propane is 50–60.The added
delocalization in the allyl anion makes propene a stronger acid than propane by a
factor of about 10
20
(Fig. 13.28). But the pK
a
of 1,3-cyclopentadiene is 15!
Cyclopentadiene is a much stronger acid than propene. The difference in acidity is
enormous. Look carefully at the structure of the cyclopentadienyl anion. Here too,
we have a planar, cyclic, and fully conjugated system.The molecular orbitals can be
derived from a Frost circle (Fig. 13.29).There are six electrons to put into the molec-
ular orbitals, and, as in the tropylium ion or benzene, they fully occupy the lowest
molecular orbital and the set of degenerate bonding molecular orbitals.The cyclopen-
tadienyl anion can be described as aromatic, and for an anion, this species is remark-
ably stable. Do not fall into the trap of expecting this anion to be as stable as benzene.
Benzene is a neutral molecule in which all of carbon’s valences are satisfied.The anion
is charged and contains unsatisfied valence. It is very stable, but only in comparison
with the rest of the family of carbon anions.
But wait. We have been at pains to equate stability with delocalization of
electrons, which results in a distribution of charge to several atoms in the molecule.
590 CHAPTER 13 Conjugation and Aromaticity
Both the tropylium ion and the cyclopentadienyl anion can be described by a most
impressive array of resonance forms (Fig. 13.30).
3
Named for the philosopher William of Ockham (1285–1349; he died during the bubonic plague), who put
it better: “What can be done with fewer is done in vain with more.”
–
+
Cyclopentadienyl
ion
Cycloheptatrienylium
ion
(tropylium ion)
=
=
–
–
–
–
–
..
..
..
..
..
+
+
+
+
+
+
+
FIGURE 13.30 Resonance descriptions
of the tropylium (cycloheptatrienylium)
cation and the cyclopentadienyl anion.
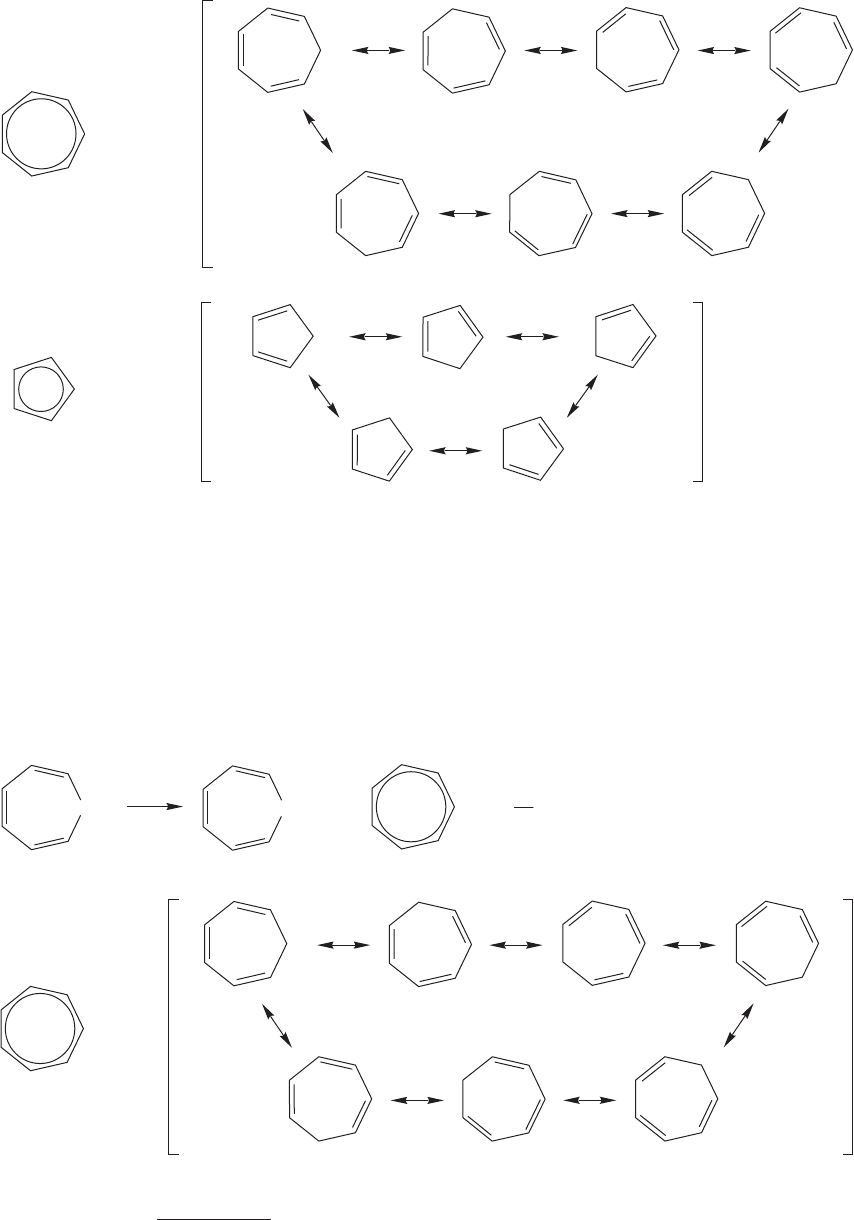
Perhaps the aromaticity concept is unnecessary. Why attribute the stability of
these species to aromaticity when simple delocalization of electrons may do as well?
That question is a good one, and needs an answer. It is not necessary to invoke spe-
cial effects if simpler concepts will suffice. The principle of “Ockham’s razor”
3
says
that when faced with a variety of equally attractive explanations, pick the simplest.
Wielding Ockham’s razor, we might say that the cyclopentadienyl anion is very
stable because of the five resonance forms so apparent in Figure 13.30. What then
would we expect of the cycloheptatrienyl anion, whose seven resonance forms are
shown in Figure 13.31? If an ion with five resonance forms is stable, surely one
base
–
B
..
..
CH
2
–
Cycloheptatrienyl
anion
=
–
CH
BH
=
+
–
..
–
..
–
..
–
..
–
..
–
..
–
..
–
FIGURE 13.31 The seven resonance forms for the cycloheptatrienyl anion.
13.6 A Generalization of Aromaticity: Hückel’s 4
n
ⴙ 2 Rule 591
with seven forms will be even more stable. Our explanation predicts a formidable
acidity for 1,3,5-cycloheptatriene; in particular, it must be more acidic than
cyclopentadiene. Its pK
a
must be . However, the pK
a
of cycloheptatriene is
about 39. Cycloheptatriene is a weaker acid than cyclopentadiene by a factor of
10
24
(Fig. 13.32)!
615
pK
a
= 15
..
CH
2
CH
BH
=
+
–
CH
2
..
CH
=
BH
+
–
pK
a
= 39
Five resonance forms
Seven resonance forms
–
–
base
–
B
..
base
–
B
..
FIGURE 13.32 Cycloheptatriene is
a weaker acid than cyclopentadiene
by a factor of 10
24
.
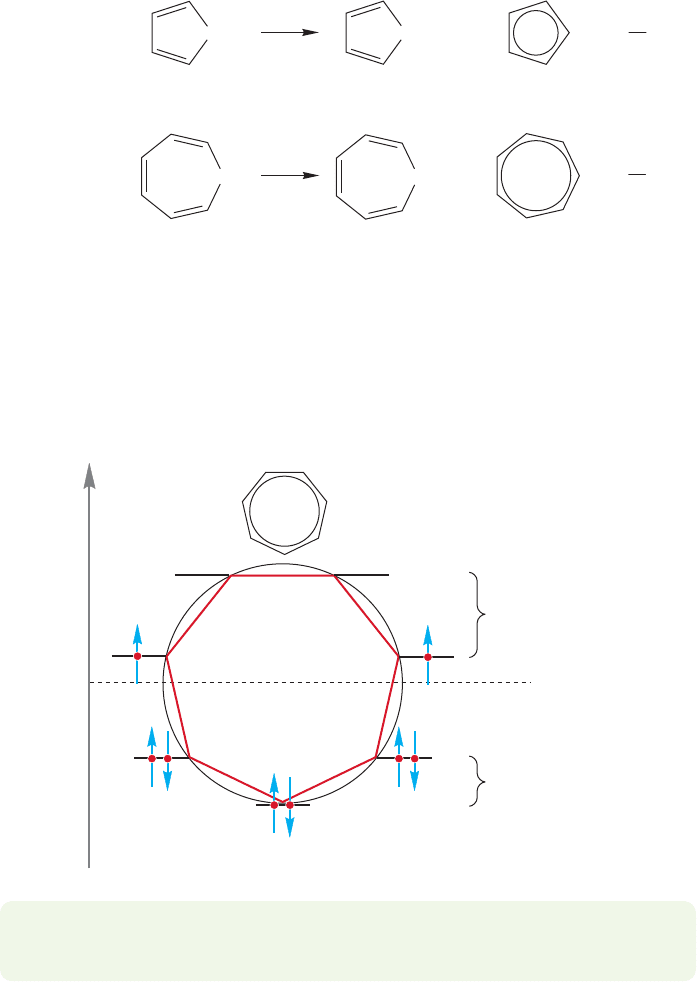
So,the number of resonance forms is not always a predictor of stability. No longer
are we faced with equally attractive explanations.The aromaticity concept is neces-
sary to make sense of the data. Aromaticity trumps resonance. A look at the molec-
ular orbitals of cycloheptatrienyl anion shows the source of the instability. The
cycloheptatrienyl anion must contain unpaired electrons in degenerate antibonding
orbitals (Fig. 13.33)! It is not aromatic, it is a diradical.
–
Energy
Antibonding
molecular orbitals
Bonding
molecular orbitals
Nonbonding
Cycloheptatrienyl anion
FIGURE 13.33 In the cyclohepta-
trienyl anion, two electrons must
occupy degenerate antibonding
orbitals.
PROBLEM 13.9 How many signals do the
13
C NMR spectra of the cyclopentadienyl
anion and the tropylium cation show?
Now let’s search for other neutral compounds exhibiting aromaticity. Of course,
all manner of substituted benzenes exist, but such aromatic compounds hardly con-
stitute exciting new examples. It is possible to combine benzene rings so that they
share an edge in what is called a fused relationship (p. 211). These molecules are
also aromatic.Atoms other than carbon can be introduced into the ring while main-
taining the sextet of electrons to produce heterobenzenes, which are fully conju-
gated, six-membered rings containing one or more noncarbon atoms (N, O, S, etc.).
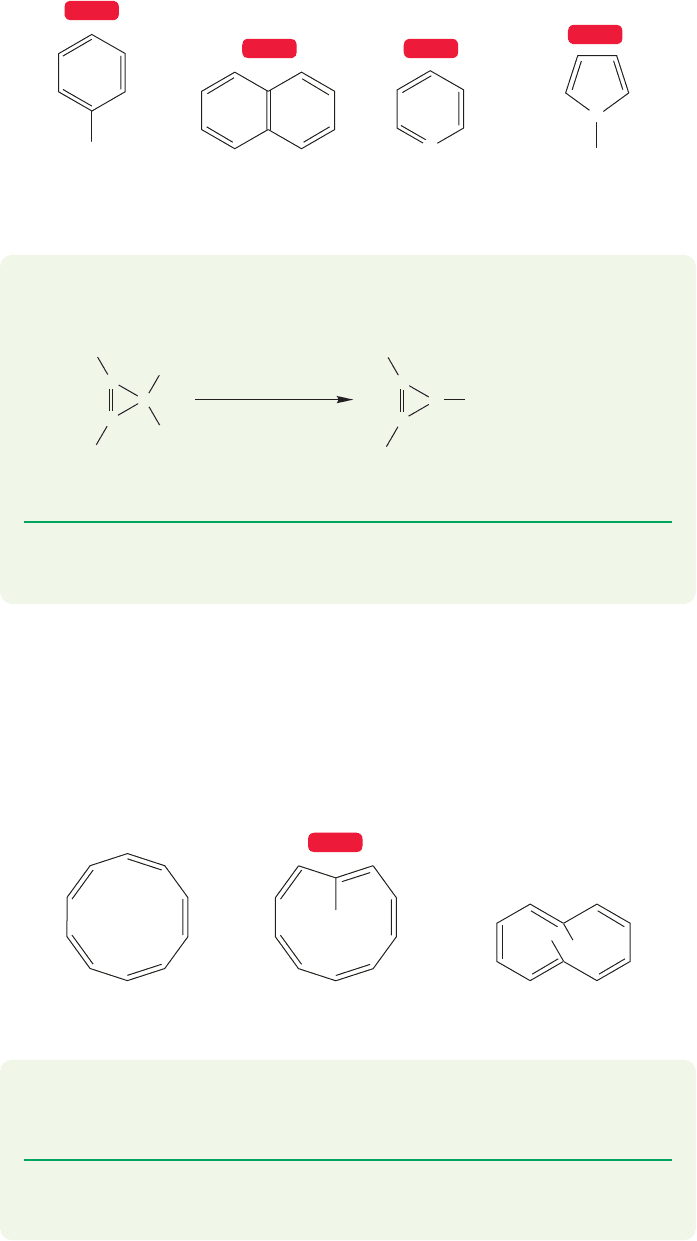
Some smaller rings also contain a sextet of π electrons; pyrrole is an example.
Molecules of these various types are shown in Figure 13.34, and will be considered
further in later sections.
592 CHAPTER 13 Conjugation and Aromaticity
Toluene
(a simple substituted
benzene)
..
CH
3
Naphthalene
(a fused, two-ring
aromatic compound)
N
Pyridine
(a six-membered
heterobenzene)
Pyrrole
(a five-membered
aromatic compound)
..
N
H
WEB 3D
WEB 3D WEB 3D
WEB 3D
FIGURE 13.34 Some simple exten-
sions of aromaticity: substituted,
fused, and heterobenzene compounds,
as well as a five-membered ring
aromatic compound.
PROBLEM 13.10 It is startlingly easy to form the cyclopropenyl cation. Explain
why. For this system, what is the value of n in Hückel’s formula?
A cyclopropenium ion
C
C
R
R
R
H
C
(C
6
H
5
)
3
CH
(C
6
H
5
)
3
C
–
BF
4
+
C
C
R
R
+
+
R
C
What we are looking for here are not examples of relatively straightforward
extensions, but new 4n 2 molecules. You have learned that n can be any integer.
We have seen many examples of n 1. In Problems 13.7 and 13.10 we have seen
n 0, 2, 3, and 4.
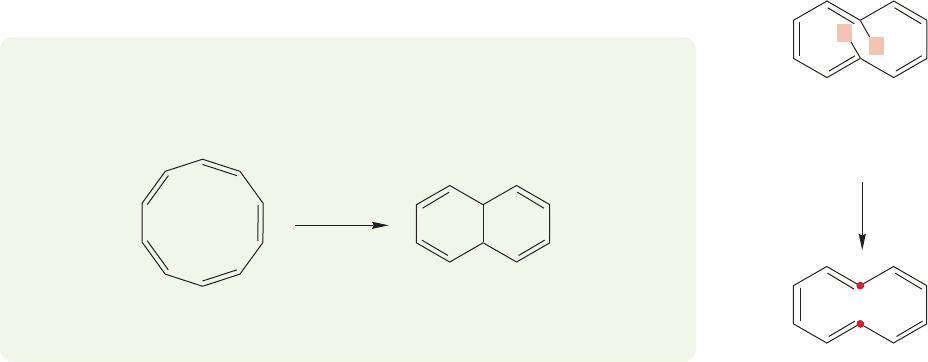
Let’s consider the cyclodecapentaenes, cyclic molecules with 10 π electrons
(4n 2, n 2) (Fig. 13.35). Are they as stable as the six-electron molecules we
have been examining?
cis,cis,cis,cis,cis-
Cyclodecapentaene
trans,cis,cis,cis,cis-
Cyclodecapentaene
H
H
H
trans,cis,trans,cis,cis-
Cyclodecapentaene
WEB 3D
FIGURE 13.35 Some
cyclodecapentaenes.These molecules
have 10 π electrons (4n 2 10,
n 2).There are two pairs of
degenerate orbitals that are filled.
PROBLEM 13.11 Do you expect the corresponding cyclopropenyl anion to be stable?
Explain.
PROBLEM 13.12 The three isomeric cyclodecapentaenes of Figure 13.35 differ in
the stereochemistries of the double bonds. Why isn’t there more than one isomer
of benzene?
PROBLEM 13.13 How many signals would be seen in the
13
C NMR spectra of the
three cyclodecapentaenes in Figure 13.35?
13.6 A Generalization of Aromaticity: Hückel’s 4
n
ⴙ 2 Rule 593
A remarkable amount of effort was expended on the synthesis of the cyclodec-
apentaenes before they were successfully made.The magnitude of this effort should
make us suspicious; aromatic compounds are everywhere and extraordinarily easy to
make. Moreover, the known cyclodecapentaenes are not only not especially stable,
they are instead strikingly unstable. So, we have some explaining to do.We have been
relying on a theory that predicts special stabilization conferred by the aromatic 4n 2
number of π electrons, and the cyclodecapentaenes are members of the 4n 2 club.
If we cannot explain why these molecules are unstable, Hückel’s rule must be aban-
doned. It turns out to be not too difficult to resolve the problem. Aromatic stability
is not some magical force. It is just one kind of stabilizing effect, which in the face
of stronger destabilizing effects will not prevail.Put simply, the planar cyclodecapen-
taenes are destabilized by an amount greater than aromaticity can overcome. What
might those destabilizing effects be? Look first at the molecule on the right in
Figure 13.35, the trans,cis,trans,cis,cis isomer. For the molecule to be flat, the two
hydrogens inside the ring must occupy the same space.This crowding induces extraor-
dinary strain that is greater than any resonance stabilization. The molecule cannot
be flat. Aromaticity cannot compensate for this strong destabilizing effect.
The trans,cis,cis,cis,cis isomer, the middle molecule in Figure 13.35, has some
of the same problems, although here only one hydrogen is inside the ring. In this
molecule,however, there is severe angle strain,and the planar form, required for aro-
maticity, is badly destabilized.
The all-cis isomer, the molecule on the left in Figure 13.35, has even more angle
strain, and it too can be planar, and therefore aromatic, only at a prohibitively high
energy cost. The benefits of delocalization are overwhelmed by the angle strain.
A good set of models will show this strain easily. Construct the planar, all-cis
cyclodecapentaene and the strain will be apparent. If you are lucky, the plastic will
mimic the molecule and the model will snap into a pretzel shape, which approxi-
mates the energy minimum form of the molecule.These molecules are best described
as strained polyenes. They fail the test of planarity for aromatic molecules because
the planar structures are much more strained than the nonplanar forms. In this case,
strain trumps aromaticity.
PROBLEM 13.14 The all-cis cyclodecapentaene forms a new, cross-ring bond (see
below). As the molecule warms up, a bicyclo[4.4.0]decatetraene is formed. Write
an arrow formalism for this reaction.
cis,cis,cis,cis,cis-
Cyclodecapentaene
Bicyclo[4.4.0]deca-
2,4,7,9-tetraene
Δ
So we haven’t really judged the limits of the theory of aromaticity by using the
cyclodecapentaenes. We might be able to if the offending strain could somehow be
removed. On paper, this transfomation is simple. For example, we might just erase
the two inside hydrogens in one of the molecules (Fig. 13.36). Unfortunately, this
erasure doesn’t give a real molecule because this structure has two trivalent carbons
at the positions marked with dots in Figure 13.36.
trans,cis,trans,cis,cis-
Cyclodecapentaene
(strain caused by the
inside red hydrogens)
H
H
magic
hydrogen
eraser
(much less strain! But…
what is going on at the
red-dot positions?)
FIGURE 13.36 The strain caused
by two inside hydrogens in one
cyclodecapentaene can be eliminated
by removing the offending
hydrogens.
594 CHAPTER 13 Conjugation and Aromaticity
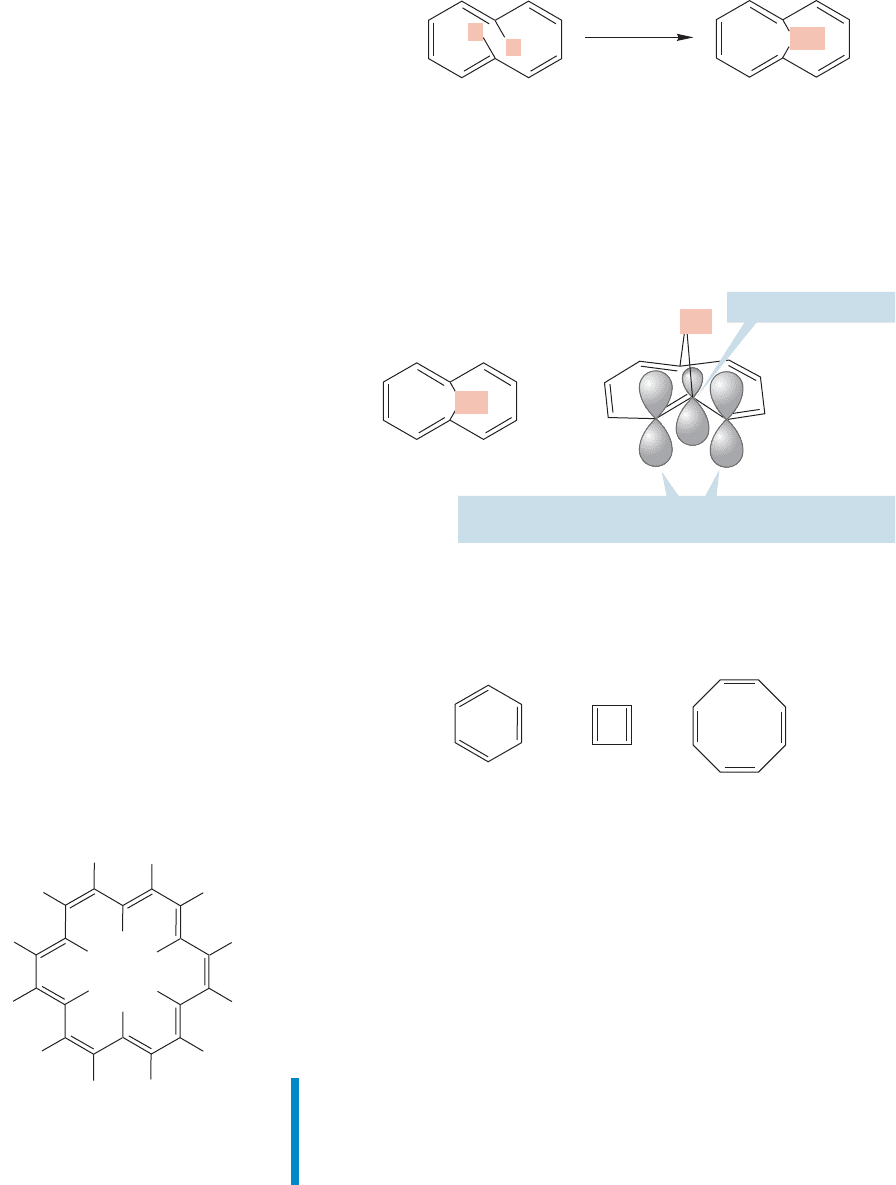
A particularly clever German chemist, Emanuel Vogel (b. 1927), found a way
to do this erasure chemically. He used a remarkable synthesis to replace the offend-
ing inside hydrogens with a bridging methylene group (Fig. 13.37). The removal
of the offending hydrogens eliminates the strain that comes from their “bumping”
into each other.
H
H
Vogel’s clever
synthesis
CH
2
FIGURE 13.37 Vogel’s bridged
cyclodecapentaene.
The resulting molecule, a bridged system containing 10 π electrons, isn’t a per-
fect cyclodecapentaene. The overlap between the 2p orbitals at the bridgehead and
adjacent positions isn’t optimal, for example (Fig. 13.38). Still, the molecule main-
tains enough orbital overlap to remain fully conjugated and satisfy the criteria for
aromaticity (cyclic, flat, conjugated, and 4n 2 π electrons). Both its chemical and
physical properties allow it to be classified as aromatic.
Bridgehead position
=
Overlap here is not ideal (make a model!), but it is good
enough to allow substantial electron delocalization
CH
2
CH
2
FIGURE 13.38 Vogel’s molecule is
fully conjugated, but orbital overlap is
not optimal, as it is, for example, in
benzene. Nonetheless, this molecule
is aromatic.
The generic term for monocyclic, fully conjugated molecules is annulene.
Benzene could be called [6]annulene, square cyclobutadiene is [4]annulene, and
planar cyclooctatetraene is [8]annulene (Fig. 13.39).
[6]Annulene
[4]Annulene [8]Annulene etc.
FIGURE 13.39 Some simple annulenes.
Some other annulenes, besides [6]annulene,appear to be aromatic.These annu-
lenes are large enough so that the destabilizing steric effects present in the cyclodec-
apentaenes ([10]annulenes) are diminished, and severe angle strain is avoided.
A nice example is [18]annulene,a planar molecule in which all carbon–carbon bond
lengths are very similar. It contains 4n 2 π electrons, where n 4 (Fig. 13.40).
The description of this molecule as aromatic derives largely from an examination of
its nuclear magnetic resonance (NMR) spectrum, and that must wait for Chapter 15,
but [18]annulene does appear to be aromatic.
[18]Annulene
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
FIGURE 13.40 [18]Annulene.
Summary
Planar, cyclic, fully conjugated molecules containing 4n 2 π electrons are espe-
cially stable and are called “aromatic.” The “4n 2 rule” works because such mol-
ecules have a set of filled degenerate molecular orbitals, in a sense a closed shell.
13.7 Substituted Benzenes 595
We have concentrated on the phenomenon of aromaticity as applied to benzene
and larger and smaller polyenes. We saw two experimental methods of evaluat-
ing the stabilization of aromaticity quantitatively, and with the aid of Hückel’s
insight, we found a generalization of the phenomenon.Now we move on to ways
of varying the structure of our prototypal example, benzene, and then take a quick
look at reactivity.
Generic name
R
Phenyl R
CH
3
CH(CH
3
)
2
NH
2
OH
Toluene
(methylbenzene or
phenylmethane)
OCH
3
Anisole
(methyl phenyl ether or
methoxybenzene)
Phenol
(benzenol or
hydroxybenzene)
Aniline
(benzenamine or
aminobenzene)
Cumene
(isopropylbenzene or
2-phenylpropane)
Ethylbenzene
(phenylethane)
COOH
CHO
CH
2
X
Benzyl X
Generic name
Biphenyl
(phenylbenzene)
Benzaldehyde
(benzenecarboxaldehyde)
Benzoic acid
(benzenecarboxylic
acid)
Styrene
(vinylbenzene or
ethenylbenzene)
Benzyl chloride
(chlorophenylmethane)
Benzyl alcohol
(phenylmethanol)
Br
Bromobenzene
HC
CH
2
CH
2
CH
3
CH
2
Cl CH
2
OH
FIGURE 13.41 Some simple
monosubstituted benzenes. Common
names are widely used (boldface).
13.7 Substituted Benzenes
13.7a Mono- and Disubstitution Monosubstituted benzenes are usually named
as derivatives of benzene, and the names are written as single words.There are numer-
ous common names that have survived a number of attempts to systemize them out
of existence. You really do have to know a number of these names in order to be lit-
erate in organic chemistry.We don’t think the systematic “benzenol”will replace “phe-
nol”in your lifetime,for example.At least we hope it doesn’t. Figure 13.41 shows some
simple benzenes with both the systematic and common names.
596 CHAPTER 13 Conjugation and Aromaticity
Two of the most often used common names are phenyl (sometimes even Ph),
which stands for a benzene ring with an open valence (the ring is attached to an R
group, for example), and benzyl, which stands for the group that has an
open valence. Both are shown in Figure 13.41.
When one Kekulé structure is drawn, you know that you must (mentally or
physically) draw the other form as well for a full picture.
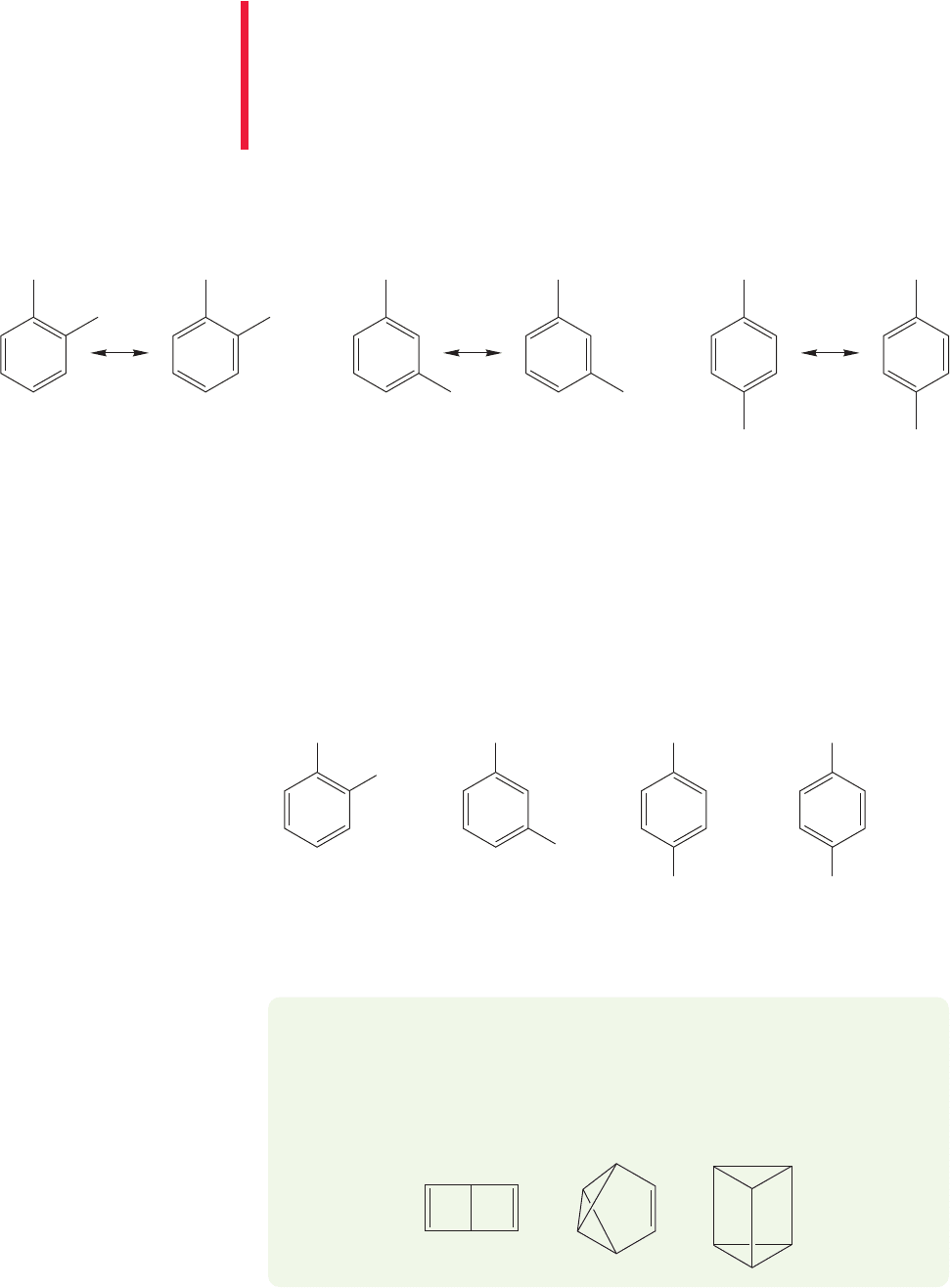
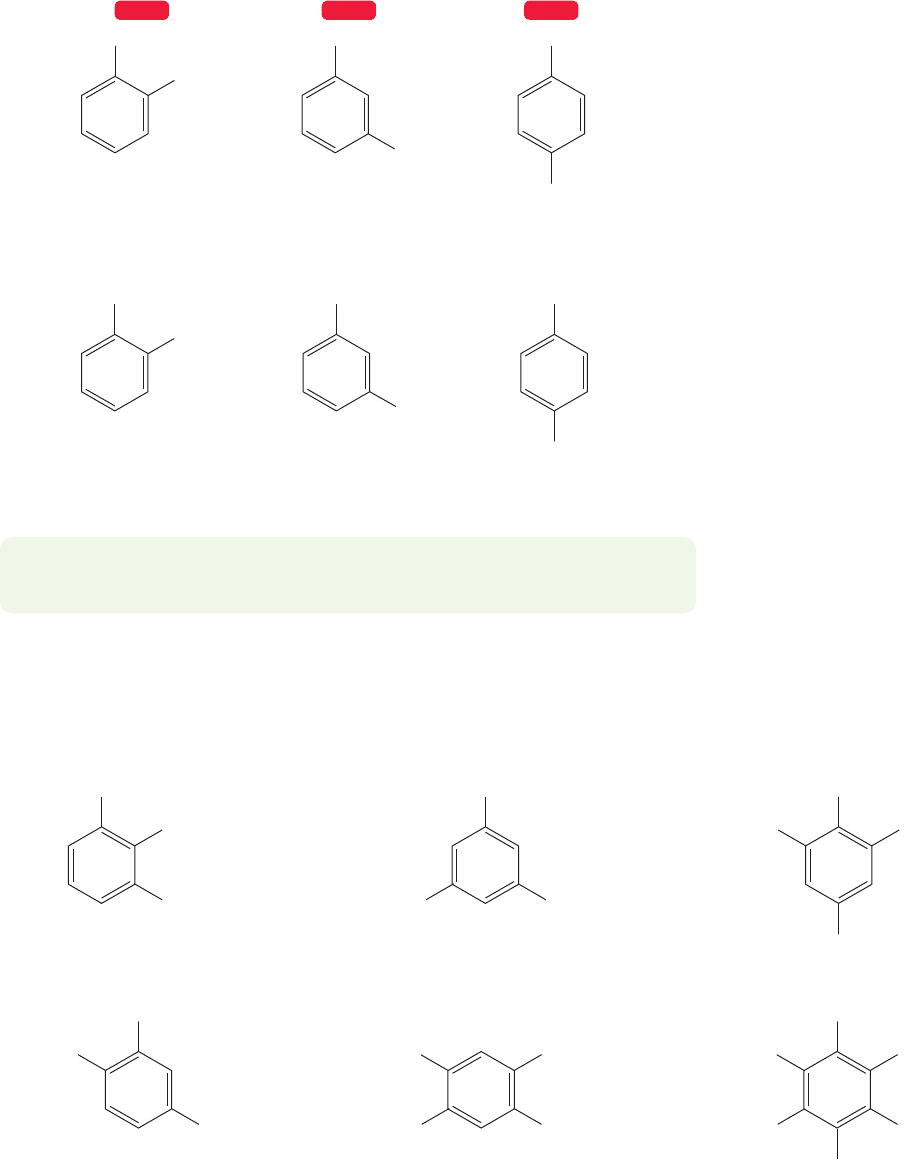
There are three possible isomers of disubstituted benzenes (Fig. 13.42).
1,2-Substitution is known as ortho (o-), 1,3-substitution as meta (m-), and
1,4-substitution as para (p-).
Ph
O
CH
2
FIGURE 13.42 The three possible substitution patterns for disubstituted benzenes.
ortho (o-)
1,2-Disubstituted
meta (m-)
1,3-Disubstituted
para (
p-)
1,4-Disubstituted
CONVENTION ALERT
In practice, either the numbers or the “ortho, meta, para” designations are used.
If there is a widely used common name, it is used as a base for the full name. For
example, the first and third molecules in Figure 13.43 are quite properly named as
o-bromotoluene and p-nitrophenol, respectively.
2-Bromotoluene
(o-bromotoluene)
CH
3
Br
1,3-Dichlorobenzene
(m-dichlorobenzene)
Cl
Cl
4-Nitrophenol
(
p-nitrophenol)
OH
NO
2
1-Ethyl-4-fluorobenzene
(
p-ethylfluorobenzene)
F
CH
2
CH
3
FIGURE 13.43 Four disubstituted
benzenes.
PROBLEM 13.15 The isolation of three, and only three isomers of disubstituted
benzenes was a crucial factor in the assignment of the Kekulé form as the correct
structure of benzene. Use the fact that there are three and only three achiral
isomers of a disubstituted benzene (C
6
H
4
R
2
) to criticize the suggestions that
benzene might have the following structures:
13.7 Substituted Benzenes 597
13.7b Polysubstitution There are disubstituted benzenes that are also known
by common names. Figure 13.44 shows a sampling of these molecules.
o -Xylene
(1,2 -dimethylbenzene)
CH
3
CH
3
m-Xylene
(1,3 -dimethylbenzene)
p-Xylene
(1,4 -dimethylbenzene)
CH
3
CH
3
CH
3
CH
3
OH
OH
Catechol
(o-dihydroxybenzene)
Resorcinol
(m-dihydroxybenzene)
Hydroquinone
(p-dihydroxybenzene)
OH
OH
OH
OH
WEB 3DWEB 3D WEB 3D
FIGURE 13.44 More common names
for disubstituted benzenes.
PROBLEM 13.16 How many signals will appear in the
13
C NMR spectra of the
three dihydroxybenzenes at the bottom of Figure 13.44?
1-Bromo-3-ethyl-2-nitrobenzene
NO
2
Br
CH
2
CH
3
1,3,5-Trichlorobenzene
ClCl
Cl
2-Bromo-4-chloro-6-fluoroaniline
FBr
NH
2
Cl
Hexachlorobenzene
ClCl
ClCl
Cl
Cl
4-Bromo-2,5-dichlorophenol
ClBr
OHCl
4-Bromo-2-chlorotoluene
H
3
C
Br
Cl
FIGURE 13.45 Some named polysubstituted benzenes.
The substituents on polysubstituted benzenes are numbered and named alpha-
betically (Fig. 13.45). Notice that a benzene with a methyl-,a hydroxy-,or an amino-
group will have the root word of toluene, phenol, or aniline respectively. Because it
is the root word, the carbon attached to that group is automatically C(1).

598 CHAPTER 13 Conjugation and Aromaticity
13.8 Physical Properties of Substituted Benzenes
The physical properties of substituted benzenes resemble those of alkanes and alkenes
of similar shape and molecular weight. Table 13.1 collects some physical properties
for a number of common substituted benzenes. Notice the effects of symmetry.
p-Xylene melts at a much higher temperature than the ortho or meta isomer, for
example. Many para isomers have high melting points,and crystallization can some-
times be used as a means of separating the para regioisomer from the others.
TABLE 13.1 Physical Properties of Some Substituted Benzenes
Name bp (°C) mp (°C) Density (g/mL)
Cyclohexane 80.7 6.5 0.78
Benzene 80.1 5.5 0.88
Methylcyclohexane 100.9 126.6 0.77
Toluene 110.6 95 0.87
o-Xylene 144.4 25.2 0.88
m-Xylene 139.1 47.9 0.86
p-Xylene 138.3 13.3 0.86
Aniline 184.7 6.3 1.02
Phenol 181.7 43 1.06
Anisole 155 37.5 1.0
Bromobenzene 156.4 30.8 1.5
Styrene 145.2 30.6 0.91
Benzoic acid 249.1
a
122.1 1.1
Benzaldehyde 178.6 26 1.0
Nitrobenzene 210.8 5.7 1.2
Biphenyl 255.9 71 0.87
a
At 10 mm pressure.
You have learned that aromatic compounds are particularly stable. This stabili-
ty is reflected in the chemical and physical properties of substances that contain aro-
matic compounds, such as graphite and coal. Aromatic molecules are hardy.
Another important physical property of aromatic compounds is their involvement
in π stacking. These flat molecules lie easily on top of each other, and this property
is a critical feature of enzyme structure–activity and in DNA double helix formation.
Aromatic compounds are called arenes and the term for an arene with an open
valence is aryl. A general abbreviation for aryl is Ar, in analogy to the R used for a
general alkyl species.
CONVENTION ALERT
Pyridine
H
H
H
H
H
N
..
H
H
H
H
H
H
Benzene
FIGURE 13.46 Pyridine, a heterobenzene.
13.9 Heterobenzenes and Other Heterocyclic
Aromatic Compounds
13.9a Contrasting Structures of Pyridine and the Five-Membered
Rings Pyrrole, Furan, and Thiophene
If a CH unit of benzene is
replaced with a nitrogen, the result is pyridine, another aromatic six-membered
ring (Fig. 13.46). From the provincial view of organic chemistry all atoms except
